Post-Border Forest Biosecurity in Australia: Response to Recent Exotic Detections, Current Surveillance and Ongoing Needs
Abstract
1. Introduction
2. Detection and Responses to Exotic Forest Pests and Pathogens in Australia
2.1. Methods
2.2. Detections of Exotic Forest Pests
2.3. Response to Detections
3. Current Biosecurity Surveillance for Early Detection of Exotic Plant Pests
Current Post-Border Surveillance for Forest Pests
4. Discussion
5. Ongoing Needs
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pheloung, P. An Australian perspective on the management of pathways for invasive species. In Invasive Species: Vectors and Management Strategies; Ruiz, G.M., Carlton, J.T., Eds.; Island Press: Washington, DC, USA, 2003; pp. 249–269. [Google Scholar]
- Anagnostakis, S.L. Chestnut blight: The classical problem of an introduced pathogen. Mycologia 1987, 79, 23–37. [Google Scholar] [CrossRef]
- Brasier, C.; Webber, J. Sudden larch death. Nature 2010, 466, 824–825. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.N. Intercontinental epidemiology of Dutch elm disease. Ann. Rev. Phytopathol. 1978, 16, 287–307. [Google Scholar] [CrossRef]
- Haack, R.A.; Hérard, F.; Sun, J.; Turgeon, J.J. Managing invasive populations of Asian longhorned beetle and citrus longhorned beetle: A worldwide perspective. Ann. Rev. Entomol. 2009, 55, 521–546. [Google Scholar] [CrossRef]
- Herms, D.A.; McCullough, D.G. Emerald ash borer invasion in North America: History, biology, impacts, and management. Ann. Rev. Entomol. 2014, 59, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.G.; Futai, K.; Sutherland, J.R.; Takeuchi, Y. Pine Wilt Disease; Springer: Tokyo, Japan, 2008. [Google Scholar]
- Craik, W.; Palmer, D.; Sheldrake, R. Priorities for Australia’s Biosecurity System: An Independent Review of the Capacity of the National Biosecurity System and Its Underpinning Intergovernmental Agreement; Commonwealth of Australia: Canberra, Australia, 2017. Available online: http://www.agriculture.gov.au/biosecurity/partnerships/nbc/intergovernmental-agreement-on-biosecurity/igabreview#final-report (accessed on 11 January 2019).
- Plant Health Australia. National Plant Biosecurity Status Report 2017; Plant Health Australia: Canberra, Australia, 2018. [Google Scholar]
- Council of Australian Governments. National Environmental Biosecurity Response Agreement (NEBRA); Commonwealth of Australia: Canberra, Australia, 2012. Available online: http://www.agriculture.gov.au/biosecurity/emergency/nebra (accessed on 11 January 2019).
- Carnegie, A.J.; Lawson, S.; Wardlaw, T.; Cameron, N.; Venn, T. Benchmarking forest health surveillance and biosecurity activities for managing Australia’s exotic forest pest and pathogen risks. Aust. For. 2018, 81, 14–23. [Google Scholar] [CrossRef]
- Mohammed, C.; Glen, M.; Walshe, T.; Wardlaw, T.; Stone, C.; Beadle, C.; Lawson, S. An Audit of Forest Biosecurity Arrangements and Preparedness in Australia; FWPA Report PNC159-0910; Forests and Wood Products Australia: Melbourne, Australia, 2011; Available online: https://www.fwpa.com.au/resources/resources/116-an-audit-of-forest-biosecurity-arrangements-and-preparedness-in-australia.html (accessed on 11 January 2019).
- Tovar, F.; Carnegie, A.J.; Collins, S.; Horwood, M.; Lawson, S.; Smith, D.; Subasinghe, R.; Wardlaw, T. Framework for National Biosecurity Surveillance of Exotic Forest Pests; Department of Agriculture and Water Resources: Canberra, Australia, 2017.
- Beale, R.; Fairbrother, J.; Inglis, A.; Trebeck, D. One Biosecurity: A Working Partnership. The Independent Review of Australia’s Quarantine and Biosecurity Arrangements; Commonwealth of Australia: Canberra, Australia, 2008; p. 244. Available online: https://web.archive.org/web/20091024200423/http:/daff.gov.au/__data/assets/pdf_file/0010/931609/report-single.pdf (accessed on 11 January 2019).
- Nairn, M.E.; Allen, P.G.; Inglis, A.R.; Tanner, C. Australian Quarantine: A Shared Responsibility; Department of Primary Industries and Energy: Canberra, Australia, 1996; p. 284. Available online: http://www.agriculture.gov.au/biosecurity/australia/reports-pubs/nairn (accessed on 11 January 2019).
- Cahill, D.M.; Rookes, J.E.; Wilson, B.A.; Gibson, L.; McDougall, K.L. Phytophthora cinnamomi and Australia’s biodiversity: Impacts, predictions and progress towards control. Aust. J. Bot. 2008, 56, 279–310. [Google Scholar] [CrossRef]
- Cameron, N.L.; Carnegie, A.J.; Wardlaw, T.; Lawson, S.A.; Venn, T. An economic evaluation of sirex wood wasp (Sirex noctilio) control in Australian pine plantations. Aust. For. 2018, 81, 37–45. [Google Scholar] [CrossRef]
- Lefoe, G. Management of Elm Pests and Diseases in Australia; Horticulture Australia Ltd.: Sydney, Australia, 2006; p. 45. [Google Scholar]
- Carnegie, A.J.; Lawson, S.; Cameron, N.; Wardlaw, T.; Venn, T. Evaluating the Costs and Benefits of Managing New and Existing Biosecurity Threats to Australia’s Plantation Industry; Project number: PNC362-1415; Forest and Wood Products Australia: Melbourne, Australia, 2017. Available online: https://www.fwpa.com.au/images/resources/-2017/Final_Report_-_Forest_Biosecurity_Benefit-Cost_PNC362-1415.pdf (accessed on 11 January 2019).
- Andow, D.D. Pathways-based risk assessment of exotic species invasions. In Invasive Species: Vectors and Management Strategies; Ruiz, G.M., Carlton, J.T., Eds.; Island Press: Washington, DC, USA, 2003; pp. 439–455. [Google Scholar]
- Ruiz, G.M.; Carlton, J.T. (Eds.) Invasive Species: Vectors and Management Strategies; Island Press: Washington, DC, USA, 2003. [Google Scholar]
- Brockerhoff, E.G.; Liebhold, A.M.; Richardson, B.; Suckling, D.M. Eradication of invasive forest insects: Concepts, methods, costs and benefits. N. Z. J. For. Sci. 2010, 40, S117–S135. [Google Scholar]
- Liebhold, A.M.; Berec, L.; Brockerhoff, E.G.; Epanchin-Niell, R.S.; Hastings, A.; Herms, D.A.; Kean, J.M.; McCullough, D.G.; Suckling, D.M.; Tobin, P.C.; et al. Eradication of invading insect populations: From concepts to applications. Ann. Rev. Entomol. 2016, 61, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Liebhold, A.M.; Kean, J.M. Eradication and containment of non-native forest insects: Successes and failures. J. Pest Sci. 2019, 92, 83–91. [Google Scholar] [CrossRef]
- Tobin, P.C.; Kean, J.M.; Suckling, D.M.; McCullough, D.G.; Herms, D.A.; Stringer, L.D. Determinants of successful arthropod eradication programs. Biol. Invasions 2014, 16, 401–414. [Google Scholar] [CrossRef]
- Pheloung, P. Plant pest surveillance in Australia. Aust. J. Emerg. Manag. 2004, 19, 13–16. [Google Scholar]
- Plant Health Australia. PLANTPLAN: Australian Emergency Plant Pest Response Plan; Version 2; Plant Health Australia: Canberra, Australia, 2010. [Google Scholar]
- Eldridge, R.H.; Simpson, J.A. Development of contingency plans for use against exotic pests and diseases of trees and timber: 3. Histories of control methods against some introduced pests and diseases of forests and forest products in Australia. Aust. For. 1987, 50, 24–36. [Google Scholar] [CrossRef]
- Walker, J.; Hartigan, D.; Bertus, A.L. Poplar rusts in Australia with comments on potential conifer rusts. Plant Dis. Rep. 1974, 4, 100–118. [Google Scholar] [CrossRef]
- Lawson, S.A.; Carnegie, A.J.; Cameron, N.; Wardlaw, T.; Venn, T. Risk of exotic pests to the Australian forest industry. Aust. For. 2018, 81, 3–13. [Google Scholar] [CrossRef]
- Department of Agriculture and Water Resources. National Forest Biosecurity Surveillance Strategy; Plant Health Australia: Canberra, Australia, 2018; p. 32. Available online: http://www.planthealthaustralia.com.au/strategies/national-forest-biosecurity-surveillance-strategy/ (accessed on 11 January 2019).
- Department of Agriculture and Water Resources. National Forest Biosecurity Surveillance Strategy—Implementation Plan; Plant Health Australia: Canberra, Australia, 2018; p. 24. Available online: http://www.planthealthaustralia.com.au/strategies/national-forest-biosecurity-surveillance-strategy/ (accessed on 11 January 2019).
- Plant Health Australia. National Plant Biosecurity Status Report 2014; Plant Health Australia: Canberra, Australia, 2015. [Google Scholar]
- Plant Health Australia. National Plant Biosecurity Status Report 2015; Plant Health Australia: Canberra, Australia, 2016. [Google Scholar]
- Kean, J.M.; Suckling, D.M.; Sullivan, N.J.; Tobin, P.C.; Stringer, L.D.; Smith, G.R.; Kimber, B.; Lee, D.C.; Flores Vargas, R.; Fletcher, J.; et al. Global Eradication and Response Database. 2019. Available online: http://b3.net.nz/gerda (accessed on 17 February 2019).
- Food and Agriculture Organization. ISPM 6. Guidelines for Surveillance; FAO, IPPC: Rome, Italy, 1997; Available online: https://www.ippc.int/static/media/files/publication/en/2016/01/ISPM_06_1997_En_2015-12-22_PostCPM10_InkAmReformatted.pdf (accessed on 11 January 2019).
- Hodda, M.; Smith, D.; Smith, I.; Nambiar, L.; Pascoe, I. Incursion management in the face of multiple uncertainties: A case study of an unidentified nematode associated with dying pines near Melbourne, Australia. In Pine Wilt Disease: A Worldwide Threat to Forest Ecosystems; Mota, M.M., Vieira, P., Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 15–40. [Google Scholar]
- Smith, D.I.; Hodda, M.; Smith, I.W.; Nambiar, L.; Pascoe, I.G.; Aldaoud, R. Bursaphelenchus hunanensis associated with dying Pinus species in Victoria, Australia. Australas. Plant Dis. Notes 2008, 3, 93–95. [Google Scholar] [CrossRef]
- Carnegie, A.J.; Lidbetter, J.R.; Walker, J.; Horwood, M.A.; Tesoriero, L.; Glen, M.; Priest, M.J. Uredo rangelii, a taxon in the guava rust complex, newly recorded on Myrtaceae in Australia. Australas. Plant Pathol. 2010, 39, 463–466. [Google Scholar] [CrossRef]
- Carnegie, A.J.; Cooper, K. Emergency response to the incursion of an exotic myrtaceous rust in Australia. Australas. Plant Pathol. 2011, 40, 346. [Google Scholar] [CrossRef]
- Carnegie, A.J.; Pegg, G.S. Lessons from the incursion of myrtle rust in Australia. Ann. Rev. Phytopathol. 2018, 56, 457–478. [Google Scholar] [CrossRef]
- Plant Health Australia. National Plant Biosecurity Status Report 2016; Plant Health Australia: Canberra, Australia, 2017. [Google Scholar]
- Carnegie, A.J.; Daly, A.; Rossiter, L.; Sargeant, D.; Nagel, M.; Laurence, M.; Seago, S.; Gillespie, P.; Sullivan, S.; Gunning, D.; et al. Response to the detection of Bursaphelenchus aff. vallesiansus/sexdentati on Pinus in NSW: Partnerships in biosecurity response. In Proceedings of the Australian Entomological Society Conference, Terrigal, Australia, 17–20 September 2017. [Google Scholar]
- Plant Health Australia. National Plant Biosecurity Status Report 2011; Plant Health Australia: Canberra, Australia, 2012. [Google Scholar]
- International Plant Protection Convention. Pest reports from Australia. Detection of Willow Black Canker. 2006. Available online: https://www.ippc.int/en/countries/australia/pestreports/ (accessed on 12 December 2018).
- Cunnington, J.H.; Powney, R.A.; Adair, R.J.; Finlay, K.J. Glomerella miyabeana on willows in Australia. Australas. Mycol. 2007, 25, 69–72. [Google Scholar]
- Dominiak, B.C.; Gillespie, P.S.; Worsley, P.; Locker, H. Survey for sycamore lace bug Corythucha ciliata (Say) (Hemiptera: Tingidae) in New South Wales during 2007. Gen. Appl. Entomol. 2008, 37, 27–30. [Google Scholar]
- International Plant Protection Convention. Pest Reports from Australia. Confirmation of Chestnut Blight in North-East Victoria. 2010. Available online: https://www.ippc.int/en/countries/australia/pestreports/ (accessed on 12 December 2018).
- Plant Health Australia. National Plant Biosecurity Status Report 2010; Plant Health Australia: Canberra, Australia, 2011. [Google Scholar]
- Carver, M.; Kent, D.S. Essigella californica (Essig) and Eulachnus thunbergia Wilson (Hemiptera: Aphididae): Lachninae) on Pinus in south-eastern Australia. Aust. J. Entomol. 2000, 39, 62–69. [Google Scholar] [CrossRef]
- Jacobs, K.; Wingfield, M.J.; Wingfield, B.D.; Yamaoka, Y. Comparison of Ophiostoma huntii and O. europhioides and description of O. aenigmaticum sp. nov. Mycol. Res. 1998, 102, 289–294. [Google Scholar] [CrossRef]
- Howick, C.D. The European house borer Hylotrupes bajulus (L.) in Australia–a 40-year update. Sber Ges Naturf Freunde 2010, 50, 57–63. [Google Scholar]
- Fletcher, M.J.; Knight, W.J. New Australian records for exotic Leafhoppers. In Proceedings of the 29th Annual General Meeting and Scientific Conference of the Australian Entomological Society, Brisbane, Queensland, Austrilia, 26–29 September 1998; 1998. [Google Scholar]
- Simpson, J.A.; Grgurinovic, C.A. First record of Lophodermium conigenum on Pinus in Australia. Australas. Plant. Pathol. 2004, 33, 447–448. [Google Scholar] [CrossRef]
- Bruzzese, E.; McFadyen, R. Arrival of leaf-feeding willow sawfly Nematus oligospilus Förster in Australia—Pest of beneficial? Plant Prot. Q. 2006, 21, 43–44. [Google Scholar]
- Caron, V.; Ede, F.; Sunnucks, P.; O’Dowd, D.J. Distribution of the introduced willow sawfly Nematus oligospilus Förster (Hymenoptera:Tenthredinidae) in Australasia. Aust. Entomol. 2014, 53, 175–182. [Google Scholar] [CrossRef]
- International Plant Protection Convention. Pest Reports from Australia. Detection of Teak Leaf Rust. 2006. Available online: https://www.ippc.int/en/countries/australia/pestreports/ (accessed on 12 December 2018).
- Daly, A.M.; Shivas, R.G.; Pegg, G.S.; Mackie, A.E. First record of teak leaf rust (Olivea tectonae) in Australia. Australas. Plant Dis. Notes 2006, 1, 25–26. [Google Scholar] [CrossRef]
- Davidson, E.M.; Drenth, A.; Kumar, S.; Mack, S.; Mackie, A.E.; McKirdy, S. Pathogens associated with nursery plants imported into Western Australia. Australas. Plant Pathol. 2006, 35, 473–475. [Google Scholar] [CrossRef]
- Plant Health Australia. National Plant Biosecurity Status Report 2013; Plant Health Australia: Canberra, Australia, 2014. [Google Scholar]
- Caon, G.; De Barro, P. Ash whitefly—A new pest. Nurs. Pap. 1999, 7, 1–2. [Google Scholar]
- Andjic, V.; Maxwell, A.; Hardy, G.E.S.; Burgess, T.I. New cryptic species of Teratosphaeria on Eucalyptus in Australia. IMA Fungus 2016, 7, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Burgess, T.I.; Andjic, V.; Wingfield, M.J.; Hardy, G.E.S.J. The eucalypt leaf blight pathogen Kirramyces destructans discovered in Australia. Australas. Plant Dis. Notes 2007, 2, 141–144. [Google Scholar] [CrossRef]
- Carnegie, A.J.; Shuey, L.S.; McTaggart, A.R.; Shivas, R.G. First record of Thyronectria pinicola on Pinus radiata in Australia. Plant Dis. 2015, 99, 1182. [Google Scholar] [CrossRef]
- Pegg, G.S.; Griffiths, M.; McDonald, J.; Bartlett, J.; Nahrung, H.; Hayes, A.; Lawson, S. Protecting Queensland’s Timber Resource from Pest and Disease Incursions; Department of Agriculture and Fisheries: Brisbane, Queensland, Australia, 2018.
- International Plant Protection Convention. Pest Reports from Australia. Mahogany Angular Leaf Spot Present in Northern Territory. 2009. Available online: https://www.ippc.int/en/countries/australia/pestreports/ (accessed on 12 December 2018).
- Commonwealth of Australia. Forests and Timber: A Field Guide to Exotic Pests and Diseases; Commonwealth of Australia: Canberra, Australia, 2000. Available online: http://www.agriculture.gov.au/pests-diseases-weeds/forestry-timber (accessed on 11 January 2019).
- van Schagen, J.; Bain, J. New Zealand’s part in Australia’s fight against European house borer. Biosecurity 2009, 89, 1. Available online: http://www.nzffa.org.nz/farm-forestry-model/the-essentials/forest-health-pests-and-diseases/Pests/hylotrupes-bajulus/new-zealands-part-in-australias/ (accessed on 11 January 2019).
- Carnegie, A.J.; Kathuria, A.; Pegg, G.S.; Entwistle, P.; Nagel, M.; Giblin, F.R. Impact of the invasive rust Puccinia psidii (myrtle rust) on native Myrtaceae in natural ecosystems in Australia. Biol. Invasions 2016, 18, 127–144. [Google Scholar] [CrossRef]
- Pegg, G.S.; Taylor, T.; Entwistle, P.; Guymer, G.; Giblin, F.G.; Carnegie, A.J. Impact of Austropuccinia psidii on Myrtaceae rich wet sclerophyll forests in south-east Queensland. PLoS ONE 2017, 12, e0188058. [Google Scholar] [CrossRef]
- Eyles, A.; Robinson, A.P.; Smith, D.; Carnegie, A.J.; Smith, I.; Stone, C.; Mohammed, C. Quantifying stem growth loss at the tree-level in a Pinus radiata plantation to repeated attack by the aphid, Essigella californica. For. Ecol. Manag. 2011, 261, 120–127. [Google Scholar] [CrossRef]
- May, B.M.; Carlyle, J.C. Effect of defoliation associated with Essigella californica on growth of mid-rotation Pinus radiata. For. Ecol. Manag. 2003, 183, 297–312. [Google Scholar] [CrossRef]
- Nahrung, H.F.; Loch, A.D.; Matsuki, M. Invasive insects in Mediterranean forest systems: Australia. In Insects and Diseases of Mediterranean Forest Systems; Paine, T., Lieutier, F., Eds.; Springer: Cham, Switzerland, 2016; pp. 475–498. [Google Scholar]
- Plant Health Aust. Plantation Forest Biosecurity Plan; Version 2.0; Plant Health Australia: Canberra, Australia, 2013. [Google Scholar]
- Gould, B. MPI surveillance and incursion management. In Proceedings of the 13th Annual FOA/MPI Forest Biosecurity Workshop, Rotorua, New Zealand, 24–25 February 2015; Available online: https://www.nzfoa.org.nz/resources/file-libraries-resources/foa-workshop-conference-reports/forest-health-workshops/fh2015 (accessed on 11 January 2017).
- Wilson, J.A.; Stephenson, B.P.; Gill, G.S.C.; Randall, J.L.; Viegelais, C.M.C. Principles of response to detections of new plant pest species and the effectiveness of surveillance. N. Z. Plant Prot. 2004, 57, 156–160. [Google Scholar]
- Anderson, C.; Low-Choy, S.; Whittle, P.; Taylor, S.; Gambley, C.; Smith, L.; Gillespie, P.; Löcker, H.; Davis, R.; Dominiak, B. Australian plant biosecurity surveillance systems. Crop Prot. 2017, 100, 8–20. [Google Scholar] [CrossRef]
- Tobin, P.C.; Berec, L.; Liebhold, A.M. Exploiting Allee effects for managing biological invasions. Ecol. Lett. 2010, 14, 615–624. [Google Scholar] [CrossRef]
- McAllister, R.R.J.; Robinson, C.J.; Brown, A.; Maclean, K.; Perry, S.; Liu, S. Balancing collaboration with coordination: Contesting eradication in the Australian plant pest and disease biosecurity system. Int. J. Commons 2017, 11, 330–354. [Google Scholar] [CrossRef]
- Berthon, K.; Esperón-Rodróguez, M.; Beaumont, L.; Carnegie, A.J.; Leishman, L. Assessment and prioritisation of plant species at risk from myrtle rust (Austropuccinia psidii) under current and future climates in Australia. Biol. Conserv. 2018, 218, 154–162. [Google Scholar] [CrossRef]
- Cannon, A.M. Myrtle Rust—Forest Industry Issues Paper; Project Number: PRC 218-1011; Forest & Wood Products Australia: Melbourne, Australia, 2011; Available online: https://www.fwpa.com.au/images/resources/PRC218-1011_Myrtle_Rust_June_2011_0.pdf (accessed on 11 January 2019).
- Cunningham, R.J.; Collins, D.; Vagg, L.; Grimm, M. Pine Timber Roof Environments in Western Australia and Its Susceptibility to European House Borer; Forest & Wood Products Australia Limited: Malbourne, Australia, 2010; Available online: https://www.fwpa.com.au/resources/market-access/218-pine-timber-roof-environments-in-western-australia-and-its-susceptibility-to-european-house-borer.html (accessed on 11 January 2019).
- Hodby, A.; Sagerer, S. European House Borer Project: Technical Review; Acil Allen Consulting: Perth, Australia, 2017. [Google Scholar]
- Wylie, F.R.; Griffiths, M.; King, J. Development of hazard site surveillance programs for forest invasive species: A case study from Brisbane, Australia. Aust. For. 2008, 71, 119–235. [Google Scholar] [CrossRef]
- Morgan, F.D. Ips grandicollis in South Australia. Aust. For. 1967, 31, 137–155. [Google Scholar] [CrossRef]
- Stone, C.; Simpson, J.A. Species associations in Ips grandicollis galleries in Pinus taeda. N. Z. J. For. Sci. 1990, 20, 75–96. [Google Scholar]
- Bashford, R. The development of a port surrounds trapping system for the detection of exotic forest insect pests in Australia. In New Advances and Contributions to Forestry Research; Oteng-Amoaka, A., Ed.; InTech: Rijeka, Croatia, 2012; pp. 86–100. [Google Scholar]
- Smith, D.; Smith, B.; Smith, I. The Use of Sentinel Plantings to Provide an Early Warning of Potential Pests of Concern to the Victorian Timber Industry; The University of Melbourne: Melbourne, Australia, 2010. [Google Scholar]
- Cook, D.; Fraser, R.; Wilby, A. Plant Biosecurity Policy Evaluation: The Economic Impact of Pests and Diseases; World Scientific: Singapore, 2017. [Google Scholar]
- National Biosecurity Committee. National Framework for the Management of Established Pests and Diseases of National Significance; Commonwealth of Australia: Canberra, Australia, 2016. Available online: http://www.agriculture.gov.au/biosecurity/partnerships/nbc/intergovernmental-agreement-on-biosecurity/national-framework (accessed on 11 January 2019).
- Colquhoun, I.; Hardy, G.E.S.J. Managing the risks of Phytophthora root and collar rot during bauxite mining in the Eucalyptus marginata (Jarrah) forest of Western Australia. Plant Dis. 2000, 84, 116–127. [Google Scholar] [CrossRef] [PubMed]
- O’Gara, E.; Howard, K.; Wilson, B.; Hardy, G.E.S.J. Management of Phytophthora cinnamomi for Biodiversity Conservation in Australia: Part 1. A Review of Current Management; Centre for Phytophthora Science and Management, Murdoch University: Murdoch, Australia, 2015. [Google Scholar]
- Klapwijk, M.J.; Hopkins, A.J.; Eriksson, L.; Pettersson, M.; Schroeder, M.; Lindelöw, Å.; Rönnberg, J.; Keskitalo, E.C.H.; Kenis, M. Reducing the risk of invasive forest pests and pathogens: Combining legislation, targeted management and public awareness. Ambio 2016, 45, 223–234. [Google Scholar] [CrossRef]
- Laidlaw, M.J.; Holland, A.; Guymer, G. Many eyes on the prize: The role of citizen scientists in active weed surveillance. In Proceedings of the 20th Australasian Weeds Conference, Perth, Australia, 11–15 September 2016; pp. 17–19. [Google Scholar]
- Carnegie, A.J.; Venn, T.; Lawson, S.A.; Nagel, M.; Wardlaw, T.; Cameron, N.L.; Last, I. An analysis of pest risk and potential economic impact of pine wilt disease to Pinus plantations in Australia. Aust. For. 2018, 81, 24–36. [Google Scholar] [CrossRef]
- Colunga-Garcia, M.; Magarey, R.A.; Haack, R.A.; Gage, S.H.; Qi, J. Enhancing early detection of exotic pests in agricultural and forest ecosystems using an urban-gradient framework. Ecol. Appl. 2010, 20, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Hulbert, J.M.; Agne, M.C.; Burgess, T.I.; Roets, F.; Wingfield, M.J. Urban environments provide opportunities for early detections of Phytophthora invasions. Biol. Invasions 2017, 19, 3629–3644. [Google Scholar] [CrossRef]
- Paap, T.; Burgess, T.I.; Wingfield, M.J. Urban trees: Bridge-heads for forest pest invasions and sentinels for early detection. Biol. Invasions 2017, 19, 3515–3526. [Google Scholar] [CrossRef]
- Bulman, L.S. Pest detection surveys on high-risk sites in New Zealand. Aust. For. 2008, 71, 242–244. [Google Scholar] [CrossRef]
- Hester, S.M.; Cacho, O.J. The contribution of passive surveillance to invasive species management. Biol. Invasions 2017, 19, 737–748. [Google Scholar] [CrossRef]
- Poland, T.M.; Rassati, D. Improved biosecurity surveillance of non-native forest insects: A review of current methods. J. Pest Sci. 2019, 92, 1–13. [Google Scholar] [CrossRef]
- Rassati, D.; Faccoli, M.; Petrucco Toffolo, E.; Battisti, A.; Marini, L. Improving the early detection of alien wood-boring beetles in ports and surrounding forests. J. Appl. Ecol. 2015, 52, 50–58. [Google Scholar] [CrossRef]
- Hauser, C.E.; McCarthy, M.A. Streamlining ‘search and destroy’: Cost-effective surveillance for invasive species management. Ecol. Lett. 2009, 12, 683–692. [Google Scholar] [CrossRef]
- Plant Health Australia. Biosecurity Manual for the Plantation Timber Industry; Plant Health Australia: Canberra, Australia, 2015. [Google Scholar]
- Eschen, R.; Britton, K.; Brockerhoff, E.; Burgess, T.; Dalley, V.; Epanchin-Niell, R.S.; Gupta, K.; Hardy, G.; Huang, Y.; Kenis, M.; et al. International variation in phytosanitary legislation and regulations governing importation of plants for planting. Environ. Sci. Policy 2015, 51, 228–237. [Google Scholar] [CrossRef]
- Wardlaw, T.J.; Bashford, R.; Wotherspoon, K.P.; Wylie, F.R.; Elliott, H.J. Efficiency of routine forest health surveillance in detecting pest and disease damage in eucalypt plantations. N. Z. J. For. Sci. 2008, 38, 253–269. [Google Scholar]
- Aukema, J.E.; Leung, B.; Kovacs, K.; Chivers, C.; Britton, K.O.; Englin, J.; Frankel, S.J.; Haight, R.G.; Holmes, T.P.; Liebhold, A.M.; et al. Economic impacts of non-native forest insects in the continental United States. PLoS ONE 2011, 6, e25487. [Google Scholar] [CrossRef] [PubMed]
- Loo, J.A. Ecological impacts of non-indigenous invasive fungi as forest pathogens. Biol. Invasions 2009, 11, 81–96. [Google Scholar] [CrossRef]
- Pimentel, D.; McNair, S.; Janecka, J.; Wightman, J.; Simmonds, C.; O’Connell, C.; Wong, E.; Russel, L.; Zern, J.; Aquino, T.; et al. Economic and environmental threats of alien plant, animal, and microbe invasions. Agric. Ecosyst. Environ. 2001, 84, 1–20. [Google Scholar] [CrossRef]
- Santini, A.; Ghelardini, L.; De Pace, C.; Desprez-Loustau, M.L.; Capretti, P.; Chandelier, A.; Cech, T.; Chira, D.; Diamandis, S.; Gaitniekis, T.; et al. Biogeographical patterns and determinants of invasion by forest pathogens in Europe. New Phytol. 2013, 197, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Lovett, G.M.; Weiss, M.; Liebhold, A.M.; Holmes, T.P.; Leung, B.; Lambert, K.F.; Orwig, D.A.; Campbell, F.T.; Rosenthal, J.; McCullough, D.G.; et al. Nonnative forest insects and pathogens in the United States: Impacts and policy options. Ecol. Appl. 2016, 26, 1437–1455. [Google Scholar] [CrossRef]
- Roy, B.A.; Alexander, H.M.; Davidson, J.; Campbell, F.T.; Burdon, J.J.; Sniezko, R.; Brasier, C. Increasing forest loss worldwide from invasive pests requires new trade regulations. Front. Ecol. Environ. 2014, 12, 457–465. [Google Scholar] [CrossRef]
- Wingfield, M.J.; Brockerhoff, E.G.; Wingfield, B.D.; Slippers, B. Planted forest health: The need for a global strategy. Science 2015, 349, 832–836. [Google Scholar] [CrossRef]
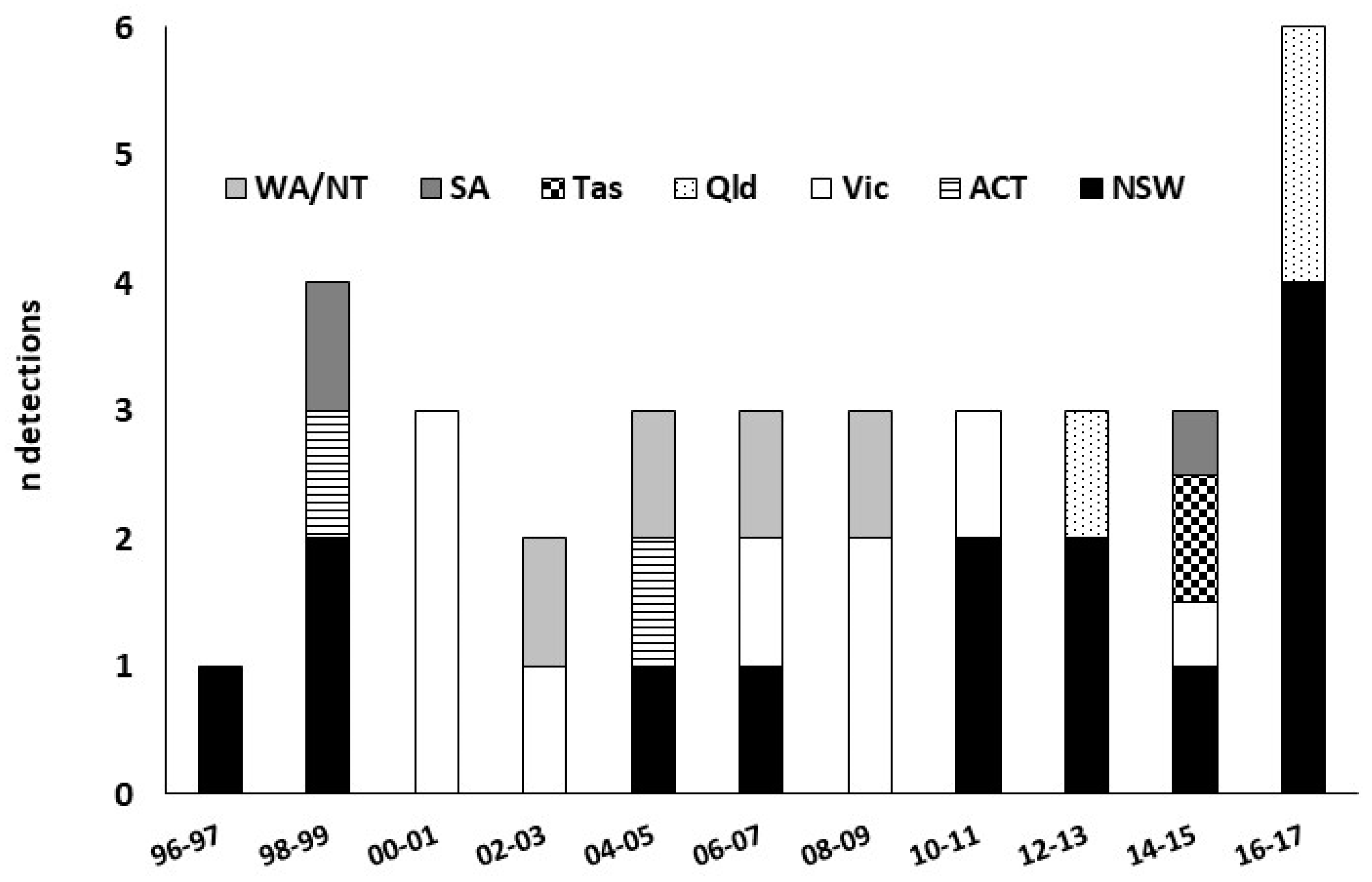
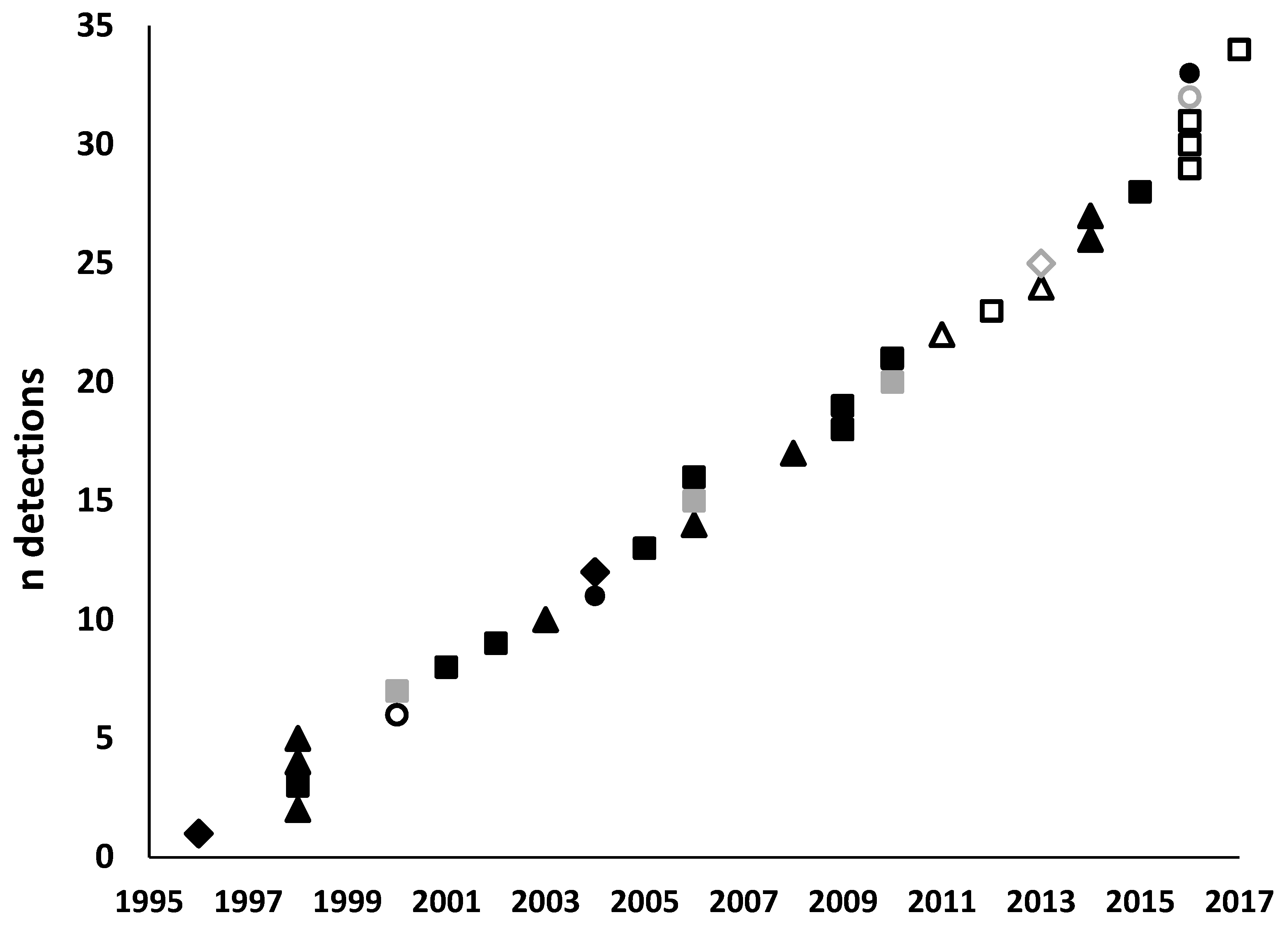
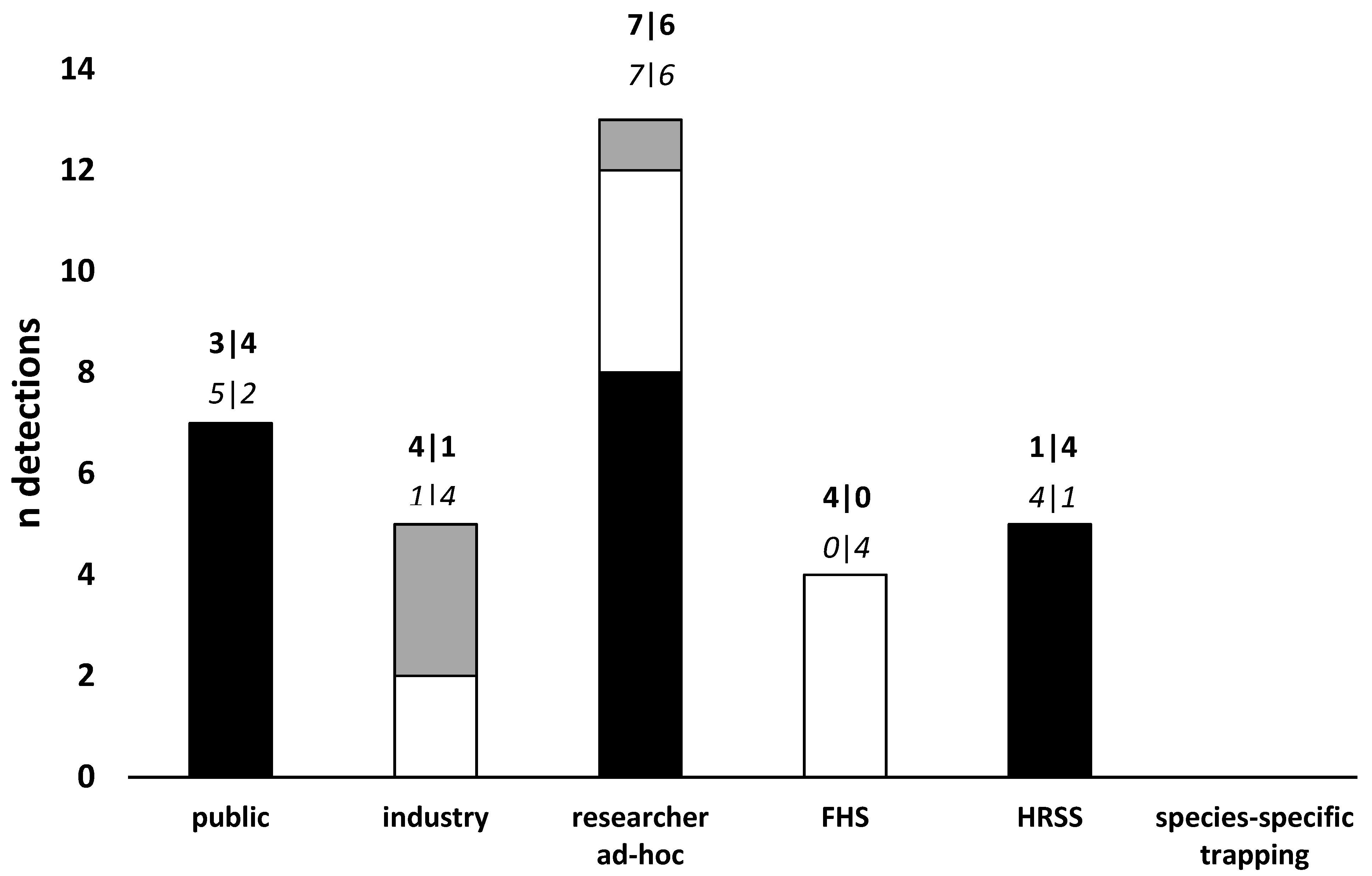
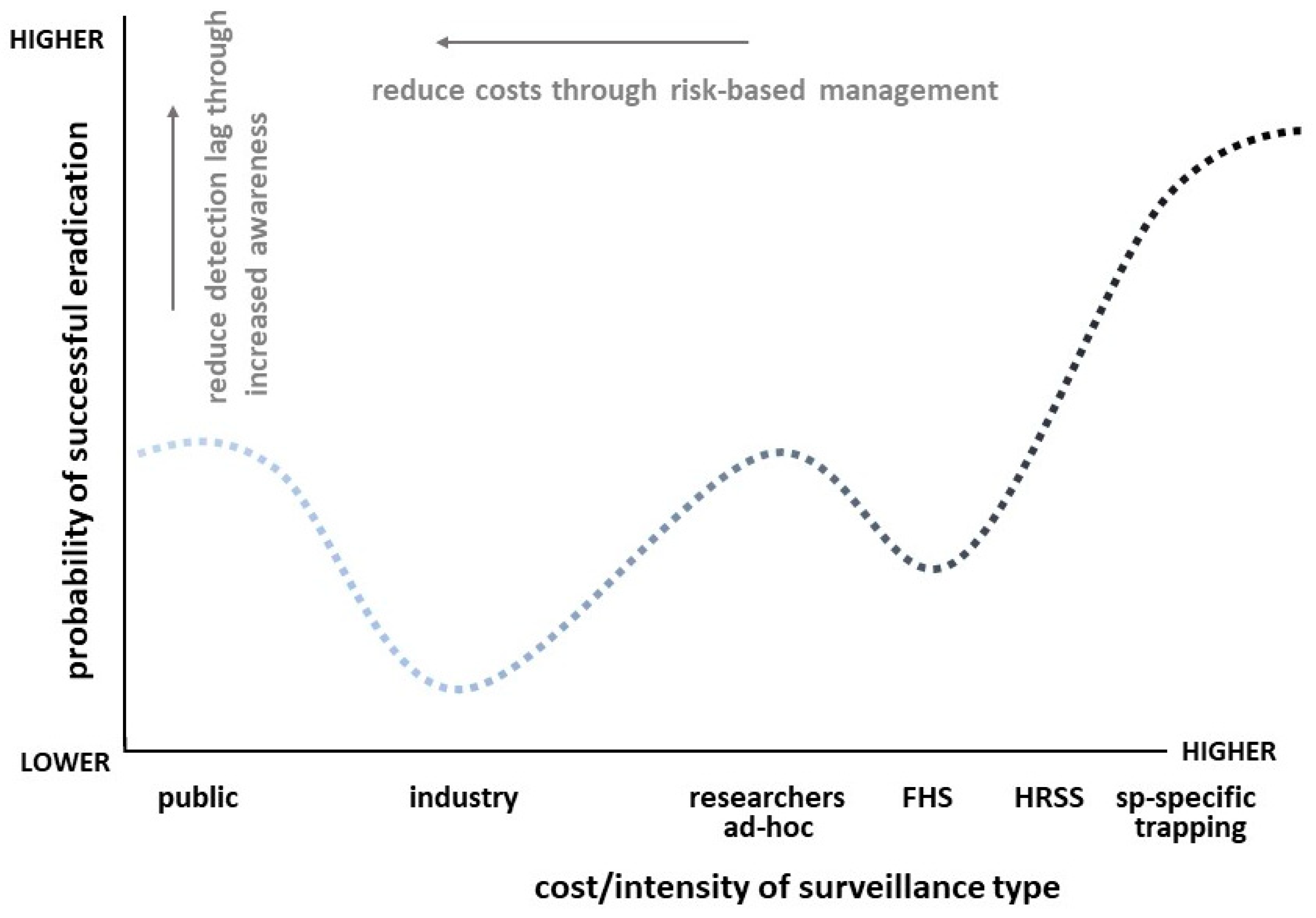
| Species | Common Name | Division/Order (Family) | Year | Main host/s | Surveillance Type a | Surveillance Level b | Detection Location c | Detection Region d | State | Response e | Status f | Impact g | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arhopalus rusticus | longicorn beetle | Coleoptera (Cerambycidae) | 2000 | Pinus | S | 5 | A | U | Vic | N * | E | L | [37,38] |
| Austropuccinia psidii | Myrtle rust | Basidiomycota/Pucciniales | 2010 | Myrtaceae | G | 2 | N ^ | R | NSW | E | E | H | [38,39,40,41] |
| Bursaphelenchus aff. vallesianus/sexdentati | pinewood nematode | Nematoda (Aphelenchidae) | 2016 | Pinus | S | 5 | A | U | NSW | N | E | L | [42,43] |
| Bursaphelenchus hildegardae | pinewood nematode | Nematoda (Aphelenchidae) | 2016 | Pinus | S | 4 | P | R | NSW | N | E | L | Carnegie et al. unpublished |
| Bursaphelenchus hunanesis | pinewood nematode | Nematoda (Aphelenchidae) | 2000 | Pinus | G | 1 | A | U | Vic | E | Er | n | [37,38] |
| Chaitophorus leucomelas | Black poplar leaf aphid | Hemiptera (Aphididae) | 2011 | Populus | S | 5 | A | U | NSW | N | E | L | [44], P. Gillespie (NSWDPI) pers. comm. |
| Cinara pilicornis | Spruce shoot aphid | Hemiptera (Aphididae) | 2008 | Picea | G | 2 | N | U | Vic | N | E | L | APPD; D. Smith (AgVic) pers. comm. |
| Colletotrichum salicis | Willow black canker | Ascomycota/Glomeralles | 2005 | Salix | G | 3 | A | R | NSW | N | E | L | [45,46] |
| Corythucha ciliata | Sycamore lace bug | Hemiptera (Tingidae) | 2006 | Platanus | G | 3 | A | U | NSW | N | E | M | [47], P. Gillespie (NSWDPI) pers. comm. |
| Cryphonectria parasitica | Chestnut blight | Ascomycota/Diaporthales | 2010 | Castanea | G | 2 | P | R | Vic | E | U | H | [42,48] |
| Diplodia africana | Diplodia canker | Ascomycota/Botryosphaeriales | 2009 | Pinus | G | 1 | A | U | Vic | N | E | L | [49], D. Smith (AgVic) pers. comm. |
| Essigella californica | Monterey pine aphid | Hemiptera (Aphididae) | 1998 | Pinus | G | 3 | A | U | ACT | N * | E | H | [50] |
| Grosmannia huntii | blue stain | Ascomycota/Ophiostomatales | 1998 | Pinus | G | 3 | P | R | NSW | N * | E | L | [51] |
| Hylotrupes bajulus | European house borer | Coleoptera (Cerambycidae) | 2004 | Pinus (dead) | G | 1 | H | U | WA | E | E | H | [35,52] |
| Kybos lindbergi | Birch leafhopper | Hemiptera (Cicadellidae) | 1998 | Betula | G | 1 | A | R | NSW | N * | E | L | [53] |
| Lophodermium conigenum | Lophodermium needle cast | Ascomycota/Rhytismatales | 2001 | Pinus | G | 3 | P | R | Vic | N * | E | L | [54] |
| Marchalina hellenica | Giant pine scale | Hemiptera (Margarodidae) | 2014 | Pinus | G | 1 | A | U | Vic/SA | E | E | H | [34,42] |
| Nematus oligospilus | Willow sawfly | Hymenoptera (Tenthredinidae) | 2004 | Salix | G | 3 | A | U | ACT | N * | E | M | [55,56] |
| Olivea tectonae | Teak leaf rust | Basidiomycota/Pucciniales | 2006 | Tectona | G | 2 | P | R | NT | N | E | L | [57,58] |
| Ophiostoma angusticollis | blue stain | Ascomycota/Ophiostomatales | 2017 | Pinus | S | 4 | P | R | NSW | N | E | L | NMG 2018 # |
| Ophiostoma pallidulum | blue stain | Ascomycota/Ophiostomatales | 2016 | Pinus | S | 4 | P | R | NSW | N | E | L | NMG 2018 # |
| Phytophthora niederhauserii | dieback | Oomycota/Peronosporales | 2002 | Polyphagous | G | 3 | N | R | NT/WA | N * | E | M | [59] |
| Psyllopsis fraxinicola | Ash leaf psyllid | Hemiptera (Psyllidae) | 2003 | Fraxinus | G | 3 | A | U | Vic | N * | E | L | APPD |
| Quadrastichus erythrinae | Erythina gall wasp | Hymenoptera (Eulophidae) | 2013 | Erythina | S | 5 | A | R | Qld | N | E * | L | [33], M. Ashton (QDAF) pers. comm. |
| Rugonectria castaneicola | Rugonectria canker | Ascomycota/Hypocreales | 2015 | Quercus | G | 3 | A | U | NSW | N | E | L | [34], Carnegie et al. unpublished |
| Shivaphis celti | Asian woolly hackberry aphid | Hemiptera (Aphididae) | 2013 | Celtis | S | 5 | A | U | NSW | N | E | L | [60], R. Rickard (DAWR) pers. comm. |
| Siphoninus phillyreae | Ash whitefly | Hemiptera (Aleyrodidae) | 1998 | Fraxinus | G | 3 | A | R | SA | N * | E | M | [61] |
| Teratosphaeria destructans‡ | Eucalypt leaf blight | Ascomycota/Capnodiales | 2006 | Eucalyptus | G | 3 | P ^ | R | NT | u | n | n | [62,63] |
| Thyronectria pinicola | canker | Ascomycota/Hypocreales | 2012 | Pinus | S | 4 | P | R | NSW | N | E | L | [64] |
| Tremex fuscicornis | Tremex wasp | Hymenoptera (Siricidae) | 1996 | Salix, Populus | G | 1 | A | R | NSW | N * | E | L | State Forests of NSW unpublished |
| Trichoferus campestris | Chineses longhorned beetle | Coleoptera (Cerambycidae) | 2016 | Polyphagous (Pinus) | S | 3 | A | U | Qld | n | D | n | [42,65] |
| Tuberolachnus salignus | Giant willow aphid | Hemiptera (Aphididae) | 2014 | Salix | G | 1 | A | R | Tas | N | E | L | [33], L. Hill (DPIPWE) pers. comm. |
| Xanthomonas axonopodis pv. unnamed | Mahogany angular leaf spot | Proteobacteria/Xanthomonadales | 2009 | Khaya | G | 2 | N | R | NT | N | E | L | [66] |
| Xylosandrus crassiusculus | Asian ambrosia beetle | Coleoptera (Curculionidae) | 2016 | Polyphagous | G | 3 | P | R | Qld | N | E | L | [42] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carnegie, A.J.; Nahrung, H.F. Post-Border Forest Biosecurity in Australia: Response to Recent Exotic Detections, Current Surveillance and Ongoing Needs. Forests 2019, 10, 336. https://doi.org/10.3390/f10040336
Carnegie AJ, Nahrung HF. Post-Border Forest Biosecurity in Australia: Response to Recent Exotic Detections, Current Surveillance and Ongoing Needs. Forests. 2019; 10(4):336. https://doi.org/10.3390/f10040336
Chicago/Turabian StyleCarnegie, Angus J., and Helen F. Nahrung. 2019. "Post-Border Forest Biosecurity in Australia: Response to Recent Exotic Detections, Current Surveillance and Ongoing Needs" Forests 10, no. 4: 336. https://doi.org/10.3390/f10040336
APA StyleCarnegie, A. J., & Nahrung, H. F. (2019). Post-Border Forest Biosecurity in Australia: Response to Recent Exotic Detections, Current Surveillance and Ongoing Needs. Forests, 10(4), 336. https://doi.org/10.3390/f10040336






