Abstract
Background and Motivation: Nitrogen content in tissues of Fagus crenata Blume is key for flowering and seed production. However, there is a lack of information on seasonal intra-plant nitrogen partitioning in this representative tree species typical of heavy snowfall regions in Japan. Therefore, the objective of this study was to elucidate Fagus crenata intra-plant nitrogen movement by means of nitrogen content, nitrogen isotope analysis, and amino acids temporal variability. Materials and Methods: Nitrogen content, isotope ratio, and free amino acids content were measured in coarse roots, sapwood, leaves, and litter in four phenological stages in nine adult Fagus crenata trees and upscaled to the whole-tree level. Results: Nitrogen was reabsorbed to and stored in coarse roots during the pre-abscission stage, as was revealed by the depletion of the δ15N ratio of coarse roots, which coincided with an enrichment of 15N found in leaves. During the post-abscission stage, N was stored in the sapwood, where an enrichment in 15N was found coinciding with the depletion of the δ15N ratio in leaves. It seemed that 15N-enriched nitrogen was initially reabsorbed from leaves to coarse roots during the pre-abscission period, followed by the reabsorption of 15N-enriched nitrogen from leaves to sapwood shortly before leaf abscission. Free amino acids content and their dynamics could mostly explain seasonal δ15N fractionation in leaves, coarse roots, and partially in sapwood. At the whole-tree level, N content stored in coarse roots and sapwood was similar. Furthermore, reabsorbed leaf N accounted for 32% of all nitrogen stored during leaf senescence. Conclusion: We found three phases of nitrogen storage revealed by δ15N fractionation during leaf senescence: (1) reabsorption of leaf 15N-depleted nitrogen to coarse roots, followed by (2) reabsorption of leaf 15N-enriched nitrogen to sapwood and (3) soil 15N-depleted nitrogen uptake to coarse roots. Further, changes in free amino acids, which are the result of enzyme activities involved in amino acids synthesis, partially explained δ15N fractionation in plant tissues.
1. Introduction
Japanese beech (Fagus crenata Blume) is the most common and widely distributed deciduous broad-leaved tree species in Japan, growing in the cool-temperate zone as a late-successional and climax species [1,2]. It usually grows in pure stands or mixed with Quercus mongolica Fisch. Ex Ledeb. and has an important ecological function, protecting soils from erosion and maintaining biodiversity. Additionally, Fagus crenata is used for afforestation and ceremonial plantations [2]. The importance of these forests is reflected in the UNESCO World Natural Heritage site of the Shirakami mountains in north-eastern Japan, consisting of a natural Fagus crenata forest covering an area of nearly 17,000 ha. Fagus crenata is found in areas with deep snow, regenerating constantly and producing more seedlings and juveniles than Fagus japonica Maxim. [3]. This is related to snow cover protecting the seeds from rodents and winter desiccation, as well as representing an important source of water in the shoot growth stage [4].
Japanese beech has developed a system of accumulating N over several years before using this storage for a controlled synchronized flowering and seed production for masting events. These events deplete the N storage of the following year, as shown in many studies [5,6,7,8]. This adaptation to N-limited conditions is common in most forests and the availability of mineral N rarely matches the demand of plants [9,10,11]. To allocate N for masting events, an efficient uptake of N and reabsorption of N from senescing leaves [12,13] is crucial. Reabsorption takes place in the autumn during leave senescence, when N is returned to woody tissues for storage, followed by remobilization after the break of dormancy during the shoot growth stage in spring [14]. Remobilized leaf N greatly contributes to the growth of new tissues in spring [8,14]. There are numerous descriptive studies on N cycling in deciduous trees, but as of now, it is still elusive how internal N sources fluctuate throughout the season in Fagus crenata in non-masting years [7]. Furthermore, there is little quantitative knowledge of seasonal allocation patterns and reabsorption efficiency [15]. Most studies have been conducted in controlled environments with seedlings [15,16,17]. However, these findings need to be validated in situ with adult trees, including the effect of soil N availability, mycorrhizae, aging of trees, and other environmental variables [14,18]. Tateno et al. [19] found that the position on the slope influenced mineralization and nitrification rates, with the bottom of the slopes showing higher nitrification and lower mineralization rates than the top, which in turn influenced ammonium and nitrate distribution along the slope. Nitrate generally has a lower δ15N than ammonium, thus, a higher availability in nitrate will cause lower δ15N values in mycorrhizal tissues in comparison to an abundance of ammonium in the soil [19]. Subsequently, mycorrhizal δ15N will influence the host plants’ δ15N. It has been shown that mycorrhizae allocate 15N and rather pass on 14N [20], depleting the isotopic composition of plant tissues. This provides valuable information about the N source and internal pathways of N [19]. Soil sampling combined with seasonal samplings and N fractionation of different plant tissues could provide insights into anabolic and catabolic processes of the nitrogen metabolism of plants [21]. Kolb and Evans [10] hypothesized that δ15N could change due to protein hydrolysis, causing fractionation within the leaf before abscission. During protein hydrolysis, soluble amino compounds are released by the degradation of proteins. These free amino acids are known to be indicators of the N status of plants [22]. Therefore, emerging free amino acids from protein hydrolysis moving to different plant tissues may be linked to this possible isotopic fractionation. Amino acids measured in different aquatic and terrestrial organisms have shown different δ15N values, and glutamic acid and phenylalanine have been proven particularly useful to reveal food-web structures [23,24].
The overall aim of this study is to qualitatively and quantitatively determine the temporal N partitioning of Fagus crenata during leaf N reabsorption in a non-masting year.
Thus, the specific objectives of this study are (1) to quantify the contribution of resorbed leaf N to the storage of the stem and roots, (2) to trace the source and sink of N intra-plant mobilization by means of δ15N at the whole-plant level, and (3) to find a relation between the release of free amino acids and the seasonal N isotopic composition of plant tissues.
2. Materials and Methods
2.1. Study Site
The study site is located in north-eastern Japan in the Yamagata prefecture at the Japanese seaside. The climate is humid, with annual precipitation of 3000 mm, and approximately half corresponds to snow, which covers the sampling sites from December to May at an elevation of ~700 m above sea level. The driest month is June, with approximately 70 mm of rain and the wettest is December, with 500 mm. Nitrogen deposition with precipitation is relatively low, with 10−15 kg total N ha−1 year−1 (e.g., Kanto region 30−38.5 kg total N ha−1 year−1) [2]. The mean annual temperature is 9.7 °C.
The sampling sites were distributed within a distance of 2 km on three slopes and selected by stand purity and accessibility in every season at the Research Forest of Yamagata University. To ensure comparability of the sites, soils and basic parameters were identified prior to tree sampling.
2.2. Sample Collection and Treatments
Soil samples were collected on August the 3rd in 2017 from three soil pits. Soil horizons were identified in the field following the FAO guidelines [25]. Genetic horizons were sampled for soil characterization, while samples for nitrogen and δ15N analysis were taken at fixed depths from 0−5 cm, 5 −15 cm, and 15−30 cm from three pits as replicates surrounding each individual tree. All the samples were transported in plastic bags after collection in the field, air-dried, sieved (< 2 mm), powdered, and stored until analysis. The samples used for the determination of inorganic N were frozen after transport and stored in the dark. Before analysis, they were thawed overnight in a refrigerator and sieved (< 2 mm).
Nine Fagus crenata trees of a similar age (70−80 years old) in the selected sites were chosen for sampling (Table 1). Coarse roots (> 2 mm), stem, and leaf samples were taken on May 20 (shoot growth stage), August 2 (green leaf stage), October 19 (pre-abscission stage), and November 29 (post-abscission stage), with fresh litter collected from litter traps (size: 1 m2). Sapwood was sampled by using an increment borer (10 mm) and not heartwood, assuming that there was no variation in N content in heartwood throughout the growing season [26]. Leaf samples were taken from the lower canopy from different leaf clusters. The position in the canopy has no significant impact on the reabsorption efficiency [12] and by sampling different leaf clusters, we covered possible variations in N content as leaf clusters’ N content is regulated independently for this species [27].

Table 1.
Characteristics of sampled Fagus crenata Blume (n = 9).
All samples were transported in plastic bags and oven-dried in the laboratory. Coarse roots, leaves, and litter were dried at 70 °C, and sapwood samples at 40 °C, for at least 48 hrs until they were dried. Subsequently, the samples were ground and stored until analysis.
2.3. Soil Analysis
Soil texture was measured with a laser diffraction particle size analyzer (Coulter LS200 with an attached Fluid Module, Beckman Coulter GmbH, Germany), after treating the samples with H2O2 and Na4P2O7. The analyses were triplicated. The pH was determined potentiometrically in a 1:2.5 soil:water suspension. The carbon (C) and N contents were analysed by dry combustion using the SUMIGRAPH NC-220F automatic high sensitive NC analyzer SCAS (Japan). The results were expressed on a dry-weight (105 °C) basis.
Total soluble N (TNb) was extracted from the soil samples by shaking them with 50 ml of 1M KCl. The supernatant was centrifuged, filtered (0.45 μm), stored in a refrigerator and analysed with a TOC/TNb analyser (vario TOC cube, elementar, Germany) for TNb determination. Ammonium content was determined using the method of Crooke et al. [28], whereas nitrate content was determined by using the method of Mulvaney [29] modified by Miranda et al. [30]. The colorimetric determination of ammonium and nitrate content was conducted with a U-2000 Spectrophotometer (Hitachi, Japan).
2.3.1. Total Nitrogen and δ15N Isotope Analysis
For the determination of total N content and δ15N in plant tissues, a Thermo Quest EA1110 Elemental Analyzer (Italy) which was connected to an IsoPrime (GV Instruments, UK) was used. The isotopic compositions of samples were expressed relative to atmospheric N2 (δ15N = 0‰) on scales normalized to the known δ15N values of laboratory working standards for glycine (δ15N = −0.3), which was normalized to L-glutamic acid distributed as USGS-40 (δ15N = −0.2‰) by SI Science Inc., Japan. Additionally a tertiary reference material was used, namely the in-house laboratory standard acetanilide (−0.89‰). The three working standards were analysed after every eight to ten samples during CF-IRMS runs to assess the replicability of the isotope measurements and normalization. One pulse of pure N2 reference gas from a tank reservoir (δ15N = −2.5‰) was discharged into the IRMS at the beginning of each chromatogram for both standards and samples. The accuracy obtained for standards and samples during the overall analytical procedure was better than ±0.2‰.
2.3.2. Amino Acid Analysis
Amino acids were extracted from the dried powders of plant materials with methanol and separated by liquid/liquid extraction with ultracentrifugation. Nineteen amino acids (Ala, Arg, Asn, Asp, Glu, Gln, Gly, His, Ile, Leu, Lys, Met, Phe, Pro, Ser, Thr, Trp, Tyr, Val) were quantified by capillary electrophoresis mass spectrometry (CE-MS). The preparation, conditions during sample extraction, and CE-MS analysis followed Oikawa et al. [31].
2.3.3. Whole-Tree N Calculations
Whole-tree biomass was calculated as follows:
For root, stem, and branch weight, the allometric equation by Ono et al. [32] for Fagus crenata was used.
where, Y is the biomass [kg], a is a normalization coefficient (roots = 0.0147, stem = 0.0946, branch = 0.0049), DBH is the tree diameter at breast height [cm], and b is a scaling exponent (roots = 2.62, stem = 2.44, branch = 2.78).
Ono et al. [32] reported that for smaller trees (DBH < 30 cm), such as those found in our study sites, leaf biomass was underestimated. Thus, for leaf biomass, the equation reported by Tateishi et al. [33] for Fagus sylvatica was used.
where, (ML) is the leaf mass [g], p is a normalization coefficient, q is a scaling exponent (2.9 and 1.7, respectively), and DBH is expressed as [cm] for Fagus crenata.
Fresh litter collected with litter traps verified the estimation of leaf biomass with Equation (2).
Lastly, for the determination of the sapwood weight, an equation by Gebauer et al. [34] developed for Fagus sylvatica was used and verified by tree core sampling.
where, As is the sapwood area (cm2), a and b are species-specific coefficients determined by regression techniques (0.778 and 1.917, respectively), and DBH is in [cm].
Furthermore, we assumed that in the post-abscission stage, all N was in the respective storage tissues (coarse roots and sapwood), while storage was considered empty during the green leaf stage as N is needed in the growing tissues. Thus, the whole-tree N storage was calculated as follows:
where, Nstorage is the amount of N stored in a certain tissue, Npost is the N content during the post-abscission stage, and Ngreen is the N content during green leaf stage. This value was calculated for all plant tissues as relative amounts (g N kg−1) and as whole-tree absolute N storage (g N).
Reabsorption efficiency (RE) was calculated as
Nitrogen was expressed as nitrogen mass per leaf dry mass (mg g−1). Litter represents the senesced leaves in November, while the green leaf is the matured August leaf. We used this value to calculate whole-tree total reabsorbed leaf N by considering the total tree leaf weight.
2.3.4. Statistical Analysis
One-way ANOVA was applied to determine the statistical significance of differences in nitrogen content, δ15N, and amino acids content. If significant differences were found, and a post-hoc multiple comparison was subsequently conducted, using the Tukey-Kramer test at the significance levels of 0.05 and 0.01. Furthermore, multiple linear regression analysis was conducted with RStudio (Version 1.1.463 – © 2009-2018 RStudio, Inc.) in order to evaluate the effect of the dependent variable (δ15N) on the independent variable (free amino acids content).
3. Results
3.1. Soil Characteristics
In all three sites, the soils were identified as brown forest soils (Cambisols), with an average depth of 80 cm and a silty-loamy texture. In the first 30 cm, we found a pH of 3.7 and a carbon to nitrogen ratio of 18 (Table 2).

Table 2.
Soil characteristics with ± denoting SD. DON stands for dissolved organic nitrogen.
3.2. Total Nitrogen Content in Soil and Tissues
Soil N content significantly decreased (min. p < 0.05) with depth from the top to deeper soil layers by 87% (Table 2, Figure 1a).
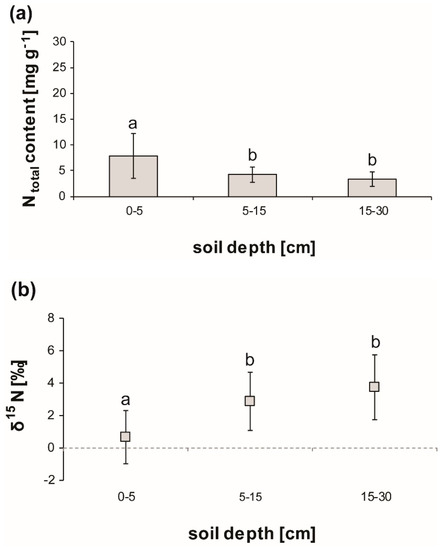
Figure 1.
Total N content (a) and δ15N (b) in soil layers under Fagus crenata Blume. The error bars denote SD (n = 9), p < 0.05.
Inorganic plant available N decreased similarly (p < 0.01) from top to bottom by 60% and was exclusively formed of ammonium. Nitrate content accounted for less than 1% of the soil total inorganic N. Total soluble N decreased by 50% (p < 0.01) from top to bottom, while dissolved organic nitrogen (DON) accounted for about 25% of that with no clear distribution pattern (Table 2).
N content of coarse roots significantly decreased (p < 0.01) from shoot growth to the green leaf stage by 32%, followed by an increase (p < 0.01) of 28% during the pre-abscission stage. Finally, during the post-abscission stage, no change in N content was observed (Figure 2a).
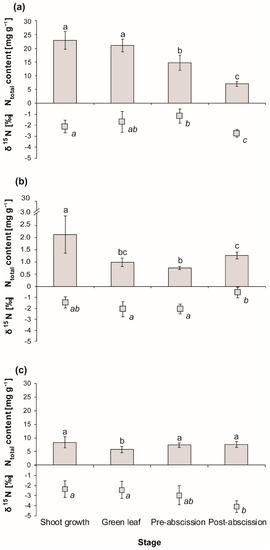
Figure 2.
Seasonal pattern of N content and δ15N in coarse roots (a), sapwood (b), and leaves (c) of Fagus crenata Blume. Leaves post-abscission stage values represent fresh litter. The error bars denote SD (n = 9), p < 0.05.
Sapwood N content decreased by 50% (p < 0.01) from shoot growth to the green leaf stage, as well as in the pre-abscission stage. However, a significant increase (p < 0.05) in N content of 62% was observed during the post-abscission stage. However, it was 39% lower (p < 0.01) than the value found during the shoot growth stage (Figure 2b).
Leaves’ N content significantly decreased (p < 0.01) from the shoot growth to the pre-abscission stage by 35%. Finally, the N content in litter was significantly (p < 0.05) lower in the post-abscission stage (Figure 2c), with an RE of 66%.
3.3. δ15N Isotope Analysis
Soil δ15N increased five-fold (p < 0.01) within the top 30 cm (Figure 1b). Coarse roots’ δ15N ratio depleted significantly from pre-abscission to the post-abscission stage (Figure 2a), while at the same time, sapwood δ15N was significantly enriched (Figure 2b).
Leaf δ15N enriched significantly during the pre-abscission stage, followed by a significant depletion in litter during the post-abscission stage (Figure 2c).
Soil δ15N was the most enriched of all measured plant tissues during any given phenological stage. During the growing season, coarse roots in terms of δ15N were the most depleted plant tissue, while leaves and sapwood showed similar δ15N values from the shoot growth to the pre-abscission stage. Only during the post-abscission stage was leaf litter significantly more depleted in 15N than sapwood.
3.4. Amino Acid Analysis in Plant Tissues
Total free amino acids content (FAAC) in coarse roots decreased dramatically (300%, p < 0.01) from the shoot growth to the green leaf stage and decreased another 60% (p < 0.05) in the pre-abscission stage. However, it increased again during the post-abscission stage by 300% (p < 0.05) (Figure 3).
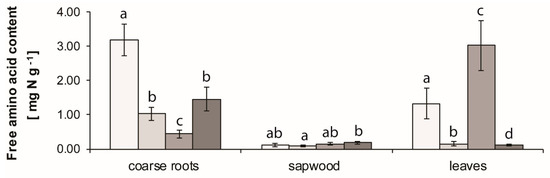
Figure 3.
Seasonal pattern of free amino acids content of coarse roots, sapwood, and leaves of Fagus crenata Blume. Leaves post-abscission stage value represents fresh litter. The error bars denote SD (n = 3), p < 0.05.
Sapwood FAAC only showed a significant increase (p < 0.05) of 50% from the green leaf stage to the post-abscission stage (Figure 3).
Leaf total FAAC decreased (p < 0.05) by 90% from the shoot growth to the green leaf stage. We observed a 15-fold increase (p < 0.01) during the pre-abscission stage and an equally sharp decrease (p < 0.01) in fresh litter during the post-abscission stage (Figure 3).
The most abundant free amino acid in coarse roots was asparagine, which accounted for 60 to 80% of total FAAC along the growing season, with the highest content (p < 0.05) in the shoot growth and post-abscission stage (Table 3). Other free amino acids of minor concentration were alanine and arginine, showing a similar distribution along the growing season as asparagin. Other amino acids were found in negligible concentrations. The results of the multiple linear regression analysis showed that roots’ asparagine content alone could not explain roots’ δ15N. However, the total pool of all amino acids revealed that asparagine content has an increasing effect (p = 0.01) on the δ15N value, while all the others have a decreasing effect (p < 0.01) (Table 4).

Table 3.
Amino acid content in different tissues of Fagus crenata Blume (n = 3). Only amino acids found in significant amounts were reported with ± denoting SD.

Table 4.
Summary statistics of the multiple regression analysis of the effect of 19 free amino acid contents on δ15N in plant tissues of Fagus Crenata Blume (n = 3).
Asparagine was also predominant in sapwood samples, with an increasing concentration (p < 0.01) from shoot growth to the post-abscission stage. Along with asparagine, alanine was found in high concentrations with similar amounts and increased (p < 0.05) throughout the growing season. Together, they accounted for at least 50% of all free amino acids found in sapwood during any given time. Further amino acids detected in minor concentrations were glutamic acid, glutamine, aspartic acid, proline, and serine. All other amino acids were found in negligible concentrations. The results of the multiple linear regression analysis showed that sapwood asparagine content had an increasing effect (p < 0.05) on sapwood δ15N (Table 4).
In leaves, alanine was the most abundant free amino acid, with peak concentrations (p < 0.05) in the shoot growth and pre-abscission stage. All other amino acids were found in negligible concentrations, except during the pre-abscission stage, where asparagine, glutamic acid, glutamine, isoleucine, leucine, phenylalanine, proline, serine, threonine, tryptophan, tyrosine, and valine were detected. In fresh litter, during the post-abscission stage, mainly tyrosine, valine, proline, threonine, isoleucine, asparagine, leucine, phenylalanine, and tryptophan remained, while other free amino acids were depleted. The results of the multiple linear regression analysis showed that leaf alanine has the strongest control (p < 0.05) on leaf δ15N than all the other free amino acids (Table 4).
3.5. Whole-Tree Level
The total biomass of all plant tissues was calculated for four trees of similar DBH in the green leaf stage (Table 5). We assumed that root, sapwood, and branch biomass did not change significantly during one growing season. Furthermore, leaf biomass was assumed not to change significantly during the pre-abscission stage and to equal the litter biomass in the post-abscission stage. We could not make any assumption on the leaf biomass during the shoot growth stage, thus it was not calculated for this stage (Table 5).

Table 5.
Biomass during the green leaf stage and seasonal changes in whole-tree N content per plant tissue of Fagus crenata Blume (n = 4) with ± denoting SD.
In the green leaf stage, the whole-tree roots’ N content was 688 ± 67 g N, while sapwood, branches’, and leaves’ total N content was 290 ± 35 g N, 54 ± 8 g N, and 193 ± 20 g N, respectively (Table 5). In the pre-abscission stage, N content in roots increased by 25% (p < 0.05), while sapwood and branches showed no variation. Furthermore, we observed a 25% decrease (p < 0.05) of N in leaves. Finally, in the post-abscission stage, roots’ N content did not change, while we found a 60% increase (p < 0.01) in sapwood and branches and another 55% decrease (p < 0.01) in litter.
Roots’ N storage was 23% higher (ns) than sapwood, while N stored in branches was the lowest (Figure 4). At the whole-tree level, N stored in all measured tissues was 402 ± 93 g, of which 32% were reabsorbed from senescing leaves during leaf abscission.
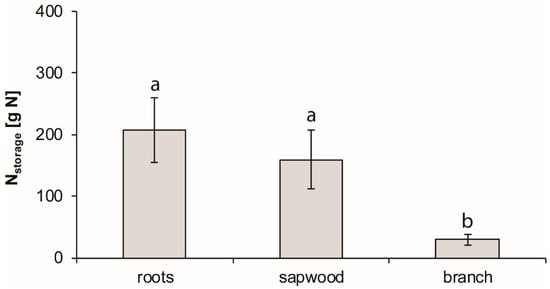
Figure 4.
Total N storage of coarse roots, sapwood, and branches of Fagus crenata Blume. The error bars denote SD (n = 9), p < 0.01.
4. Discussion
4.1. Reabsorption, Storage, and Remobilization of N in Plant Tissues
The N cycle in Fagus crenata followed a well-established pattern: in the green leaf stage, most N was bound in leaves’ photosynthetic apparatus [35]. In the pre-abscission stage, leaf N was reabsorbed until leaf abscission. Subsequently, fresh litter in the post-abscission stage was depleted in N content (RE 66%). Enta et al. [36] and Yasumura et al. [12] reported a similar value (RE 68-71%) for this species. Subsequently, N was stored in a sequential temporal pattern: first, it was stored in coarse roots during the pre-abscission stage, followed by N storage in the sapwood in the post-abscission stage. In previous studies [14,16,35,37], this temporal pattern of N storage in coarse roots and sapwood of deciduous trees has not been shown. In the post-abscission stage, when most N was stored, roots tended to store more N (ns) than sapwood. N reabsorption from leaves contributed 32% to overall N storage in the post-abscission stage.
During the shoot growth stage, N contents peaked in roots (ns) and sapwood (p < 0.01). Together with soil N taken up in late April, stored N supported shoot growth in the shoot growth stage [38] despite heavy snow cover, as is typical for this region. The continuous uptake of soil N during the dormant phase of the tree in the post-abscission stage could also contribute to the high N content found in the shoot growth stage. However, N uptake during dormancy has been reported to be minimal and only few studies have found it for boreal species during warm periods of the winter season [35,39]. Tree internal N storage strongly determines the beech masting period [8,15] and thus, the quantification of N uptake during the dormant period of this species needs to be explored in more detail. In the green leaf stage, after stored N was remobilized to support new shoot and leaf growth, coarse roots and sapwood were left depleted [37,40]. We did not quantify the contribution of stored N to new growth, but Dyckman and Flessa [15] reported that previous-year N contributes to leaf N with about 15% in Fagus sylvatica.
4.2. Effect of Soil N Uptake on Plant δ15N
Soil total N content, as well as inorganic N content, decreased with depth as δ15N became heavier due to the input of fresh N on the soil surface: precipitation and microbial turnover of litter, as well as illuviation of 15N-rich materials to deeper soil layers, are the main mechanisms [41,42,43,44]. Tateno et al. [19] found that foliar δ15N of the green leaf stage depends on ammonium and nitrate availability. In this latter study, Fagus crenata trees on upper slopes took up ammonium due to a lack of nitrate, leading to higher foliar δ15N values (−1.0‰) than at the bottom of the slope, where nitrate was more abundant, leading to 15N depletion in leaves (−2.0 ‰). In our study, we found a high availability of ammonium and a negligible amount of nitrate, which explained the δ15N value of −1.2 ± 0.6‰ in agreement with Tateno et al.’s [19] findings for trees in upper slope positions. Additionally, we measured low potential mineralization rates (1mg kg−1 soil in 30 days, data not published) in a nearby Cryptomeria japonica stand being similar to Tateno et al.’s [19] results. Thus, we concluded that our trees rather relied on ammonium rather than nitrate, although under different conditions, they would preferably acquire nitrate [45,46], but climatic conditions and the acidic pH strongly limited the nitrification process.
The inorganic soil N taken up by tree roots and mycorrhiza undergoes isotopic fractionation as mycorrhizal fungi selectively accumulate 15N and thus, a rather depleted 15N fraction passes to the host plants [19,20,47,48]. Leberecht et al. [18] demonstrated that the ectomycorrhizal fungi (EMF) associated with Fagus sylvatica preferably uptake ammonium from the soil, resulting in a more enriched 15N accumulation in the EMF in comparison to EMF acquiring nitrate. In both cases, δ15N values of the host plants will be lower, but the depletion in 15N will be less pronounced for N deriving from ammonium. This suggests a stronger control of N supply to the host tree by the EMF acquiring ammonium than would be the case with EMF taking up nitrate. Soil δ15N was 5‰ greater than root δ15N, revealing the effect of microbial turn over and uptake of inorganic N via mycorrhiza [20].
4.3. Effect of Intra-Plant N Movement on δ15N
In general, leaves’ δ15N ratio falls in line with the findings of Tateno et al. [19], who found similar values for Fagus crenata in lower slope positions under similar soil conditions. In the pre-abscission stage, leaves were significantly enriched in 15N by 0.5‰. At the same time, coarse roots’ δ15N tended to decrease (ns) by 0.5‰. Depleted reabsorbed leaf N seemed to be transported and stored in coarse roots during the pre-abscission stage, possibly together with taken up soil N, forming the bulk of coarse roots’ N storage. During the post-abscission stage, N storage only increased marginally (ns); however, a significant decrease in coarse roots’ δ15N suggests that only 15N-depleted soil N was stored, since the reabsorption of leaf N was already concluded.
After leaf abscission, N content significantly decreased in litter, depleting the δ15N value by 1.5‰. Thus, shortly before leaf senescence, 15N-enriched N was reabsorbed and subsequently stored in sapwood, increasing it by 1.5‰. In contrast to the results of Kolb and Evans [10], we found N isotope fractionation during reabsorption in leaves.
Thus, three phases of N storage could be observed:
(1) 15N-depleted N was reabsorbed from leaves to coarse roots in the pre-abscission stage, which was followed by (2) 15N-enriched leaf N reabsorption, significantly enriching sapwood in the heavier isotope during the post-abscission stage. (3) At the same time, coarse roots’ δ15N decreased, suggesting a strong N isotope fractionation in the EMF during the uptake and storage of soil N, as it occurs under ammonium-rich soil conditions [18].
In the transition from the post-abscission to the shoot growth stage, coarse roots were enriched in 15N, while sapwood was depleted. This suggests a movement of depleted N from coarse roots to sapwood, which eventually would be used for new shoot growth and leaf production. Only during the green leaf stage did all measured tissues show similar δ15N values. Further analysis is necessary to confirm whether foliar δ15N during the green leaf stage could be used as a proxy for whole-tree δ15N in other tree species, as has been suggested in previous studies [10].
4.4. Plant Tissues Free Amino Acids
During the green leaf stage, free amino acid content (FAAC) was generally low in all tissues. However, coarse roots’ FAAC was the highest among all tissues, possibly because of soil N uptake [49]. DON represents a major soluble N pool in the soil and is mostly composed of amino acids, which can be directly taken up by roots [50]. In our study, DON made up 21% of all soluble N of the top 30 cm. Roberts and Jones [51] reported that DON made up about 40% of all plant available N in a eutric cambisol, with free amino acids accounting for 10%. The low amino acid concentration in the soil solution contributed to the extremely fast rate at which these are removed from the soil, thus explaining the high values found in coarse roots of our study.
During the pre-abscission stage, FAAC increased sharply in leaves as a result of protein hydrolysis [37]. During the post-abscission stage, as leaf N reabsorption ended, a sharp decrease in total N and FAAC content in the fresh litter was observed, corresponding with significant increases in sapwood and coarse roots. This was likely linked to the continuous uptake of soil N [48] and further storage during the dormant phase of trees in the post-abscission stage. FAAC was high in all plant tissues during the shoot growth stage, suggesting that in coarse roots, N was internally remobilized within the tree and also absorbed from the soil. N is transported via sapwood to the emerging leaves to synthesise proteins for building cell walls and the photosynthetic apparatus [35].
In coarse roots, the most abundant free amino acid throughout the phenological stages was asparagine. Asparagine is known to act as a transport and storage form of N in plants [22] and its peak in the shoot growth stage revealed its high importance for N remobilization in spring, as has also been found in the case of apple trees [52]. Other major compounds were alanine and arginine. Alanine is closely connected with N metabolism [53] and is commonly found under stress conditions (e.g., temperature stress), yet its precise function is unclear. As alanine is high in all tissues in the shoot growth, as well as in the pre- and post-abscission stage, it might be linked not only to temperature stress caused by the long snow cover in our plots, but also to the transport of N. Arginine seemed to be linked to stress and transport as well, as it contributes to building and degrading proteins [22], which coincides with its occurrence during the shoot growth and pre-abscission stage.
Similar patterns were found in the sapwood for asparagine and alanine being linked to N transport during remobilization and storage. Although only low contents of glutamic acid and glutamine were found, these two amino acids are also associated with the transport and storage of N [51]. The occurrence of proline in the shoot growth stage was likely linked to snow-related temperature stress [54].
Finally, leaves predominantly contained alanine, which largely occurred during the shoot growth and pre-abscission stage, supporting our findings of its transport function linked to N storage and remobilization.
4.5. Correlation between Free Amino Acid Content and Plant Tissues δ15N
We observed four synchronized events between FAAC variation and δ15N fractionation in leaves. (1) In the pre-abscission stage, free amino acids are released after protein hydrolysis [37] and fractionation of 15N occurred. (2) In the post-abscission stage, as all the released amino acids were reabsorbed from leaves, δ15N significantly decreased. (3) During the shoot growth stage, 15N increased in leaves and leaf FAAC increased in comparison to litter. (4) During the green leaf stage, FAAC was low, as free amino acids were synthesised into proteins [35], and leaf δ15N showed no variation.
Therefore, the seasonal increase in leaf FAAC appears to be the reason for the seasonal enrichment of leaf 15N. However, a decrease in FAAC, as observed during the green leaf and post-abscission stage, does not automatically mean a depletion in leaf 15N. A decrease in leaf δ15N in the green leaf stage was not observed because the free amino acids were bound in proteins and are present in the leaf [55]. In contrast, in the post-abscission stage, the small leaf FAAC reflects their absence because they have been reabsorbed to storage tissues. The single most abundant free amino acid in leaves was alanine, which suggests that the seasonal variability of this amino acid is mainly responsible for leaf δ15N fractionation. Singling out seasonal measurements of alanine δ15N during the pre-abscission stage could clarify its contribution to leaf 15N fractionation [23].
We found a less clear connection between FAAC and δ15N in sapwood and coarse roots, since the source of stored N originates from leaf N reabsorption and soil N uptake, as reflected by the difference in free amino acids’ composition in these tissues [56], resulting in a lower adjusted R2 in the linear regression analysis. However, we could link root and sapwood asparagine content to cause an enrichment in δ15N. In contrast to leaves and roots where FAAC were found in abundance, sapwood seemed to be dominated by other N-containing compounds, e.g., linked to the incomplete breakdown of leaf N storage to free amino acids [10,56]. Future studies should include measurements of protein in order to increase the R2, especially in the case of sapwood.
5. Conclusions
The results obtained in this study showed that, on average, adult Fagus crenata trees had an N reabsorption efficiency of 66%. The reabsorbed foliar N was sequentially stored in coarse roots during the pre-abscission stage and in the sapwood in the post-abscission stage, from where it was remobilized to growing tissues during the shoot growth stage. Leaf N made up 32% of the total storage in roots and sapwood, making leaf N reabsorption vital for the species’ survival. Nitrogen was evenly stored between coarse roots and sapwood. N isotope fractionation occurred when N reabsorption took place before leaf abscission. First, 15N-depleted N from leaves was traced to 15N-depleted N stored in coarse roots, followed by the reabsorption of 15N-enriched N that was traced to 15N-enriched N stored in sapwood. Free amino acids’ dynamics could partially explain changes in δ15N in all measured tissues.
Author Contributions
F.S. and M.L.L.C. formulated the conceptualization and methodology. F.S. conducted the investigation, formal analysis, data curation, and visualization and prepared the original draft. M.L.L.C. was supervising this study. A.O. measured the amino acid content, while T.Y. measured the N content and isotope ratio. M.L.L.C., A.O., E.B., and L.C. contributed to data interpretation and writing of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We want to thank the staff of the University Research Forest of Yamagata University for keeping the site accessible in summer and winter, as well as the laboratory of Professor Oikawa for measuring amino acids. We also thank Cristina Lerda from the Università degli Studi di Torino, for the measurements related to soil N forms.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liang, N.; Nagayama, M.; Nakata, M.; Maruyama, K. Growth, photosynthesis and nitrogen content in Japanese beech (Fagus crenata Bl.) seedlings grown under five irradiances. Photosynthetica 1995, 31, 257–268. [Google Scholar]
- Watanabe, M.; Yamaguchi, M.; Matsumura, H.; Kohno, Y.; Izuta, T. Risk assessment of ozone impact on Fagus crenata in Japan: Consideration of atmospheric nitrogen deposition. Eur. J. For. Res. 2012, 131, 475–484. [Google Scholar] [CrossRef]
- Fukushima, T.; Takasuna, H.; Matsui, T.; Nishio, T.; Kyan, T.; Tsunetomi, Y. New phytosociological classification of beech forests in Japan. Jpn. J. Ecol. 1995, 45, 79–98, (In Japanese with English Synopsis). [Google Scholar]
- Shimano, K.; Masuzawa, T. Effects of snow accumulation on survival of beech (Fagus crenata) seed. Plant Ecol. 1998, 134, 235–241. [Google Scholar]
- Yasumura, Y.; Hikosaka, K.; Hirose, T. Resource allocation to vegetative and reproductive growth in relation to mast seeding in Fagus crenata. For. Ecol. Manag. 2006, 229, 228–233. [Google Scholar] [CrossRef]
- Han, Q.; Kabeya, D.; Iio, A.; Kakubari, Y. Masting in Fagus crenata and its influence on the nitrogen content and dry mass of winter buds. Tree Physiol. 2008, 28, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y.; Maruyama, Y.; Chiba, Y.; Kobayashi, M.J.; Joseph, B.; Shimizu, K.K.; Mochida, K.; Hiura, T.; Kon, H.; Satake, A. Nitrogen as a key regulator of flowering in Fagus crenata: Understanding the physiological mechanism of masting by gene expression analysis. Ecol. Lett. 2014, 17, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Kabeya, D.; Iio, A.; Inagaki, Y.; Kakubari, Y. Nitrogen storage dynamics are affected by masting events in Fagus crenata. Oecologia 2014, 174, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Aerts, R.; Chapin, F.S. The mineral nutrition of wild plants revisited: A re-evaluation of processes and patterns. Adv. Ecol. Res. 2000, 30, 1–67. [Google Scholar]
- Kolb, K.J.; Evans, R.D. Implication of leaf nitrogen recycling on the nitrogen isotope composition of deciduous plant tissues. New Phytol. 2002, 156, 57–64. [Google Scholar] [CrossRef]
- Yasumura, Y.; Hikosaka, K.; Matsui, K.; Hirose, T. Leaf-level nitrogen-use efficiency of canopy and understorey species in a beech forest. Funct. Ecol. 2002, 16, 826–834. [Google Scholar] [CrossRef]
- Yasumura, Y.; Onoda, Y.; Hikosaka, K.; Hirose, T. Nitrogen resorption from leaves under different growth irradiance in three deciduous woody species. Plant Ecol. 2005, 178, 29–37. [Google Scholar] [CrossRef]
- Lopez, M.L.; Mizota, C.; Nobori, Y.; Sasaki, T.; Yamanaka, T. Temporal changes in nitrogen acquisition of Japanese black pine (Pinus thunbergii) associated with black locust (Robinia Pseudoacacia). J. For. Res. 2014, 25, 585–589. [Google Scholar]
- Millard, P.; Grelet, G.-A. Nitrogen storage and remobilization by trees: Ecophysiological relevance in a changing world. Tree Physiol. 2010, 30, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Dyckmans, J.; Flessa, H. Influence of tree internal N status on uptake and translocation of C and N in beech: A dual 13C and 15N labeling approach. Tree Physiol. 2001, 21, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Millard, P.; Hester, A.; Wendler, R.; Baillier, G. Interspecific defoliation responses of trees depend on sites of winter nitrogen storage. Funct. Ecol. 2001, 15, 535–543. [Google Scholar] [CrossRef]
- Kayama, M.; Makoto, K.; Nomura, M.; Satoh, F.; Koike, T. Nutrient dynamics and carbon partitioning in larch seedlings (Larix kaempferi) regenerated on a serpentine soil in northern Japan. Landsc. Ecol. Eng. 2009, 5, 125–135. [Google Scholar] [CrossRef]
- Leberecht, M.; Dannenmann, M.; Tejedor, J.; Simon, J.; Rennenberg, H.; Polle, A. Segregation of nitrogen use between ammonium and nitrate of ectomycorrhizas and beech trees. Plant Cell Environ. 2016, 39, 2691–2700. [Google Scholar] [CrossRef]
- Tateno, R.; Osada, N.; Terai, M.; Tokuchi, N.; Takeda, Y. Inorganic nitrogen source utilization by Fagus crenata on different soil types. Trees 2005, 19, 477–481. [Google Scholar] [CrossRef]
- Hobbie, E.A.; Högberg, P. Nitrogen isotopes link mycorrhizal fungi and plants to nitrogen dynamics. New Phytol. 2012, 196, 367–382. [Google Scholar]
- Gebauer, G.; Dietrich, P. Nitrogen Isotope Ratios in Different Compartments of a Mixed Stand of Spruce, Larch and Beech Trees and of Understorey Vegetation Including Fungi. Isot. Environ. Health Stud. 1993, 29, 35–44. [Google Scholar] [CrossRef]
- Fotelli, M.N.; Rennenberg, H.; Geßler, A. Effects of Drought on the Competitive Interference of an Early Succsessional Species (Rubus fruticosus) on Fagus sylvatica L. Seedlings: 15N Uptake and Partitioning, Responses of Amino Acids and other N Compounds. Plant Boil 2002, 4, 311–320. [Google Scholar] [CrossRef]
- Chikaraishi, Y.; Ogawa, N.O.; Kashiyama, Y.; Takano, Y.; Suga, H.; Tomitani, A.; Miyashita, M.; Kitazato, H.; Ohkouchi, N. Determination of aquatic food-web structure based on compound-specific nitrogen isotopic composition of amino acids. Limnol. Oceanogr. Methods 2009, 7, 740–750. [Google Scholar] [CrossRef]
- Chikaraishi, Y.; Ogawa, N.O.; Doi, H.; Ohkouchi, N. 15N/14N ratios of amino acids as a tool for studying terrestrial foodwebs: A case study of terrestrial insects (bees, wasps, and hornets). Ecol. Res. 2011, 26, 835–844. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). Guidelines for Soil Description, 4th ed.; FAO: Rome, Italy, 2006. [Google Scholar]
- Tomlinson, G.; Siegwolf, R.T.W.; Buchmann, N.; Schleppi, P.; Waldner, P.; Weber, P. The mobility of nitrogen across tree-rings of Norway spruce (Picea abies L.) and the effect of extraction method on tree-ring δ 15N and δ 13C values. Rapid Commun. Mass Sprectrometry 2014, 28, 1258–1264. [Google Scholar] [CrossRef]
- Osada, N.; Yasumura, Y.; Ishida, A. Leaf nitrogen distribution in relation to crown architecture in the tall canopy species, Fagus crenata. Oecologia 2014, 175, 1093–1106. [Google Scholar] [CrossRef]
- Crooke, W.M.; Simpson, W.E. Determination of ammonium in Kjedahl digest of crops by an automated procedure. J. Sci. Food Agric. 1971, 22, 9–10. [Google Scholar] [CrossRef]
- Mulvaney, R.L. Nitrogen—Inorganic Forms. In Methods of Soil Analysis. Part 3, Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpoor, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; SSSA Book Series No. 5; SSSA: Madison, WI, USA, 1996; pp. 125–1184. [Google Scholar]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, A.; Ostuka, T.; Nakabayashi, R.; Jikumaru, Y.; Isuzugawa, K.; Murayama, H.; Saito, K.; Shiratake, K. Metabolic Profiling of Developing Pear Fruits Reveals Dynamic Variation in Primary and Secondary Metabolites, Including Plant Hormones. PLoS ONE 2015, 10, e0131408. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Yasuda, Y.; Matsuo, T.; Hoshino, D.; Chiba, Y.; Mori, S. Estimating forest biomass using allometric model in a cool-temperate Fagus crenata forest in the Appi highlands, Iwate, Japan. For. Res. Inst. Res. Rep. 2013, 12, 125–141. [Google Scholar]
- Tateishi, M.; Kumagai, T.; Suyama, Y.; Hiura, T. Differences in transpiration characteristics of Japanese beech trees, Fagus crenata, in Japan. Tree Physiol. 2010, 30, 748–760. [Google Scholar] [CrossRef][Green Version]
- Gebauer, T.; Horna, V.; Leuschner, C. Variability in radial sap flux density patterns and sapwood area among seven co-occurring temperate broad-leaved tree species. Tree Physiol. 2008, 28, 1821–1830. [Google Scholar] [CrossRef]
- Enta, A.; Hayashi, M.; Lopez, C.M.L.; Fujiyoshi, L.; Yamanaka, T.; Oikawa, A.; Seidel, F. Nitrogen resorption and fractionation during leaf senescence in typical tree species in Japan. J. For. Res. 2019, in press. [Google Scholar]
- Ueda, M.U.; Mizumachi, E.; Tokuchi, N. Foliage nitrogen turnover: Differences among nitrogen absorbed at different times by Quercus serrata saplings. Ann. Bot. 2011, 108, 169–175. [Google Scholar] [CrossRef]
- Warren, C.R.; Dreyer, E.; Adams, M.A. Photosynthesis-Rubisco relationships in foliage of Pinus sylvestris in response to nitrogen supply and the proposed role of Rubisco and amino acids as nitrogen stores. Trees 2003, 17, 359–366. [Google Scholar]
- Gessler, A.; Schneider, S.; von Sengbusch, D.; Weber, P. Field and laboratory experiments on net uptake of nitrate and ammonium by the roots of spruce (Picea abies) and beech (Fagus sylvatica) trees. New Phytol. 1998, 138, 275–285. [Google Scholar] [CrossRef]
- Cooke, J.E.K.; Weih, M. Nitrogen storage and seasonal nitrogen cycling in Populus: Bridging molecular physiology and ecophysiology. New Phytol. 2005, 167, 19–30. [Google Scholar] [CrossRef]
- Wyka, T.P.; Zytkowiak, R.; Oleksyn, J. Seasonal dynamics of nitrogen level and gas exchange in different cohorts of Scots pine needles: A conflict between nitrogen mobilization and photosynthesis? Eur. J. For. Res. 2016, 135, 483–493. [Google Scholar] [CrossRef]
- Koba, K.; Tokuchi, N.; Wada, E.; Nakajima, T.; Iwatsubo, G. Intermittent denitrification: The application of a 15N natural abundance method to a forested ecosystem. Gerchimica Cosmochim. Acta 1997, 61, 5043–5050. [Google Scholar] [CrossRef]
- Koba, K.; Tokuchi, N.; Yoshioka, T.; Hobbie, E.A.; Iwatsubo, G. Natural Abundance of Nitrogen-15 in a Forest Soil. Soil Sci. Soc. Am. J. 1998, 62, 778–781. [Google Scholar] [CrossRef]
- Shi, J.; Ohte, N.; Tokuchi, N.; Imamura, N.; Nagayama, M.; Oda, T.; Suzuki, M. Nitrate isotopic composition reveals nitrogen deposition and transformation dynamics along the canopy-soil continuum of a suburban forest in Japan. Rapid Commun. Mass Spectrom. 2014, 28, 2539–2549. [Google Scholar] [CrossRef]
- Zhou, W.-J.; Sha, L.-Q.; Schaefer, D.A.; Zhang, Y.-P.; Song, Q.-H.; Tan, Z.-H.; Deng, Y.; Deng, X.-B.; Guan, H.-L. Direct effects of litter decomposition on soil dissolved organic carbon and nitrogen in a tropical rainforest. Soil Biol. Biochem. 2014, 81, 255–258. [Google Scholar] [CrossRef]
- Rennenberg, H.; Dannenmann, M. Nitrogen nutrition of trees and temperate forests – the significance of nitrogen availability in pedosphere and atmosphere. Forests 2015, 6, 2820–2835. [Google Scholar] [CrossRef]
- Dannenmann, M.; Bimüller, C.; Gschwendtner, S.; Leberecht, M.; Tejedor, J.; Bilela, S.; Gasche, R.; Haewinkel, M.; Baltensweiler, A.; Kögel-Knabner, I.; et al. Climate change impairs nitrogen cycling in European beech forests. PLoS ONE 2016, 11, e0158823. [Google Scholar] [CrossRef]
- Högberg, P.; Johannisson, C.; Yarwood, S.; Callesen, I.; Näsholm, T.; Myrold, D.D.; Högberg, M.N. Recovery of ectomycorrhizal after ‘nitrogen saturation’ of a conifer forest. New Phytol. 2011, 189, 515–525. [Google Scholar] [CrossRef]
- Clemmensen, K.E.; Bahr, A.; Ovaskainen, O.; Dahlberg, A.; Ekblad, A.; Wallander, H.; Stenlid, J.; Finlay, R.D.; Wardle, D.A.; Lindahl, B.D. Roots and Associated Fungi Drive Long-Term Carbon Sequestration in Boreal Forests. Science 2013, 339, 1615–1618. [Google Scholar] [CrossRef]
- Finzi, A.C.; Berthrong, S.T. The uptake of amino acids by microbes and trees in three cold temperate forests. Ecology 2005, 86, 3345–3353. [Google Scholar] [CrossRef]
- Jones, D.L.; Shannon, D.; Murphy, D.V.; Farrar, J. Role of dissolved organic nitrogen (DON) in soil N cycling in grassland soils. Soil Biol. Biochem. 2004, 36, 749–756. [Google Scholar] [CrossRef]
- Roberts, P.; Jones, D.L. Microbial and plant uptake of free amino sugars in grassland soils. Soil Biol. Biochem. 2012, 49, 139–149. [Google Scholar] [CrossRef]
- Malaguti, D.; Millard, P.; Wendler, R.; Hepburn, A.; Tagliavini, M. Translocation of amino acids in the xylem of apple (Malus domestica Borkh.) trees in spring as a consequence of both N remobilization and root uptake. J. Exp. Bot. 2001, 52, 1665–1671. [Google Scholar]
- Miyashita, Y.; Dolferus, R.; Ismond, K.P.; Goog, A.G. Alanine aminotransferase catalyses the breakdown of alanine after hypoxia in Arabidopsis thaliana. Plant J. 2007, 49, 1108–1121. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef]
- Näsholm, T. Removal of nitrogen during needle senescence in Scots pine (Pinus sylvestris L.). Oecologia 1994, 99, 290–296. [Google Scholar] [CrossRef]
- Näsholm, T.; Ericsson, A. Seasonal changes in amino acids, protein and total nitrogen in needles of fertilized Scots pine trees. Tree Physiol. 1990, 6, 267–281. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).