Functional Crown Architecture of Five Temperate Broadleaf Tree Species: Vertical Gradients in Leaf Morphology, Leaf Angle, and Leaf Area Density
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Stand Structure and Crown Volume
2.3. Statistical Analyses
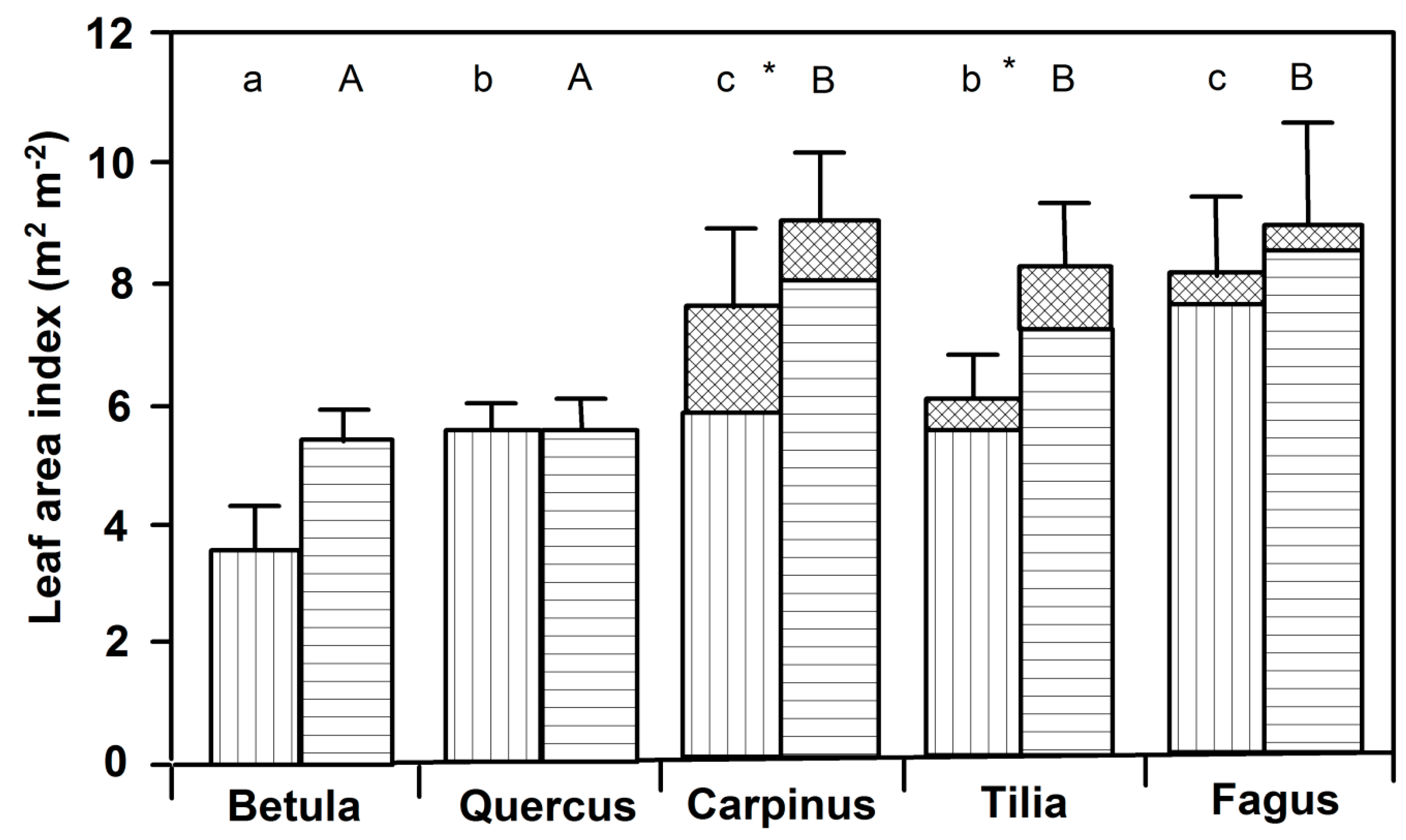
3. Results
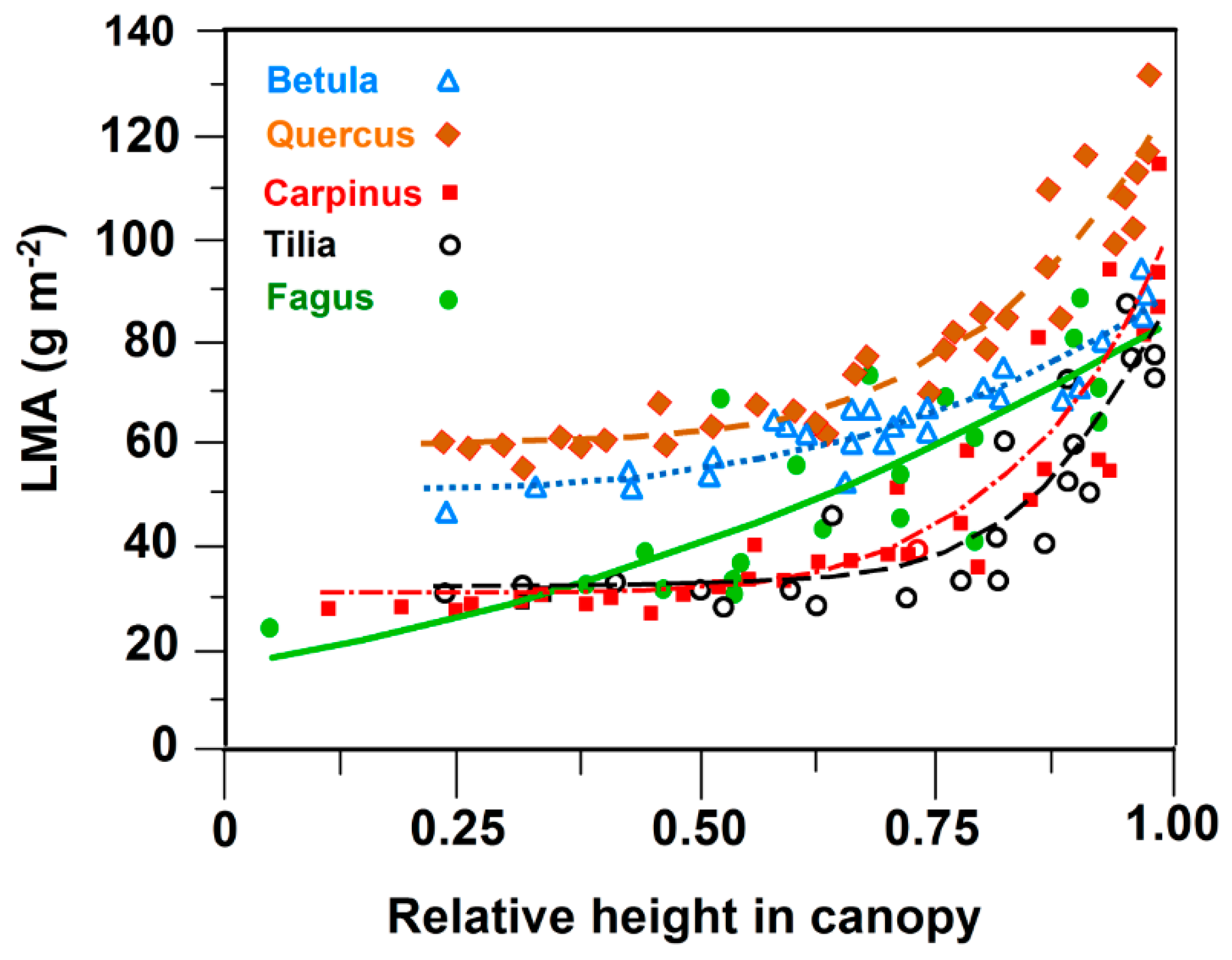
3.1. Leaf Size and Leaf Mass Area
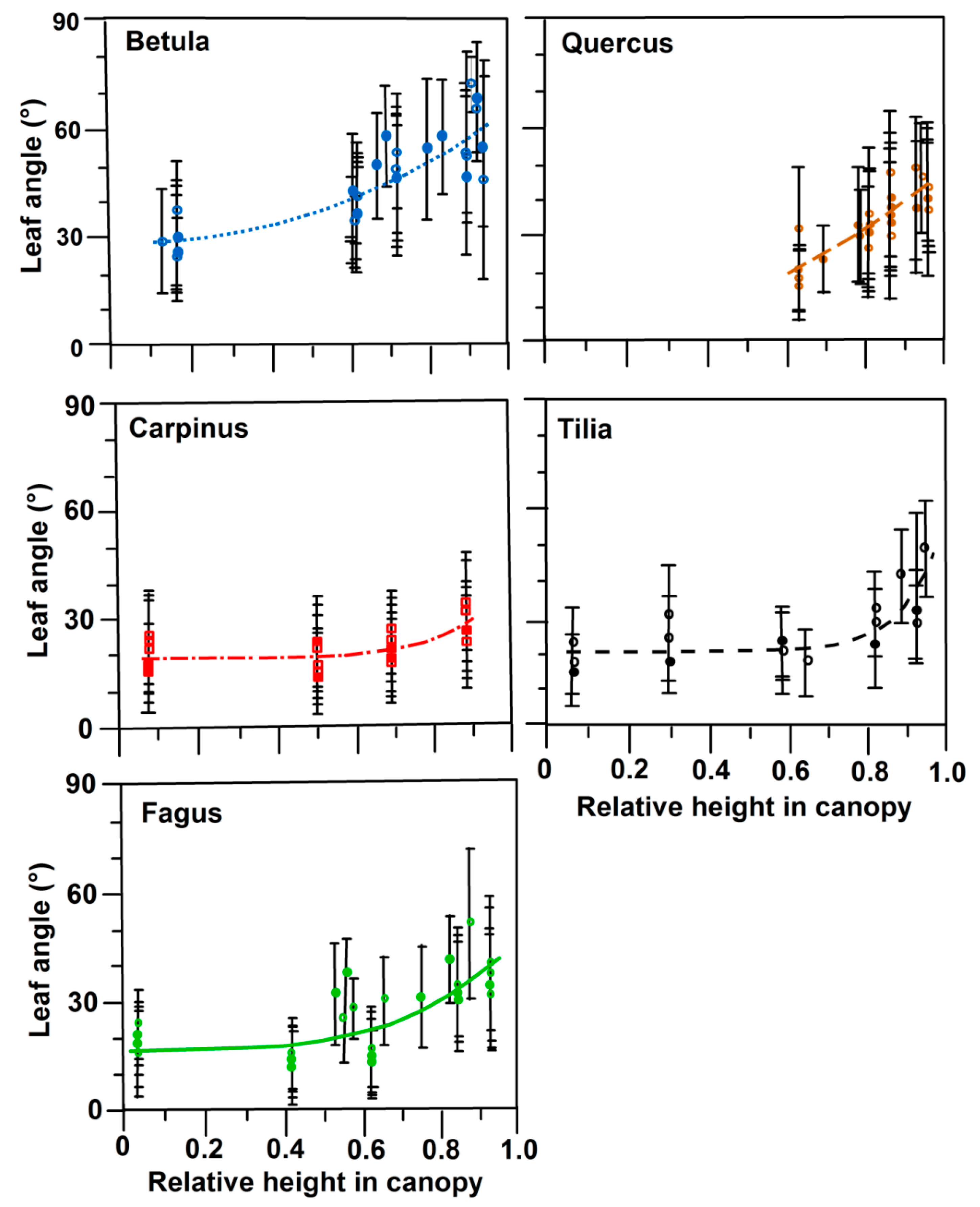
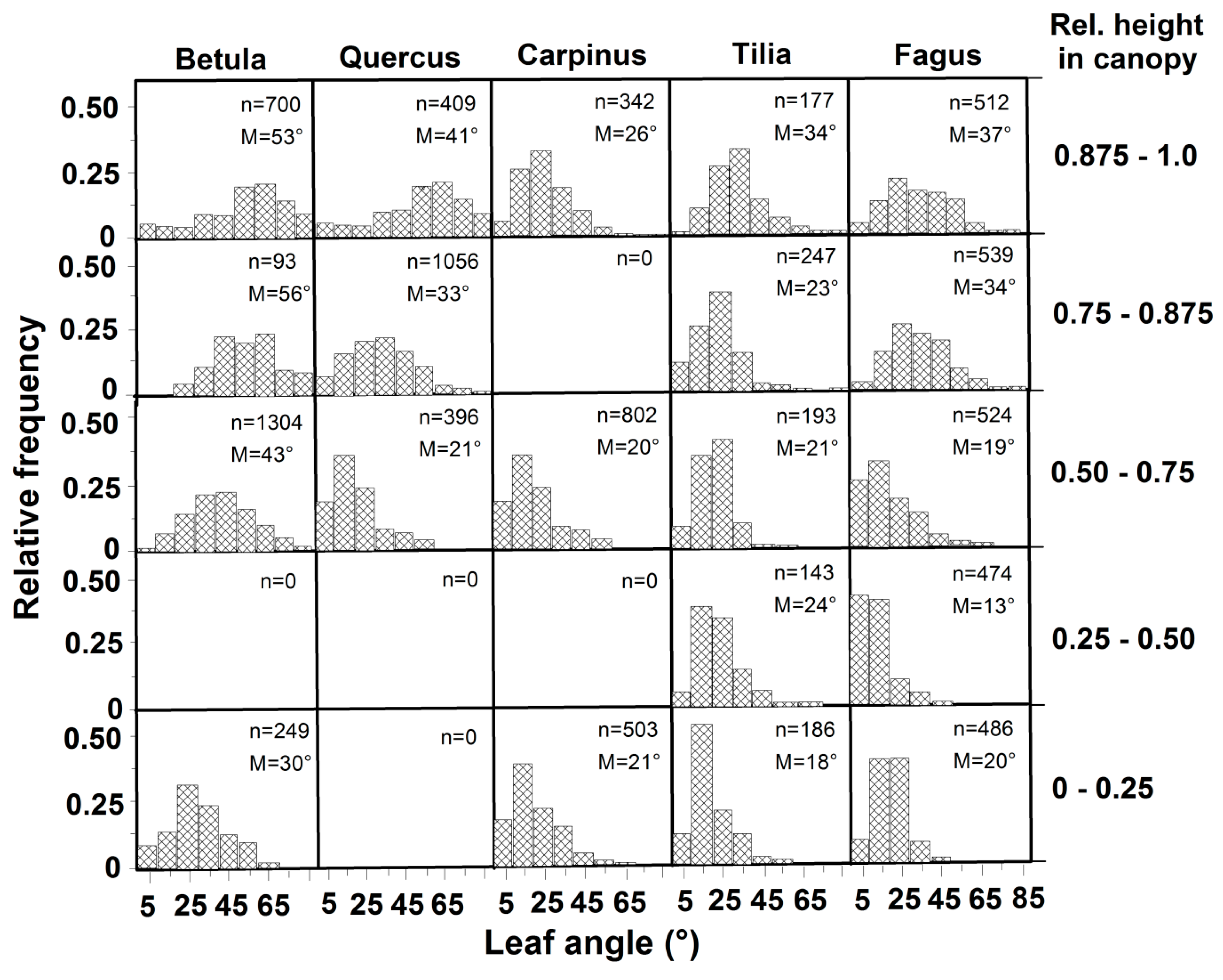
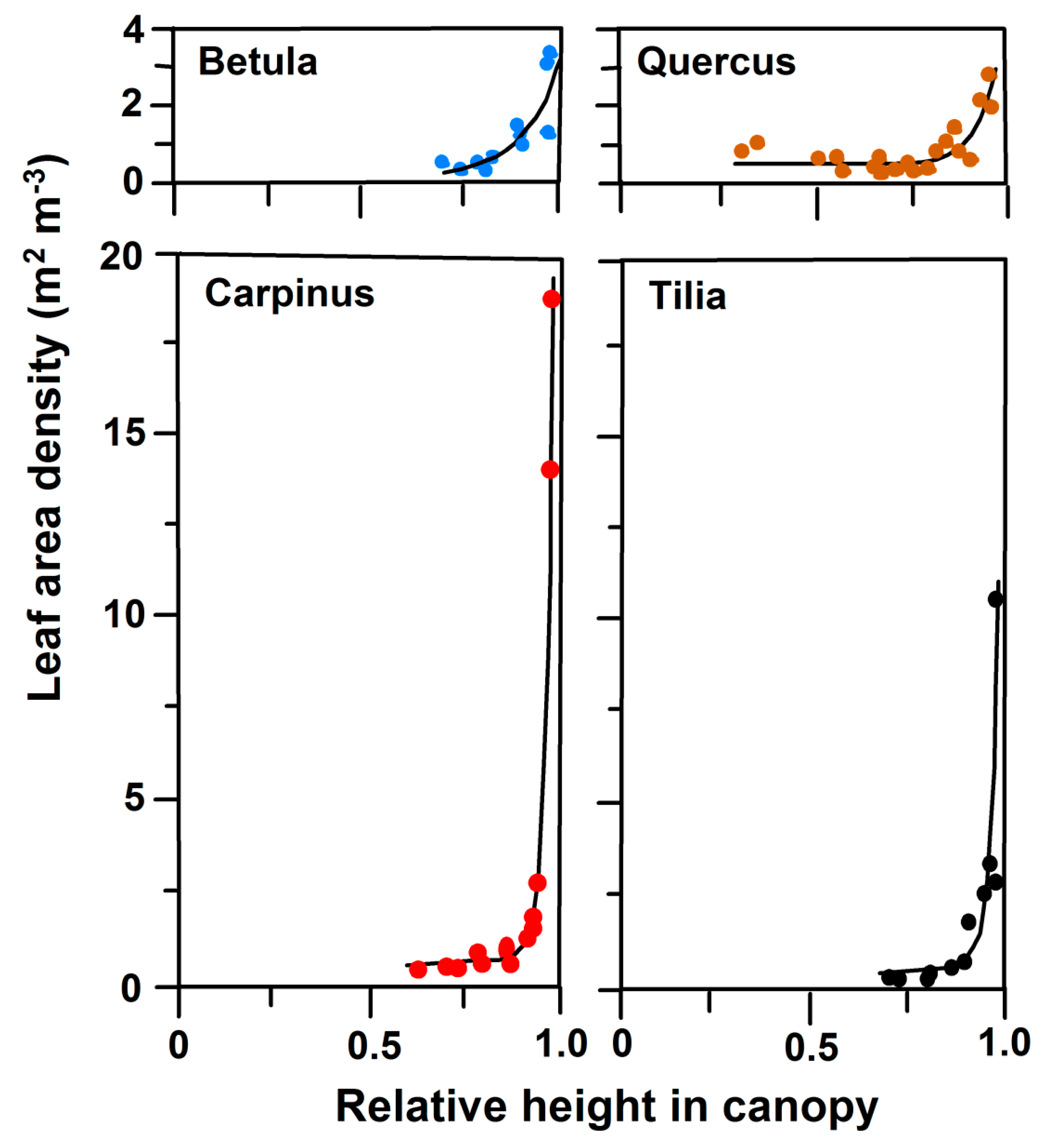
3.2. Leaf Angles and Leaf Area Distribution in the Crown
4. Discussion
4.1. Sun Crown Foliage
4.2. Shade Crown Foliage
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Elias, P.; Kratochvilova, I.; Janous, D.; Marek, M.; Masarovicova, E. Stand microclimate and physiological activity of tree leaves in an oak-hornbeam forest. I. Stand microclimate. Trees 1989, 4, 227–233. [Google Scholar] [CrossRef]
- Niinemets, Ü. A review of light interception in plant stands from leaf to canopy in different plant functional types and in species with varying shade tolerance. Ecol. Res. 2010, 25, 693–714. [Google Scholar] [CrossRef]
- Niinemets, Ü. Adjustment of foliage structure and function to a canopy light gradient in tow co-existing deciduous trees. Variability in leaf inclination angles in relation to petiole morphology. Trees 1998, 12, 446–451. [Google Scholar] [CrossRef]
- Hutchison, B.A.; Matt, D.R.; McMillan, R.T.; Gross, L.J.; Tajchman, S.J.; Norman, J.M. The architecture of a deciduous forest canopy in Eastern Tennessee, U.S.A. J. Ecol. 1986, 74, 635–646. [Google Scholar] [CrossRef]
- Elias, P. Effects of the canopy position on shoots and leaves in various trees of an oak-hornbeam forest. Biologia (Bratislava) 1990, 45, 31–42. [Google Scholar]
- Kull, O.; Broadmeadow, M.; Kruijt, B.; Meir, P. Light distribution and foliage structure in an oak canopy. Trees 1999, 14, 55–64. [Google Scholar] [CrossRef]
- Falster, D.S.; Westoby, M. Leaf size and angle vary widely across species: What consequences for light interception? New Phytol. 2003, 158, 509–525. [Google Scholar] [CrossRef]
- Larcher, W. Physiological Plant Ecology, 4th ed.; Springer: Berlin, Germany, 2003. [Google Scholar]
- McMillen, G.G.; McClendon, J.H. Leaf angle: An adaptive feature of sun and shade plants. Bot. Gaz. 1979, 140, 437–442. [Google Scholar]
- Baldocchi, D.; Hutchison, B.A.; Matt, D.R.; McMillen, R.T. Canopy radiative transfer models for spherical and known leaf inclination distributions: A test in an oak-hickory forest. J. Appl. Ecol. 1985, 22, 539–555. [Google Scholar] [CrossRef]
- Fleck, S.; Niinemets, Ü.; Cescatti, A.; Tenhunen, D. Three dimensional lamina architecture alters light harvesting efficiency in Fagus: A leaf-scale analysis. Tree Physiol. 2003, 23, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Reiter, I.M.; Häberle, K.-H.; Nunn, A.J.; Heerdt, C.; Reitmayer, H.; Grote, R.; Matyssek, R. Competitive strategies in adult beech and spruce: Space-related foliar carbon investment versus carbon gain. Oecologia 2005, 146, 337–349. [Google Scholar] [CrossRef]
- Pisek, J.; Sonnentag, O.; Richardson, A.D.; Mottus, M. Is the spherical leaf inclination angle distribution a valid assumption for temperate and boreal broadleaf tree species? Agric. For. Meteorol. 2013, 169, 186–194. [Google Scholar] [CrossRef]
- Bazzaz, F.A. The physiological ecology of plant succession. Ann. Rev. Ecol. Syst. 1979, 10, 351–371. [Google Scholar] [CrossRef]
- Leuschner, C.; Meier, I.C. The ecology of Central European tree species: Trait spectra, functional trade-offs, and ecological classification of adult trees. Perspec. Plant Ecol. Evol. Syst. 2018, 33, 89–103. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Lohbeck, M.; Poorter, L.; Lebrija-Trejos, E.; Martínez-Ramos, M.; Meave, J.A.; Paz, H.; Pérez-García, E.A.; Romero-Pérez, I.E.; Tauro, A.; Bongers, F. Successional changes in functional composition contrast for dry and wet tropical forest. Ecology 2013, 94, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Lohbeck, M.; Lebrija-Trejos, E.; Martinez-Ramos, M.; Meave, J.A.; Poorter, L.; Bongers, F. Functional trait strategies of trees in dry and wet tropical forests are similar but differ in their consequences for succession. PLoS ONE 2015, 10, e0123741. [Google Scholar] [CrossRef] [PubMed]
- Horn, H.S. The Adaptive Geometry of Trees; Princeton University Press: Princeton, NJ, USA, 1971. [Google Scholar]
- Gratzer, G.; Darabant, A.; Chhetri, P.B.; Rai, P.B.; Eckmüllner, O. Interspecific variation in the response of growth, crown morphology, and survivorship to light of six tree species in the conifer belt of the Bhutan Himalayas. Can. J. For. Res. 2004, 34, 1093–1107. [Google Scholar] [CrossRef]
- Bazzaz, F.A.; Pickett, S.T.A. Physiological ecology of tropical succession: A comparative review. Ann. Rev. Ecol. Syst. 1980, 11, 287–310. [Google Scholar] [CrossRef]
- Turner, I.M. The Ecology of Trees in the Tropical Rain Forest; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Bartels, H. Gehölzkunde: Einführung in die Dendrologie; Ulmer: Stuttgart, Germany, 1995. [Google Scholar]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Feild, T.S.; Gleason, S.M.; Hacke, U.G.; et al. Global convergence in the vulnerability of forests to drought. Nature 2012, 491, 752–755. [Google Scholar] [CrossRef]
- Ellenberg, H. Vegetation Mitteleuropas mit den Alpen, 5th ed.; Ulmer: Stuttgart, Germany, 1996. [Google Scholar]
- Hölscher, D.; Hertel, D.; Leuschner, C.; Hottkowitz, M. Tree species diversity and soil patchiness in a temperate broad-leaved forest with limited rooting space. Flora 2002, 197, 118–125. [Google Scholar] [CrossRef]
- Köcher, P.; Gebauer, T.; Horna, V.; Leuschner, C. Leaf water status and stem xylem flux in relation to soil drought in five temperate broad-leaved tree species with contrasting water use strategies. Ann. For. Sci. 2009, 66, 101–110. [Google Scholar] [CrossRef]
- Leuschner, C.; Ellenberg, H. Ecology of Central European Forests; Springer Natur: Cham, Swzerlands, 2017. [Google Scholar]
- Zimmermann, J.; Hauck, M.; Dulamsuren, C.; Leuschner, C. Climate warming-related growth decline affects Fagus sylvatica, but not other broad-leaved tree species in Central European mixed forests. Ecosystems 2015, 18, 560–572. [Google Scholar] [CrossRef]
- Lovy, D. WinDIG. University of Geneva: Geneva, Switzerland, 1996. Available online: http://www.unige.ch/sciences/chifi/cpb/windig.html (accessed on 6 February 2019).
- Norman, J.M.; Campbell, H.S. Canopy Structure; Chapman and Hall: London, UK; New York, NY, USA, 1989. [Google Scholar]
- Hollinger, D.Y. Canopy organization and foliage photosynthetic capacity in broad-leaved evergreen montane forest. Funct Ecol. 1989, 3, 53–62. [Google Scholar] [CrossRef]
- Hajek, P.; Seidel, D.; Leuschner, C. Mechanical abrasion, and not competition for light, is the dominant canopy interaction in a temperate mixed forest. For. Ecol. Manag. 2015, 348, 108–116. [Google Scholar] [CrossRef]
- Tüffers, A.V.; Naidoo, G.; Willert, D.J. The contribution of leaf angle to photoprotection in the mangroves Avicennia marina (Forssk.) Vierh. and Bruguiera gymnorrhiza (L.) Lam. under field conditions in South Africa. Flora 1999, 194, 267–275. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Kull, O. Leaf weight per area and leaf size of 85 Estonian woody species in relation to shade tolerance and light availability. For. Ecol. Manag. 1994, 70, 1–10. [Google Scholar] [CrossRef]
- Ross, J.; Mottus, M. Statistical treatment of umbra length inside willow coppice. Agric. For. Meteorol. 2000, 100, 89–102. [Google Scholar] [CrossRef]
- Vierling, L.A.; Wessman, C.A. Photosynthetically active radiation heterogeneity within a monodominant Congolese forest canopy. Agric. For. Meteorol. 2000, 103, 265–278. [Google Scholar] [CrossRef]
- Kutsch, W.; Wirth, C.; Kattge, J.; Nöllert, S.; Herbst, M.; Kappen, L. Ecophysiological characteristics of mature trees and stands—Consequences for old-growth forest productivity. In Old-Growth Forests. Ecological Studies 207; Wirth, C., Ed.; Springer: Berlin/Heidelber, Germnay, 2009; pp. 57–79. [Google Scholar]
- Niklas, K.J. The elastic moduli and mechanics of Populus tremuloides (Salicaceae) petioles in bending and torsion. Am. J. Bot. 1991, 78, 989–996. [Google Scholar] [CrossRef]
- Roden, J.S.; Pearcy, R.W. Effect of leaf flutter on the light environment of poplars. Oecologia 1993, 93, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Planchais, I.; Sinoquet, H. Foliage determinants of light interception in sunny and shaded branches of Fagus sylvatica (L.). Agric. For. Meteorol. 1998, 89, 241–253. [Google Scholar] [CrossRef]
- Planchais, I.; Pontailler, J.-Y. Validity of leaf areas and angles estimated in a beech forest from analysis of gap frequencies, using hemispherical photographs and a plant canopy analyzer. Ann. For. Sci. 1999, 56, 1–10. [Google Scholar] [CrossRef]


| Betula pendula | Quercus petraea | Carpinus betulus | Tilia Cordata | Fagus sylvatica | |
|---|---|---|---|---|---|
| Family | Betulaceae | Fagaceae | Betulaceae | Malvaceae | Fagaceae |
| Successional status | early | mid/late | mid/late | late | late |
| Shade production of adults 1 | V | III | I | II | I |
| Shade tolerance of saplings 2 | V | IV | II | III | I |
| Light demand of shade leaves 3 | 20 | 7 | 2.5 | 3 | <1 |
| Drought tolerance 4 | mid | high | mid/high | mid | low |
| Type of mycorrhiza 5 | ECM | ECM | ECM | ECM | ECM |
| Xylem anatomy 6 | diffuse | ring | diffuse | diffuse | diffuse |
| Species | Site # | Location | Coordinates | Slope | Elevation (m a.s.l.) | Soil Substrate | Admixed Species |
|---|---|---|---|---|---|---|---|
| Betula pendula | 1 | Lehrer Wald (Helmstedt county), Lower Saxony | 10°42′ E 52°19′ N | level | 95 | Loamy sand | Alnus glutinosa |
| Betula pendula | 2 | Lappwald (Helmstedt county), Lower Saxony | 11°01′ E 52°16′ N | level | 147 | Loamy sand | Pinus sylvestris |
| Quercus petraea | 3 | Breitenhees, Lüneburg Heath, Lower Saxony | 10°30′ E 52°49′ N | level | 120 | Loamy sand | Picea abies |
| Quercus petraea | 4 | Breitenhees, Lüneburg Heath, Lower Saxony | 10°30′ E 52°49′ N | level | 123 | Loamy sand | Picea abies |
| Carpinus betulus | 5 | Ziegelrodaer Forst (near Mansfeld), Thuringia | 11°32′ E 51°26′ N | 5% NW | 225 | Sandy loam | Tilia cordata, Acer ssp. |
| Carpinus betulus | 6 | Ziegelrodaer Forst (near Mansfeld), Thuringia | 11°32′ E 51°26′ N | 11% N | 235 | Sandy loam | Fraxinus excel., Tilia cordata |
| Tilia cordata | 7 | Colbitz-Letzlinger Heide (county of Wolmirstedt), Saxony-Anhalt | 11°32′ E 52°21′ N | level | 85 | Loamy sand | Quercus robur, Fraxinus excel. |
| Tilia cordata | 8 | Colbitz-Letzlinger Heide (county of Wolmirstedt), Saxony-Anhalt | 11°33′ E 52°20′ N | level | 78 | Loamy sand | Quercus robur, Fraxinus excel. |
| Fagus sylvatica | 9 | Unterlüss, Lüneburg Heath, Lower Saxony | 10°16′ E 52°50′ N | level | 115 | sand | Quercus petraea |
| Fagus sylvatica | 10 | Unterlüss, Lüneburg Heath, Lower Saxony | 10°19′ E 52°49′ N | level | 113 | Loamy sand | - |
| Species | Site # | Stand Age (year) | Basal Area (m2 ha−1) | Stem Density (ha−1) | Abundance of Main Species (% of Stems) | Mean Height (m) | Mean dbh 1 (cm) |
|---|---|---|---|---|---|---|---|
| Betula pendula | 1 | 45 | 25.2 | 880 | 85 | 20 ± 3 | 19 ± 6 |
| Betula pendula | 2 | 67 | 28.5 | 420 | 98 | 25 ± 3 | 25 ± 3 |
| Quercus petraea | 3 | 154 | 27.5 | 260 | 94 | 23 ± 3 | 37 ± 6 |
| Quercus petraea | 4 | 149 | 23.8 | 190 | 95 | 23 ± 2 | 44 ± 6 |
| Carpinus betulus | 5 | 116 | 31.5 | 345 | 68 | 27 ± 5 | 29 ± 1 |
| Carpinus betulus | 6 | 95 | 37.0 | 420 | 77 | 26 ± 3 | 32 ± 9 |
| Tilia cordata | 7 | 83 | 35.9 | 370 | 96 | 23 ± 4 | 23 ± 9 |
| Tilia cordata | 8 | 65–81 | 37.7 | 780 | 81 | 22 ± 4 | 22 ± 9 |
| Fagus sylvatica | 9 | 95–115 | 30.8 | 445 | 92 | 26 ± 2 | 38 ± 7 |
| Fagus sylvatica | 10 | 96 | 24.2 | 375 | 100 | 26 ± 2 | 38 ± 6 |
| Species | a | b | c | r | p | df |
|---|---|---|---|---|---|---|
| Betula | 50.5 | 38.7 | 3.3 | 0.94 | <0.001 | 26 |
| Quercus | 59.9 | 65.2 | 4.9 | 0.94 | <0.001 | 32 |
| Carpinus | 30.7 | 71.3 | 5.9 | 0.91 | <0.001 | 29 |
| Tilia | 30.3 | 60.5 | 7.4 | 0.91 | <0.001 | 21 |
| Fagus | 23.2 | 59.9 | 1.8 | 0.79 | 0.011 | 18 |
| Species | a | b | c | r | p | df |
|---|---|---|---|---|---|---|
| Betula | 29.0 | 34.5 | 2.13 | 0.84 | <0.001 | 28 |
| Quercus | (0) | 45.4 | 1.73 | 0.86 | <0.001 | 24 |
| Carpinus | 19.2 | 18.8 | 6.44 | 0.72 | 0.035 | 20 |
| Tilia | 20.7 | 36.8 | 10.2 | 0.79 | 0.022 | 16 |
| Fagus | 17.0 | 27.7 | 3.61 | 0.75 | 0.001 | 30 |
| Species/Community | Shade Crown | Crown Average | Sun Crown | Source |
|---|---|---|---|---|
| Betula pendula | 30 | 49 | 53 | This study |
| Populus deltoides | 32.3 | 75.7 | McMillen and McClendon (1979) [9] | |
| Quercus petraea | 21 | 25 | 41 | This study |
| 29 | 37 | 38 | Schlünder (unpubl.) | |
| Quercus robur and petraea | 26 | 46 | Kull et al. (1999) [6] | |
| Quercus rubra | 11 | 10.1 | McMillen and McClendon (1979) [9] | |
| Oak–hickory forest | 10 | 33 | 38 | Hutchison et al. (1986) [4] |
| Carpinus betulus | 21 | 22 | 26 | This study |
| 13 | 21 (mid) | 42 | Elias (1990) [5] | |
| Tilia cordata | 18 | 24 | 34 | This study |
| Acer saccharum | 7.8 | 14.6 | McMillen and McClendon (1979) [9] | |
| Fagus sylvatica | 20 | 22 | 37 | This study |
| 15 | 34 | 53 | Schlünder (unpubl.) | |
| 22.2 | 26 (mid) | 34 | Planchais & Sinoquet (1998) [41], Planchais & Pontailler (1999) [42] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hagemeier, M.; Leuschner, C. Functional Crown Architecture of Five Temperate Broadleaf Tree Species: Vertical Gradients in Leaf Morphology, Leaf Angle, and Leaf Area Density. Forests 2019, 10, 265. https://doi.org/10.3390/f10030265
Hagemeier M, Leuschner C. Functional Crown Architecture of Five Temperate Broadleaf Tree Species: Vertical Gradients in Leaf Morphology, Leaf Angle, and Leaf Area Density. Forests. 2019; 10(3):265. https://doi.org/10.3390/f10030265
Chicago/Turabian StyleHagemeier, Marc, and Christoph Leuschner. 2019. "Functional Crown Architecture of Five Temperate Broadleaf Tree Species: Vertical Gradients in Leaf Morphology, Leaf Angle, and Leaf Area Density" Forests 10, no. 3: 265. https://doi.org/10.3390/f10030265
APA StyleHagemeier, M., & Leuschner, C. (2019). Functional Crown Architecture of Five Temperate Broadleaf Tree Species: Vertical Gradients in Leaf Morphology, Leaf Angle, and Leaf Area Density. Forests, 10(3), 265. https://doi.org/10.3390/f10030265




