Abstract
Pinus contorta-dominated montane forests of western Canada with relatively dense tree canopies have ground layers with abundant bryophytes, especially the feather mosses (Pleurozium schreberi and Hylocomium splendens), while those with more open canopies are dominated by species of reindeer lichens, especially Cladonia arbuscula s.l. and C. rangiferina s.l. Woodland caribou (Rangifer tarandus caribou), which are a threatened species in Alberta, prefer open, Cladonia-dominated forests for their winter food supply. This study investigated if opening the canopy by thinning mature montane forests of the Canadian Rocky Mountain foothills would change the abundance of lichens and bryophytes. In 1997, forests were thinned by removing 20%, 40%, and 60% by volume. In 2016, 19 years after treatment, we re-surveyed a subset of these plots (n = 97) for lichen and bryophyte abundance and species richness by utilizing the amount of canopy opening at the plot level as our prime gradient. We then used ordination to determine the relationship of control plots to treatment plots. In uncut forest, the control plots were highly variable, but were mostly dominated by feather mosses, with little or no bare ground. Feather moss abundance was lower in treatment plots when compared to control plots, while cover of bare ground was greater. Overall, 19 years after treatment, we found that, in treatment plots, lichen abundance remained stable or slightly increased, feather mosses decreased markedly, and unoccupied space was double that of the control plots. We conclude that the canopy opening had little effect on understory and ground layer diversity, but considering species abundance (1) bryophytes have not recovered after canopy opening, (2) populations of reindeer lichens increased marginally, but have not colonized areas left bare from bryophyte dieback, and (3), after 19 years there, remains unoccupied areas of bare ground in plots with a reduced canopy cover. Our study demonstrated that, with canopy cover reduction resulting from forest thinning operations, the ground layer diversity is maintained, but recovery of ground layers in old-growth pine-dominated forests is not promoted. Therefore, timber harvest that partially opens the tree canopy is unlikely to benefit caribou by augmenting or accelerating winter food availability and habitat suitability for caribou.
Keywords:
bryophyte; caribou; Cladonia; ground layer; lichen; moss; Pinus contorta; reindeer lichen; feather moss 1. Introduction
Upland coniferous forests in boreal western Canada often have a ground layer composed of a mosaic of mosses and lichens. Young stands with relatively open canopies have ground layers comprised mostly of lichens, especially the “reindeer lichens” Cladonia arbuscula/mitis and C. rangiferina/stygia, while older, more mesic stands have abundant feather mosses (Pleurozium schreberi, Hylocomium splendens, and Ptilium crista-castrensis). However, ground layers in many stands are a mosaic of patches of lichens and mosses [1]. Most lichens and bryophytes that are abundant on the boreal forest floor are long-lived perennials and colonization of these species may be limited by a number of factors such as diaspore availability [2] or by limited representation in the buried diaspore bank [3]. Additionally, Pharo and Vitt [4] found that few environmental factors explained local variation in bryophyte and lichen distributions, which suggests that species distributions may be largely affected by factors other than current environmental patterns, and may instead be, in part, the result of past environmental changes.
Lichens and bryophytes have similar ecological strategies including being small in stature, lacking roots and protective cuticles, and having limited control of water loss, which makes them susceptible to desiccation [5,6]. Although shade-adapted bryophytes have limited ability to recover from prolonged periods of drying [7], fruticose lichens of the ground layer are adapted for surviving long periods of drought and high light environments [8]. These attributes of lichens and bryophytes contribute to sensitivity to disturbance, including sensitivity to mechanical damage from logging operations and changing environmental conditions post-harvest. In particular, altered humidity, moisture, and temperature regimes resulting from canopy removal [9,10,11] may exceed the tolerance limits of terrestrial bryophytes and lichens. Substrate diversity is also important for bryophytes and lichens. In undisturbed forests, varying substrates (e.g., downed woody material [DWM]) are recruited regularly, and these diverse substrates provide the basis for maintaining bryophyte and lichen species richness [12]. Disturbances at the watershed level, whether natural (e.g., fire) or anthropogenic (e.g., timber harvest), remove the tree canopy and the few remaining trees will not continue to contribute to the input of DWM. Furthermore, shade-tolerant species of bryophytes, both on woody and terrestrial substrates, fail to respond to a shift in habitat conditions and are unable to reproduce, and die following disturbance [12]. Other characteristics of the substrate also play a limiting role for ground layer species, including the amount of organic matter, moisture availability, and soil chemistry and texture [13].
The abundant bryophytes and lichens occurring in the ground layer of boreal forests play a key role in nutrient cycling and growth of forests [14,15] and have high species richness [16,17]. In many parts of the boreal zone, epiphytic lichens form the main winter food source for local caribou herds ([18]-Quebec and [19,20]-British Columbia). However, in more continental areas such as the Rocky Mountain foothills of West-Central Alberta, ground lichens (e.g., species of Cladonia) are an important winter food source for threatened woodland caribou - Rangifer tarandus caribou [21].
Studies in a number of different forest types have shown that ground layer bryophytes and lichens react quickly to disturbances from timber harvesting, in general with decreased abundance and diversity, e.g., Picea and mixed wood forests [22], west-coast Pseudotsuga forests [23], and Acadian forests [24]. Previous studies that included information on responses of the ground layer include those of Mills and Macdonald [25] who examined the bryophyte community structure in conifer-dominated forests in boreal Alberta, and reported that forest floor moisture, light, and temperature were important in determining species composition. Macdonald and Fenniak [26] examined responses of understory vascular plant communities to variable retention harvesting and reported that, in conifer-dominated forests, neither 20% nor 75% retention treatments significantly altered cover and richness of shrubs and herbs. Caners et al. [27] reported that increased canopy retention correlated to increased bryophyte epiphyte richness and abundance, but there was some loss of species in areas where variable retention occurred when compared to the undisturbed forest. Likewise, Craig and Macdonald [28] showed similar results for the vascular plant understory and for bryophytes as a whole [29]. However, a study in Finland could not demonstrate that tree retention preserved the pre-harvest vegetation nor post-harvest succession [30]. All of these studies monitored ground layer changes after a relatively short time period (less than five years) and few if any studies have examined long-term responses of ground layer vegetation to anthropogenic disturbance. Yet, some research has been carried out specifically assessing lichen responses to a variety of forest management practices in eastern Canada [31] as well as responses of vascular plants to forest management [28]. Based on our knowledge, few studies have had the opportunity to assess changes in lichen abundance after canopy opening from timber harvesting, and it remains unknown how lichens respond to forest management in the Rocky Mountain foothills of West-Central Alberta.
This study was based on the concerns that changes in the ground lichen cover (especially reindeer lichens) of aging pine-dominated forests may decrease habitat suitability for caribou [20,30,32,33]. Additionally, clear-cut logging, which has long been a standard practice in western Canada, has severe consequences for the ground layer of bryophytes and lichens [29,34,35] and may conflict with caribou recovery efforts [31,36]. The project was initiated in 1997 to evaluate the value of commercial thinning as a tool for maintaining or improving caribou habitat suitability. Focused within nine Pinus contorta-dominated stands in the Rocky Mountain foothills of West-Central Alberta, the study was designed to assess changes in understory communities after tree removal by volume of 20%, 40%, and 60%. Before harvesting, Pharo and Vitt [4] measured vegetation and environmental variables in 180 plots across nine stands, and found that stands with open canopies had higher lichen cover, while those with dense canopies had higher bryophyte cover. In 2005, Mooneyhan-McClelland [37] compared photosynthetic active radiation, relative humidity, and evaporation differences in plots with approximately 60% canopy cover to plots with approximately 40% canopy cover. She reported that plots with higher canopy cover were significantly more humid, had less light, and were cooler than plots with less canopy cover. She also reported that microsites with bryophytes were significantly more humid and had less light than those with lichens, but that bryophytes had been severely impacted and lichens had not expanded into areas left bare from bryophyte dieback. This short-term study (7-years post-harvest) provided the framework for the present long-term study.
In the present study, we used data collected in 2016 (19 years after commercial thinning occurred) collected from 97 plots to assess how ground layer and understory vegetation had responded to canopy opening. Our goals were to determine whether (1) ground layer components (lichens and bryophytes) and understory vascular plants would remain abundant after canopy opening and (2) if abundance of reindeer lichens would increase with canopy opening. We predicted that, as the result of opening of the forest canopy and increased solar radiation, (1) reindeer lichens would increase in abundance, (2) terrestrial bryophytes, especially feather mosses, would initially decrease in abundance and species richness or be extirpated, but would gradually recolonize as the altered tree canopy recovered, and (3) vascular plant understory vegetation abundance would increase. We also predicted that (4) the understory plant community would differ from undisturbed control plots.
2. Materials and Methods
2.1. Study Area
The study area was 375 km2 in size, and was in mature Pinus contorta-dominated forests in the Upper Foothills and Subalpine Natural Subregions of the eastern slopes of the Rocky Mountains [38]. Forests in the study area have regenerated from wildfire between 61 and 183 years ago. Individual trees range in size from 6.6 to 30.6 cm diameter at breast height. Younger stands are open and pine-dominated, while older stands on more mesic sites have a secondary canopy of Picea glauca. The understory is open, with dominant shrubs of Rhododendron groenlandicum and Vaccinium vitis-idaea and an herb layer of Cornus canadensis, Elymus innovata, and Lycopodium (s.l.) spp. The ground layer is mostly covered by a mosaic of reindeer lichens (mainly Cladonia arbuscula/mitis, C. rangiferina/stygia (see References [39,40] for discussion of nomenclature), and C. stellaris–collectively termed “reindeer lichens” and feather mosses (Pleurozium schreberi, Hylocomium splendens, and Ptilium crista-castrensis). Other lichens occurring on the forest floor (including dead wood and DWM) are Cladonia gracilis, Flavocetraria nivalis, Parmeliopsis ambigua, Peltigera aphthosa, and Vulpicida pinastri. Abundant bryophytes include Dicranum acutifolium, D. polysetum, Polytrichum juniperinum, Pohlia nutans, and Ptilidium pulcherrimum. Soils of the area are sandy loam or loamy sand in texture [41] and acidic with little variation in pH (mean 4.88, ±0.16 s.d.). The climate of the region is continental, with cool, moist summers and cold, snowy winters. Average decadal (2008–2017) precipitation is 606 mm, with average annual minimum temperatures of −6.1 °C and average annual maximum temperatures +8.2 °C. Elevation varies from 1350 to 1476 m.
2.2. Experimental Design and Methodology
In the summer of 1997, we delimited nine stands each 30–40 ha in size, located at three sites within the study area for experimental thinning treatments (Table 1, Figure S1 Supplementary Material). The three study sites were representative of the age, canopy cover, and stand structure of the study area, and were chosen based on (i) dominance of Pinus contorta, (ii) at least 30% ground cover of bryophytes and lichens, (iii) initially undisturbed and approximately 100 years old, and (iv) proximity to road access. We divided each of the nine stands into a control, and three treatments, each about 10 ha in size.

Table 1.
Locations of the three study sites and the companies associated with each site located in pine-dominated forests in western Alberta, Canada that were commercially thinned to reduce canopy cover. ANC = Alberta Newsprint Co.
During the winter of 1997–1998 ANC (Alberta Newsprint Co.), Weldwood Canada Ltd., and Weyerhaeuser Canada independently harvested timber from the three sites using tree removal by volume (RV) of 20%, 40%, and 60% (treatments), and 0% (control). The three treatments and one control were randomly placed within each stand. Sites were commercially thinned using single grip processors and forwarders harvesting at the stump. Forwarding trails were laid out on set intervals (~20 m, but modified according to the equipment used), and the areas between trails were thinned. Tree removals were operator selected to prioritize an even-target tree spacing in each treatment area, while cutting across the stem diameter profile (pers. communication - M. Vitt). This study was initiated to determine the impact of canopy opening on ground layer vegetation. As a result, we excluded the mechanical effects of commercial timber harvest. The tops of felled trees and other debris resulting from harvesting were excluded from the plots by careful removal at the time of harvesting, and disturbance from logging equipment was restricted to outside plot boundaries. Post-harvest changes in DWM was surveyed in 2016.
2.3. Plot Set-Up
In 1997, before thinning operations and canopy opening, we established twenty 6.5 × 6.5 m plots (42.25 m2) (180 in total) in a double restricted random design (both along and perpendicular to the transect)—five in each of the treatment and control areas. Plots were marked with a short length of rebar and tagged with flagging tape and a unique ID for later field data collection. Vegetation responses to the thinning treatments were carried out in 1998, directly after thinning was completed [4] and again in 2005 [37]. Canopy cover (%) at the four corners of each plot was recorded using a Lemmon Model C concave spherical densiometer.
In 2016, we randomly selected 97 of the 180 plots, distributed among the controls (n = 27) and three thinning treatments (n = 70), and assessed changes in a variety of plant responses. In treatment plots, we used “percent reduction in canopy cover” as our key variable in regressions and ordinations. We explored the canopy opening effects and functional group responses within the treatments and controls using either one-way ANOVA followed by Tukey’s pairwise comparison or when data were non-normal Kruskal-Wallis one-way ANOVA on ranks followed by Dunn’s pairwise comparison.
2.4. Species Diversity Responses
We estimated species occurrence and abundance of all terrestrial bryophyte, lichen, and vascular plant species (including cover from tree species less than 2-meters high) within five 1.5 × 1.5 m subplots placed at the corners and center of the 42.25 m2 plot (Figure S2, Supplementary Material). We recorded abundance using a scale of 1 to 4 that emphasizes species with less abundance and considers cover over 50% as abundant in the plot (1 = rare, only a few stems or found only once, 2 = locally abundant, 5%–25% cover, 3 = abundant, 25%–50% cover, and 4 = very abundant, 50%–100% cover). We evaluated species diversity within the treatment (n = 70) and control plots (n = 27) using three measures [42]: (i) gamma diversity: the total number of species from all plots, (ii) alpha diversity: the mean number of species within each plot, and (iii) beta diversity as a measure of species turnover, calculated by dividing gamma diversity by alpha diversity. We evaluated these diversity measures for three groups: (1) understory vascular plants, (2) lichens, and (3) bryophytes. We used a t-test or Mann-Whitney U test for non-normal data to evaluate differences in alpha diversity.
2.5. Plant Community Responses
We also used species abundance estimates from the 97 plots in a Non-Metric Multi-Dimensional Scaling (NMDS) ordination [43] to examine similarities in vegetation among control and treatment plots. Ordination is an indirect gradient analysis approach that arranges plots by similarity of plant abundance measures, and can be coupled with cluster analysis to determine groups of plots that are sufficiently unique to form significantly different clusters. We ran the NMDS analysis on Bray-Curtis similarity data with 25 restarts with a minimum stress of 0.01 and Kruskal fit scheme one. The resulting ordination was two dimensional. We then used PERMANOVA (Type III) to examine if treatment plot plant community differed from the control community and used a similarity profile analysis (SIMPROF) with the program Primer v6 [43] to carry out a group average cluster analysis to assess if there were significant groups within treatment plots. SIMPROF arranges raw data, creates a ‘mean permutated similarity profile’, and then tests whether the data set differs from that profile, or whether cluster group differences are more than those expected by chance (α set at 0.05). The cut level for significantly different clusters is dependent on where the first non-significant cluster is formed. Thus, different data sets can have different cut levels for significantly different clusters. For these data, we used a cut level of 65% similarity.
2.6. Functional Group Responses
We estimated abundances of the major functional groups including feather mosses (Pleurozium schreberi, Hylocomium splendens, and Ptilium crista-castrensis), reindeer lichens (Cladonia arbuscula/mitis, C. rangiferina/stygia, and C. stellaris), other lichens, understory vascular plants (shrubs, herbs, fern allies), and surface features of bare soil, rocks, dead moss, and DWM (collectively termed “bare ground”) using a line transect placed along the perimeter of each plot (line totaling 2600 cm). We calculated abundance as the total length of line touched by each function group/surface feature divided by 2600 (ground layer attributes and functional groups totaled to 2600, while above ground vegetation totals were variable depending on the cover of plants). We then evaluated responses of functional groups to canopy opening using linear regression and ordination. We also assessed differences in responses of functional groups to canopy opening between control plots and treatment plots with a t-test or a Mann-Whitney U test for non-normal data.
3. Results
In 1997, timber thinning regimes were implemented to produce tree removal at the stand level by volume of 20%, 40%, and 60% removal. However, field assessment revealed that, in practice, canopy cover changes at the local (plot) scale were extremely variable. By 2005, canopy cover change (pre-harvest-post-harvest) varied from 1% to 79% (mean 30%). Pre-harvest canopy cover ranged from 54% to 79% (mean 63%), while post-harvest (2005) canopy cover varied from 38% to 53% (mean 34%). We examined treatment effects as follows: Canopy cover in 2016 within the three treatments differed from the controls (H = 30.908, p < 0.001), but there were no differences between the treatments (f = 1.175, p = 0.315). Reindeer lichen abundance in the treatments slightly differed from that of the controls (H = 7.838, p = 0.049), while feather moss abundances in the treatments were different from the controls (H = 37.907, p < 0.001), but neither group had differences within the treatments H = 1.905, p = 0.386, and H = 0.490, p = 0.783, respectively). Abundance of young pine individuals in the treatments (those recorded within our perimeter monitoring plots) was similar to that in the control plots (t = 1.03, p = 0.299), and, between 1997 and 2016, canopy cover slightly decreased (t = 1.76, p = 0.049). It appears that these old-growth pine stands that characteristically lack a dispersed seed source until the occurrence of wildfire are unable to recruit new individuals. Additionally, wind related tree falling in open stands has served to maintain open canopies continually since timber harvest (DWM in treatment plots was 7.3% (±0.66) cover in 2016 compared to 4.8% (±1.1) in control plots t = 2.28, p = 0.013).
3.1. Species Diversity Responses
In 2016, we recorded 138 species across the 97 plots. Furthermore, 36% of species recorded were vascular plants, 38% were lichens, and 26% were bryophytes (Appendix A). The total number of species in treatment plots (gamma diversity) was higher for all species groups (bryophytes, lichens, and vascular plants) when compared to the control plots (Table 2). The ratio of bryophytes to lichens remained similar between treatment and control plots (0.65 vs. 0.63). However, the ratio of understory vascular plants to ground layer species (bryophytes, lichens) in the treatment plots increased substantially compared to the controls (0.57 vs. 0.49). Generally, treatment plot gamma diversity did not decrease with canopy opening.

Table 2.
Species diversity measures (means ± S.E.M.) for the treatments (n = 70) and control plots (n = 27) for three species groups. Data are from the five subplots within each of the 97 plots (see methods for details).
The mean number of species occurring in the plots (alpha diversity) for all three groups of plants (bryophytes, lichens, understory vascular plants) was not significantly different between treatment and control plots (bryophyte alpha—U = 1260.0, p = 0.923, lichen alpha—t = 1.05, p = 0.298, understory vascular alpha—U = 1156.5 p = 0.442). Lichen plot richness was consistently about three-times that of bryophytes and two-times that of vascular plants (Table 2).
Species turnover (beta diversity –gamma/alpha) between plots was higher in the treatment plots for all three groups of plants. Vascular plant turnover between plots increased by 31% (vs. 22% for bryophytes and 16% for lichens) in treatment plots versus controls (Table 2). When all species of bryophytes are considered together, abundance significantly decreased in treatment plots (t = 8.02, p < 0.001), whereas, when all species of lichens are taken together, abundance marginally increased by 8.2% (U = 1063.5, p = 0.087), while understory vascular plants remained similar to control plots (t = 0.175, p = 0.861). The ratio of abundance to alpha diversity for treatment vs. control plots remained relatively stable for lichens 0.31/0.35) and vascular plants (0.27/0.26, while this ratio for bryophytes changed dramatically (0.35 vs. 0.20 for treatment plots), which indicates that the canopy opening has a greater effect on bryophyte abundance rather than bryophyte species richness. Yet, both lichens and vascular plants are less affected.
3.2. Plant Community Responses
Control plots and most treatment plots form one large cluster on the NMDS ordination, with most control plots positioned to the right and most treatment plots to the left. Treatment plots are associated with increasing bare ground and greater canopy removal (as vectors—Figure 1A,B). Treatment plots are significantly different from control plots (PERMANOVA - p = 0.001), and form three significant groups (SIMPROF at 65% similarity). All treatment plots except five outliers form a central cluster, with four plots included in a cluster to the left of the ordination. These four plots all have high amount of bare ground and little abundance of bryophytes and lichens. Feather mosses dominate in control plots and decrease in treatment plots (Figure 1C), while reindeer lichens have higher abundance in the treatment plots compared to the control plots (Figure 1D).
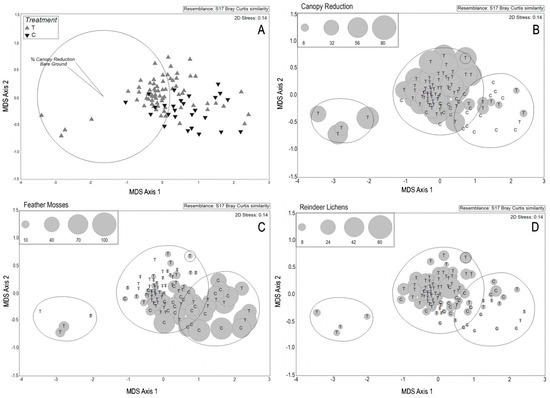
Figure 1.
(A) Results of non-metric multi-dimensional scaling ordination differentiating treatments and controls with vectors for increasing bare ground and canopy opening in pine-moss-lichen forests in western Alberta, Canada. Each triangle represents one of 97 plots. (B–D) Plot abundances overlain on NMDS ordination of 97 plots. Centered letters indicate treatment (T) or control (C). (B) Percent canopy cover reduction. (C) Feather mosses (Pleurozium schreberi, Hylocomium splendens, and Ptilium crista-castrensis). (D) Reindeer lichens Cladonia arbuscula/mitis, C. rangiferina/stygia, and C. stellaris). Sizes of the bubbles were derived from either 2016 densiometer readings (B) or perimeter line transect sums (C-D -see methods).
3.3. Functional Group Responses
Feather moss abundance in the treatment plots was significantly less than in the control plots (U = 2060.0, p < 0.001) and had a significant cubic relationship with the canopy opening (p = 0.0001, Figure 2A). Most treatment plots with less than 20% canopy reduction maintained relatively high feather moss cover, while plots with canopy reduction was greater than 20%, which generally had feather moss cover of less than 20% (Figure 2A). In comparison, abundance of reindeer lichens in treatment plots was significantly greater compared to the controls (U = 1009.5, p = 0.015), and generally increased (p = 0.022, Figure 2B) with reduced canopy cover. However, higher abundances only occasionally exceeded those in the control plots. Although abundance of other lichens in treatment plots was significantly different than the control plots (U = 979.5, p = 0.007), there was no relationship with a reduction in canopy cover (p = 0.199, Figure 2C). Abundance of understory vascular plants in the treatment plots was not different from the controls (U = 1229.0, p = 0.524) and was highest at intermediate values of canopy cover reduction (p = 0.0432, Figure 2D). Associated with these functional group changes, the bare ground of treatment plots was significantly greater in treatment plots (mean = 61.3% ( ± 3.05) compared to control plots (mean = 31.7 ( ± 3.69)) (t = −7.706, p < 0.0001).
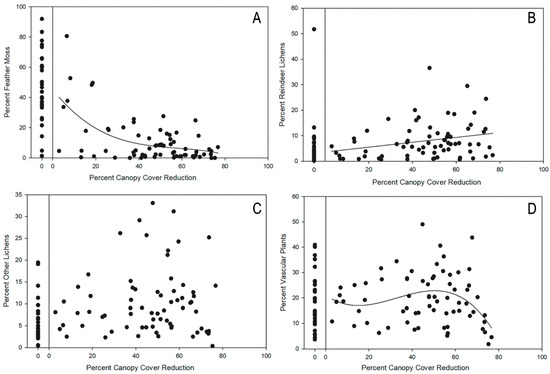
Figure 2.
Regression of percent cover of: (A) feather mosses, (R2 = 0.340, p < 0.0001, f = 45.2 + (−1.8)*x + 0.03*x2 + (−0.0002)*x3). (B) Reindeer lichens (R2 = 0.070, p = 0.028, f = 3.6 + 0.1*x). (C) Other lichens (p = 0.199). (D) Understory vascular plants (R2 = 0.12, p = 0.043, f = 21.2 + (−0.6)*x + 0.03*x2 + (-0.0003)*x3) in treatment plots as a function of percent reduction in canopy cover in pine-moss-lichen forests in western Alberta, Canada. Variation in the percent cover of the variable in control plots is shown to be left of regression and was not included in the regression analysis.
4. Discussion
4.1. Ground Layer Responses
Previous research in the study area found that undisturbed forests have variable canopies, which vary from 45% to 87% canopy cover [4] and ground layers are composed of mosaics of lichens and bryophytes, interspersed with small areas of bare ground. Down woody material provides microhabitats for a variety of lichens and bryophytes, which increases species richness [12]. More open stands have ground layers dominated by lichens, especially Cladonia arbuscula (s.l.), while stands with more closed canopies have ground layers dominated by bryophytes, especially the feather moss, Pleurozium schreberi. The degree of canopy cover is associated with changes in the environment, especially temperature and amount of light and humidity [37].
In undisturbed forest plots, we found that lichens had higher species richness in plots than bryophytes and understory vascular plants. We also found that lichen species turnover between plots was much lower than that of vascular plants or bryophytes. Similar to the pre-harvest study [4], we found overall abundance of lichens was considerably less than that of bryophytes. Changes in overall lichen species richness within the canopy cover treatment were minimal; however, lichen abundance including both reindeer lichens and other lichens increased. In only a few plots did reindeer lichen abundance exceed control levels.
Overall, the small increases in lichen abundances in the treatment plots (8.2%) suggest that these small increases are due to some expansion of existing populations and not the establishment of new populations in areas of bare ground. These findings demonstrate that lichens respond minimally to opening the canopy, and after 19 years, lichen abundances have not increased substantially and reindeer lichens have not yet expanded into areas left bare by feather mosses, which leaves large areas of the bare ground.
When we compared plots in undisturbed forests to treatment plots, we found that bryophyte plot diversity was similar across control and treatment plots, but species richness (gamma) was substantially higher in treatment plots, while overall species abundance was substantially lower in treatment plots. These differences are similar to those reported by Fenton et al. [24] wherein species composition changes were related to the severity of forest floor disturbance, and different from those reported by Nelson and Halpern [23] who found declines in species richness and cover of liverworts and bryophytes one year after timber harvesting. High beta diversity in the treatment plots provides evidence that, although bryophyte abundances may have decreased, overall species richness of the landscape has been maintained, with individual plots each having somewhat different floras. The observed lower abundance of feather mosses in our study is in accordance with our prediction that feather mosses would decrease in cover with the canopy opening; however, contrary to our prediction that feather mosses would recolonize areas over time, our results demonstrated that nearly 20 years after thinning, site conditions appear to be beyond the tolerance levels of these species and feather mosses have not recovered to their pre-disturbance abundances. As a result, substantial areas of bare ground remain that have not been colonized by bryophytes or lichens. Overall, response of bryophytes to canopy opening in our study appear to mirror those reported for retention harvesting [44] with canopy opening creating long-term impacts on site conditions that hinder the recovery of bryophytes.
In summary, our results suggest that, under natural conditions in this forest type, feather mosses and reindeer lichens dominate the ground layer with relatively little bare ground. The increase in diversity of bryophytes is due mainly to the establishment of ruderal species on the ground left bare by feather moss dieback—(e.g., Bryum caespiticium, Ceratodon purpureus, Pohlia nutans, Polyrichum juniperinum) including species largely not present or rare under undisturbed situations. Even after 19 years, the lack of pine seedling recruitment and long-term stability of an open canopy limit the gradual changes in light and moisture normally expected in succession. As a result, feather mosses are unable to tolerate these open canopy conditions and cannot re-colonize previously occupied microsites.
Contrary to our prediction, we found in general, that understory vascular plant abundance in the treatment plots was not different from control plots, but, like bryophytes and lichens, overall species richness increased in the treatment plots in comparison to the plots in the undisturbed forest. However, it is noteworthy that these increases in gamma (and beta) diversity may be an artifact of the higher number of treatment plots compared to the control plots.
With the exception of four outliers, the 97 plots sampled in 2016 ordinate in one cluster, with variation associated with the reduction in canopy cover. We found that feather mosses were more abundant in plots with lower canopy cover reduction, while reindeer lichens are more abundant in plots with higher canopy reduction. We also found that bare ground increased as canopy opening increased. Feather mosses remained abundant in most plots with canopy reduction of less than 20%, while canopy reduction greater than 20% reduced feather moss abundance drastically. These results are somewhat similar to those of Caners et al. [17] who reported differences between tree retention levels for a number of bryophyte criteria in mixed wood and boreal white spruce-dominated coniferous forests with higher understory diversity. Overall, with little pine recruitment and with canopy cover remaining largely unchanged since assessments carried out post-harvest in 1997, it is likely that the increased shade required for feather moss colonization has not occurred within our treatment sites. Additionally, our results indicate that lichens have not responded to canopy opening by colony establishment in plots that had the greatest percentage of canopy removed, and there remain strong differences in understory plant community after 19-years post-harvest.
4.2. Implications for Ground Layer Recovery after Canopy Opening
Both lichens and bryophytes have distributions at the stand level largely controlled by variations in microclimate, moisture, substrate availability, and stand age [12,45,46,47,48] with microclimate and moisture availability strongly influenced by canopy cover. Despite the apparent influence of canopy cover on lichens and bryophytes, it is likely that other factors besides canopy cover were responsible for the responses of the dominant species observed in our study. For example, in an experimental study, Mooneyhan-McClelland [37] found that lichen and moss diaspores (fragments of dominant species and bryophyte spores) were both abundant in diaspore traps and apparently available on all substrates. In addition, Caners et al. [49] demonstrated that pleurocarpous mosses germinated frequently. In reciprocal transplant studies, Mooneyhan-McClelland [37] found that feather mosses were established only in areas where mosses were located before disturbance (i.e., organic substrates), whereas lichens were established best on bare mineral soils under an open canopy. Therefore, in our study, it is possible that the presence of an organic substrate left from moss dieback inhibited lichen expansion and also prevented the establishment of new lichen colonies 19 years after treatment. An open canopy is only one of two critical limiting factors for high lichen abundance. The second factor, which is maybe even more important than canopy conditions, is a mineral soil substrate, in particular a mineral soil that was not previously colonized by moss before disturbance. Thus, the “legacy” of the soil substrate may be a key condition to be considered in creating a disturbance regime, and is likely an important factor explaining the local mosaic pattern of lichens and mosses that we observed. Associated with areas of high moss abundance may be the presence of higher amounts of organic litter [1], greater moisture availability [13], and a shallower water table that may facilitate long-term favorable conditions for bryophyte occurrence and lichen absence [50]. These deterministic processes appear to play a key role in the local spatial positions of lichens and mosses and also provide the background for successional changes. In natural old-growth pine forests, Gendreau-Berthiaume [51] proposed a similar set of processes to explain late-successional changes.
Historically in western Alberta, timber harvest has been carried out by clear-cutting stands, and the severe conditions post-harvest are detrimental to both lichens and bryophytes [52], and may reduce winter forage for threatened woodland caribou. To help inform forest practices in caribou ranges, our research examined the long-term effects of canopy removal on the various components of the ground layer with the intention of determining responses of ground layer components to opening the forest canopy. We were especially interested in determining if lichen cover would increase and replace feather mosses in areas of moss dieback after canopy opening. Although our study failed to demonstrate that the ground layer recovery after canopy opening substantially increased lichen cover for caribou winter forage, it did find evidence that canopy opening had little effect on the understory vascular plant, bryophyte, and lichen diversity. Thus, diversity of the ground layer was preserved. Although some populations of feather mosses remained intact after canopy opening, we found that these dominant ground layer species did not recover after disturbance, and the reindeer lichens, as well as vascular plants, remained mostly unchanged. We also found that there was no colonization of empty space left by bryophyte dieback, and large unoccupied areas of bare ground remain.
Our treatment for canopy opening of thinning by volume did not necessarily translate to a consistent reduction in canopy cover at the local (plot) scale, and, thus, future timber harvesting that aimed to facilitate ground layer recovery could consider substrate and existing ground cover as well as thinning volumes to maximize success. For example, stands dominated by feather mosses may prohibit future lichen expansion, while undisturbed lichen-dominated stands may promote post-harvest lichen expansion. Thus, harvesting approaches that retain some canopy cover may be an effective way to promote bryophyte and lichen survival across a managed landscape. In comparison to clear-cut forest stands, Dynesius [53] could not find support for bryophyte assemblages approaching those in uncut forests after 15 years. Our treatment plots maintained a similar set of species assemblages, and support recommendations by Fenton et al. [54] that maintained that some canopy cover could reduce the impacts of forest harvesting on understory species in pine-moss-lichen forests.
Supplementary Materials
The following are available online at https://www.mdpi.com/1999-4907/10/3/233/s1, Figure S1: Map of three study sites. 1 = Weldwood. 2 = Alberta Newsprint. 3 = Weyerhaeuser. Sites are located in western Alberta between Hinton and Grande Cache along Hwy 40 (sites 1 and 2) and along Huckleberry Road (site 3). Figure S2: Plot design for the 42.25 m2 sample plots containing 5 -1.5 x 1.5 m subplots established in pine-dominated forests in western Alberta, Canada where 2,600 cm long line transect formed the perimeter.
Author Contributions
Conceptualization, D.H.V. Data curation, M.H. Funding acquisition, D.H.V., L.F. Investigation, L.F. Methodology, D.H.V. Writing—original draft, D.H.V. Writing—review & editing, L.F. and M.H.
Funding
This research was funded by the Sustainable Forestry Initiative (2015-005), Canfor Corporation, Norbord Inc., Weyerhaeuser Company, the Alberta Conservation Association (030-00-90-258), West Fraser Timber Co. Ltd, fRI Research, and the Aseniwuche Winewak Nation of Canada through the Environment and Climate Change Canada Aboriginal Fund for Species at Risk. Funds for open access provided by SIU COPE Fund.
Acknowledgments
We thank Rebecca Mooneyhan-McClelland for making her data available to us from her previous study. We are grateful for field data collection by Leonie Brown, Brent McDonald, Dakota Moberly, Kelsey Ridley, and Rebecca Viejou, project management and GIS by Barry Nobert, Doug MacNearney, and Karine Pigeon, and Sandi Vitt for graphics and data management. We are indebted to Rick Bonar (Weldwood, Canada) and Mike Vitt (Weyerhaeuser, Canada) who initiated and helped develop the original project in 1997.
Conflicts of Interest
The authors declare no conflict of interest. The study design was developed and implemented by D.H.V. The collection, analysis, interpretation of data, writing of the manuscript, and decision to publish was done without input from the funding sponsors.
Appendix A

Table A1.
List of all species found in the 2017 survey of 97 plots in pine-dominated forests in western Alberta, Canada that were commercially thinned to reduce canopy cover. * = hepatic.
Table A1.
List of all species found in the 2017 survey of 97 plots in pine-dominated forests in western Alberta, Canada that were commercially thinned to reduce canopy cover. * = hepatic.
| Vascular Plants | Lichens | Bryophytes |
|---|---|---|
| Abies balsamea | Alectoria spp. | Aulacomnium palustre |
| Aconitum delphinifolium | Bryoria spp. | Barbilophozia barbata * |
| Agrostis scabra | Cetraria ericetorum | Barbilophozia hatcheri * |
| Antennaria parviflora | Cetraria islandica | Barbilophozia lycopodioides * |
| Arctostaphylos uva-ursi | Cladina arbuscula/mitis | Buxbaumia sp. |
| Arnica angustifolia | Cladina rangiferina/stygia | Cephaloziella rubella * |
| Arnica cordifolia | Cladina stellaris | Ceratodon purpureus |
| Aster sp. | Cladonia botrytes | Dicranum acutifolium |
| Betula pumila | Cladonia cariosa | Dicranum elongatum |
| Calamagrostris canadensis | Cladonia carneola | Dicranum fragilifolium |
| Campanula rotundifolia | Cladonia cenotea | Dicranum muehlenbeckii |
| Cornus canadensis | Cladonia chlorophaea | Dicranum polysetum |
| Deschampsia caespitosa | Cladonia coccifera | Dicranum scoparium |
| Diphasiastrum complanatum | Cladonia coniocraea | Dicranum spadiceum |
| Elymus innovatus | Cladonia cornuta | Dicranum undulatum |
| Empetrum nigrum | Cladonia crispata | Dicranum brevifolium |
| Epilobium angustifolium | Cladonia deformis | Hylocomium splendens |
| Equisetum scirpoides | Cladonia ecmocyna | Hypnum revolutum |
| Festuca saximontana | Cladonia fimbriata | Lepidozia reptans * |
| Fragaria virginiana | Cladonia cervicornus | Lophozia guttulata * |
| Galium boreale | Cladonia gracilis | Lophozia kunzeana * |
| Hedysarum alpinum | Cladonia macilenta | Lophozia longidens * |
| Linnaea borealis | Cladonia multiformis | Lophozia exsecta * |
| Lycopodium annotinum | Cladonia pleurota | Lophozia ventricosa * |
| Lycopodium clavatum | Cladonia pyxidata | Pleurozium schreberi |
| Maianthemum canadensis | Cladonia sulphurina | Pohlia nutans |
| Orthilia secunda | Cladonia uncialis | Polytrichum commune |
| Pedicularis labradorica | Dactylina arctica | Polytrichum juniperinum |
| Petasites palmatus | Flavocetraria cucullata | Polytrichum piliferum |
| Picea glauca | Flavocetraria nivalis | Polytrichum strictum |
| Picea mariana | Hypogymnia physodes | Ptilidium ciliare * |
| Pinus contorta | Icmadophila ericetorum | Ptilidium pulcherrimum * |
| Pyrola asarifolia | Letharia vulpina | Ptilium crista-castrensis |
| Pyrola virens | Nephroma arcticum | Splachnum sphaericum |
| Rhododendron groenlandicum | Nephroma expallidum | Tetraplodon mnioides |
| Rosa acicularis | Parmeliopsis ambigua | Tritomaria exsectiformis * |
| Rubus acaulis | Parmeliopsis hypertopta | |
| Rubus pedatus | Peltigera aphthosa | |
| Salix spp. | Peltigera leucophlebia | |
| Senecio sp. | Peltigera malacea | |
| Spiraea alba | Lecidea cinnabarina | |
| Taraxacum officinale | Evernia mesomorpha | |
| Vaccinium caespitosum | Amandinea punctata | |
| Vaccinium membranaceum | Peltigera scabrosa | |
| Vaccinium myrtilloides | Parmelia sulcata | |
| Vaccinium myrtillus | Peltigera rufescens | |
| Vaccinium vitis-idaea | Solorina crocea | |
| Viburnum edule | Stereocaulon alpinum | |
| Viola adunca | Stereocaulon tomentosum | |
| Viola sp. | Tuckermannopsis americana | |
| Usnea spp. Vulpicida pinastre |
References
- Sulyma, R.; Coxson, D.S. Microsite displacement of terrestrial lichens by feather moss mats in late seral pine-lichen woodlands of North-central British Columbia. Bryologist 2001, 104, 505–516. [Google Scholar] [CrossRef]
- Miles, C.J.; Longton, R.E. Deposition of moss spores in relation to distance from parent gametophytes. Jour. Bryol. 1992, 17, 355–368. [Google Scholar] [CrossRef]
- Ross-Davis, A.L.; Frego, K.A. Propagule sources of forest floor bryophytes: spatiotemporal compositional patterns. Bryologist 2004, 107, 88–97. [Google Scholar] [CrossRef]
- Pharo, E.J.; Vitt, D.H. Local variation in bryophyte and macro-lichen cover and diversity in montane forests of western Canada. Bryologist 2000, 103, 455–466. [Google Scholar] [CrossRef]
- Kershaw, K.A. Studies on lichen-dominated systems. XX. An examination of some aspects of the northern boreal lichen woodlands in Canada. Can. J. Bot. 1997, 55, 393–410. [Google Scholar] [CrossRef]
- Vitt, D.H.; Crandall-Stotler, B.J.; Wood, A. Survival in a dry world through avoidance and tolerance. In Plant Ecology and Evolution in Harsh Environments; Rajakaruna, N., Boyd, R., Harris, T., Eds.; Nova Publishers: Hauppauge, NY, USA, 2014; pp. 267–295. [Google Scholar]
- Proctor, M.C.F. Physiological ecology. In Bryophyte Biology; Shaw, A.J., Goffinet, B., Eds.; Cambridge University Press: Cambridge, UK, 2000; pp. 225–247. [Google Scholar]
- Kershaw, K.A.; Rouse, W.R. Studies on lichen-dominated systems. I. The water relations of Cladonia alpestris in spruce-lichen woodland in northern Quebec. Can. J. Bot. 1971, 49, 1389–1399. [Google Scholar] [CrossRef]
- Saunders, D.; Hobbs, R.; Margules, C. Biological consequences of ecosystem fragmentation: A review. Cons. Biol. 1991, 5, 18–32. [Google Scholar] [CrossRef]
- Chen, J.; Franklin, J.F.; Spies, T.A. Contrasting microclimates among clearcut, edge, and interior of old-growth Douglas-fir forest. Agric. For. Met. 1993, 63, 219–237. [Google Scholar] [CrossRef]
- Nyland, R. Silviculture: Concepts and Applications; McGraw-Hill: New York, NY, USA, 1996. [Google Scholar]
- Söderström, L. The occurrence of epixylic bryophyte and lichen species in an old and a managed forest stand in northeast Sweden. Biol. Cons. 1988, 45, 169–178. [Google Scholar] [CrossRef]
- Haughian, S.R.; Burton, P.J. Microhabitat associations of lichens, feathermosses, and vascular plants in a caribou winter range, and their implications for understory development. Botany 2015, 93, 1–11. [Google Scholar] [CrossRef]
- DeLuca, T.H.; Zackrisson, O.; Nilsson, M.C.; Sellstedt, A. Quantifying nitrogen fixation in feather moss carpets of boreal forests. Nature 2002, 419, 917–920. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.-C.; Wardle, D.A. Understory vegetation as a forest ecosystem driver: evidence from the northern Swedish boreal forest. Front. Ecol. Environ. 2005, 3, 421–428. [Google Scholar] [CrossRef]
- Crites, S.; Dale, M. Relationship between nonvascular species and stand age and stand structure in aspen mixedwood forests in Alberta. In Relationships between Stand, Stand Structure, and Biodiversity in ASPEN Mixedwood Forests in Alberta; Stelfox, J.B., Ed.; Alberta Environmental Centre: Vegreville, AB, Canada; Canadian Forest Service: Edmonton, AB, Canada, 1995; pp. 91–144. [Google Scholar]
- Caners, R.T.; Macdonald, S.E.; Belland, R.J. Bryophyte assemblage structure after partial harvesting in boreal mixedwood forest depends on residual canopy abundance and composition. For. Ecol. Manag. 2013, 289, 489–500. [Google Scholar] [CrossRef]
- Boudreault, C.; Coxson, D.S.; Bergeron, Y.; Stevenson, S.; Bouchard, M. Do forests treated by partial cutting provide growth conditions similar to old-growth forests for epiphytic lichens? Biol. Cons. 2013, 159, 458–467. [Google Scholar] [CrossRef]
- Coxson, D.S.; Stevenson, S.K. Arboreal forage lichens in partial cuts—A synthesis of research results from British Columbia, Canada. Rangifer 2007, 17, 155–165. [Google Scholar]
- Terry, E.L.; Mclelland, B.; Watts, G.S. Winter habitat ecology of mountain caribou in relation to forest management. J. Appl. Ecol. 2000, 37, 589–602. [Google Scholar] [CrossRef]
- Edmonds, E.J. Populations status, distribution, and movements of woodland caribou in west-central Alberta. Can. J. Zool. 1988, 66, 817–826. [Google Scholar] [CrossRef]
- Jalonen, J.; Vanha-Majamaa, I. Immediate effects of four different felling methods on mature boreal spruce forest understorey vegetation in southern Finland. For. Ecol. Manag. 2001, 146, 25–34. [Google Scholar] [CrossRef]
- Nelson, C.R.; Halpern, C.B. Short-term effects of timber harvest and forest edges on ground-layer mosses and liverworts. Can. J. Bot. 2005, 83, 610–620. [Google Scholar] [CrossRef]
- Fenton, N.J.; Frego, K.A.; Sims, M.R. Changes in forest floor bryophyte (moss and liverwort) communities 4 years after forest harvest. Can. J. Bot. 2003, 81, 714–731. [Google Scholar] [CrossRef]
- Mills, S.E.; Macdonald, S.E. Factors influencing bryophyte assemblage at different scales in the western Canadian boreal forest. Bryologist 2005, 108, 86–100. [Google Scholar] [CrossRef]
- Macdonald, S.E.; Fenniak, T.E. Understory plant communities of boreal mixedwood forests in western Canada: natural patterns and response to variable-retention harvesting. For. Ecol. Manag. 2007, 242, 34–48. [Google Scholar] [CrossRef]
- Caners, R.T.; Macdonald, S.E.; Belland, R.J. Responses of boreal epiphytic bryophytes to different levels of partial canopy harvest. Botany 2010, 88, 315–328. [Google Scholar] [CrossRef]
- Craig, A.; Macdonald, E. Threshold effects of variable retention harvesting on understory plant communities in the boreal mixedwood forest. For. Ecol. Manag. 2009, 258, 2619–2627. [Google Scholar] [CrossRef]
- Bartels, S.F.; Macdonald, S.E.; Johnson, D.; Caners, R.T.; Spence, J.R. Bryophyte abundance, diversity and composition after retention harvest in boreal mixedwood forest. J. Appl. Ecol. 2018, 55, 947–957. [Google Scholar] [CrossRef]
- Johnson, C.; Parker, K.L.; Heard, D.C. Foraging across a variable landscape: Behavioural decisions made by woodland caribou at multiple spatial scales. Oecologia 2001, 127, 590–602. [Google Scholar] [CrossRef] [PubMed]
- Nadeau Fortin, M.-A.; Sirois, L.; St. Laurent, M.-H. Extensive forest management contributes to maintain suitable characteristics for the endangered Atlantic-Gaspesie caribou. Can. J. For. Res. 2016, 46, 933–942. [Google Scholar] [CrossRef]
- Armleder, H.M.; Stevenson, S.K.; Brown, K.; Cichowski, J.; Edmonds, D.; Seip, D.; Stevenson, S.; Thomas, D.; Wood, M. 19Using alternative silvicultural systems to integrate mountain caribou and timber management in British Columbia. In Proceedings of the Sixth North American Caribou Workshop, Prince George, BC, Canada; Nordic Council for Reindeer Research: Tromsoe, Norway, 1996; pp. 141–148. [Google Scholar]
- Schaefer, J.A. Canopy, snow, and lichens on woodland caribou range in southeastern. Manitoba. Rangifer Special Issue 1996, 9, 239–244. [Google Scholar] [CrossRef]
- Johnson, S.; Strengbom, J.; Koubi, J. Low levels of tree retention do not mitigate the effects of clearcutting on ground vegetation dynamics. For. Ecol. Manag. 2014, 330, 67–74. [Google Scholar] [CrossRef]
- Newmaster, S.G.; Bell, F.W. The effects of silvicultural disturbances in cryptogam diversity in the boreal–mixedwood forest. Can. J. For. Res. 2002, 32, 38–51. [Google Scholar] [CrossRef]
- Environment Canada. Recovery Strategy for the Woodland Caribou (Rangifer tarandus caribou), Boreal Population, in Canada; Species at Risk Act Recovery Strategy Series; Environment Canada: Ottawa, ON, Canada, 2012; 138p.
- Mooneyhan-McClelland, R. Ground Layer Response to Disturbance in the Pine-Dominated Eastern Foothill Region of Alberta, Canada. Ph.D. Dissertation, Southern Illinois University, Carbondale, IL, USA, 2011. [Google Scholar]
- Beckingham, J.D.; Corns, I.G.W.; Archibald, J.H. Field Guide to Ecosites of West-Central Alberta; Special Report 9; Natural Resources Canada, Canadian Forest Service, Northwest Region, Northern Forest Centre: Edmonton, AB, Canada, 1996.
- Athukorala, S.N.P.; Doering, J.; Piercey-Normore, M.D. Morphological and genetic polymorphism in two North American reindeer lichens: Cladonia arbuscula and C. rangiferina. Ceyl. J. Sci. (Bio. Sci.) 2015, 44, 55–65. [Google Scholar] [CrossRef]
- Athukorala, S.N.P.; Pino-Bodas, R.; Stenroos, S.; Ahti, T.; Piercey-Normore, M.D. Phylogenetic relationships among reindeer lichens of North America. Lichenologist 2016, 48, 209–227. [Google Scholar] [CrossRef]
- Kalra, Y.P.; Maynard, D.G. Methods manual for forest soil and plant analysis. In Information Report NOR-X.319; Canadian Forest Service, Northwest Region, Northern Forest Centre: Edmonton, AB, Canada, 1991. [Google Scholar]
- Whittaker, R.H. Evolution and measurement of species diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2006. [Google Scholar]
- Halpern, C.B.; Halaj, J.; Evan s, S.A.; Dovciak, M. Level and pattern of overstory retention interact to shape long-term responses of understories to timber harvest. Ecol. Appl. 2012, 22, 2049–2064. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.D.; LaRoi, G.H. Bryophyte and understory vascular plant beta diversity in relation to moisture and elevation gradients. Vegetatio 1979, 40, 29–38. [Google Scholar] [CrossRef]
- Larson, D.W. Habitat overlap/niche segregation in two Umbilicaria lichens: A possible mechanism. Oecologia 1984, 62, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Lesica, P.; McCune, B.; Cooper, S.V.; Hong, W.S. Differences in lichen and bryophyte communities between old-growth and managed second-growth forests of the Swan Valley, Montana. Can. J. Bot. 1991, 69, 1745–1755. [Google Scholar] [CrossRef]
- Selva, S.B. Lichen diversity and stand continuity in the northern hardwoods and spruce-fir forests of northern New England and western New Brunswick. Bryologist 1994, 97, 424–429. [Google Scholar] [CrossRef]
- Caners, R.T.; Macdonald, S.E.; Belland, R.J. Recolonization potential of bryophyte diaspore banks in harvested boreal mixed-wood forest. Plant Ecol. 2009, 204, 55–68. [Google Scholar] [CrossRef]
- Keim, J.L.; DeWitt, P.D.; Fitzpatrick, J.J.; Jenni, N.S. Estimating plant abundance using inflated beta distributions: Applied learnings from a Lichen-caribou ecosystem. Ecol. Evol. 2016, 7, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Gendreau-Berthiaume, B.; Macdonald, S.E.; Stadt, J.J.; Hnatiuk, R.J. How dynamic are understory communities and the processes structuring them in mature conifer forests? Ecosphere 2015, 6, 1–49. [Google Scholar] [CrossRef]
- Kranrod, K. The Effects of Timber Harvesting Methods on Terrestrial Lichens and Understory Plants in West-Central Alberta. Master’s Thesis, University of Alberta, Edmonton, AB, Canada, 1996. [Google Scholar]
- Dynesius, M. Slow recovery of bryophyte assemblages in middle-aged boreal forests regrown after clear-cutting. Biol. Cons. 2015, 191, 101–109. [Google Scholar] [CrossRef]
- Fenton, N.J.; Frego, K.A. Bryophyte (moss and liverwort) conservation under remnant canopy in managed forests. Biol. Cons. 2005, 122, 417–430. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).