Seasonal Variations and Thinning Effects on Soil Phosphorus Fractions in Larix principis-rupprechtii Mayr. Plantations
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Experimental Design
2.2. Field Sampling
2.3. Soil Analysis
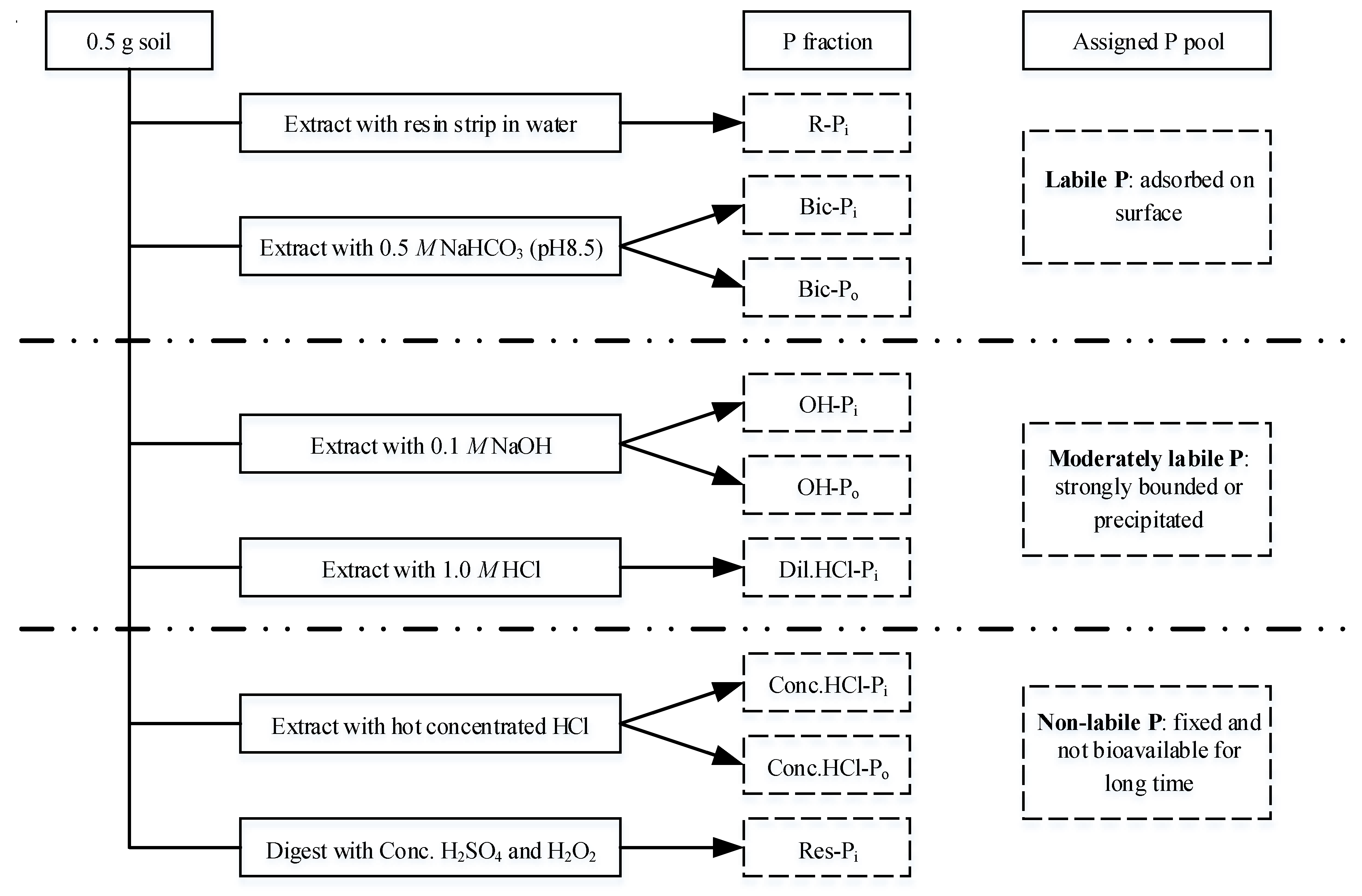
2.4. Phosphorus Fractionation
2.5. Statistical Analysis
3. Results
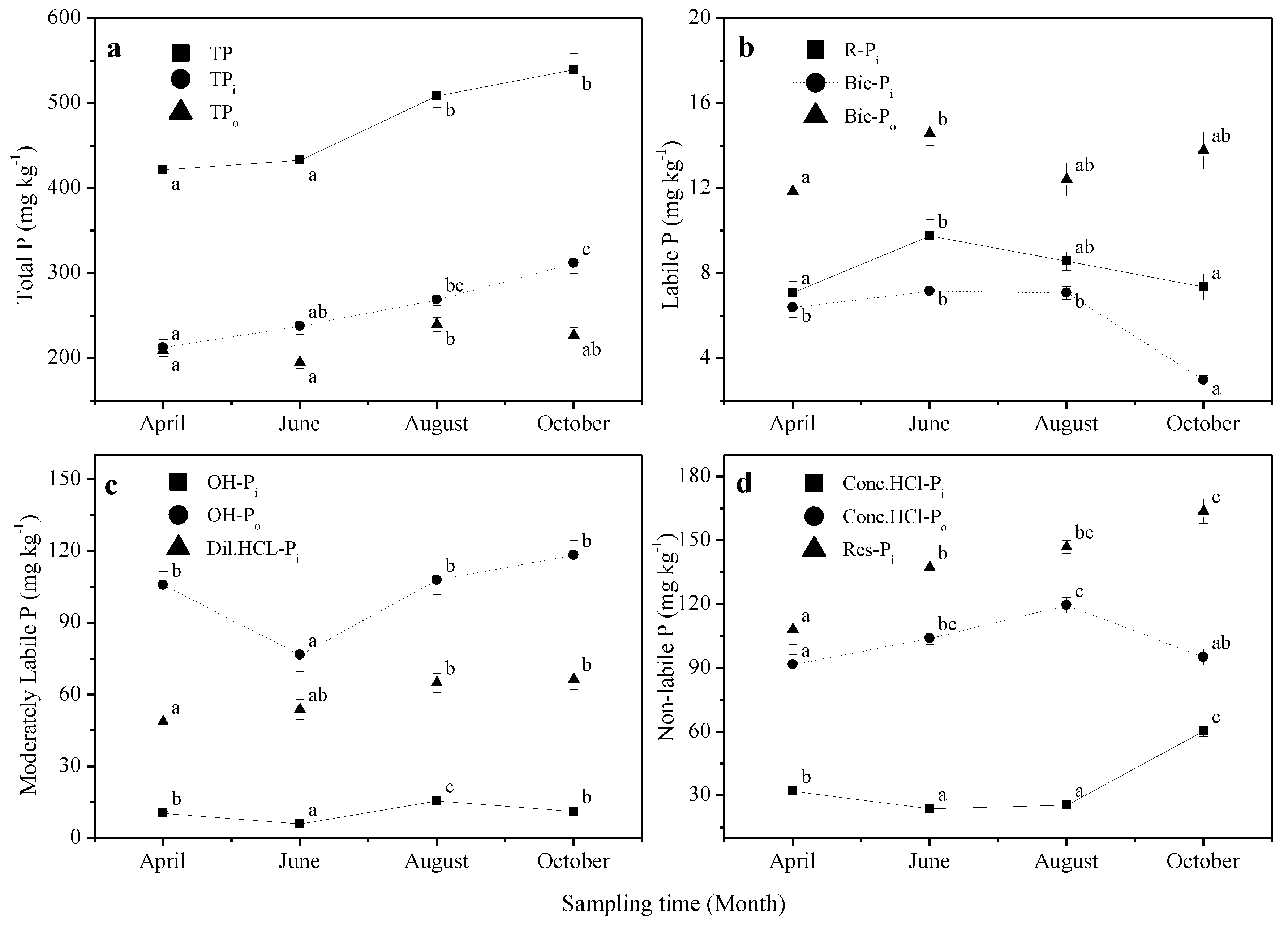
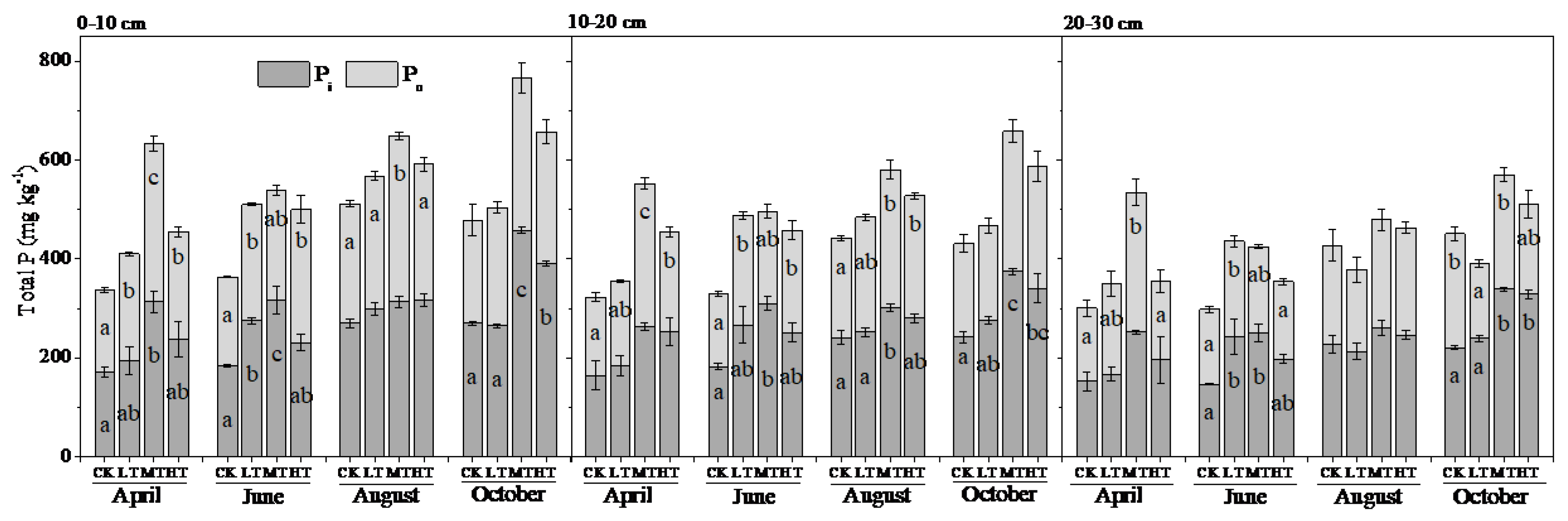
3.1. Soil Total P, Pi and Po Concentrations
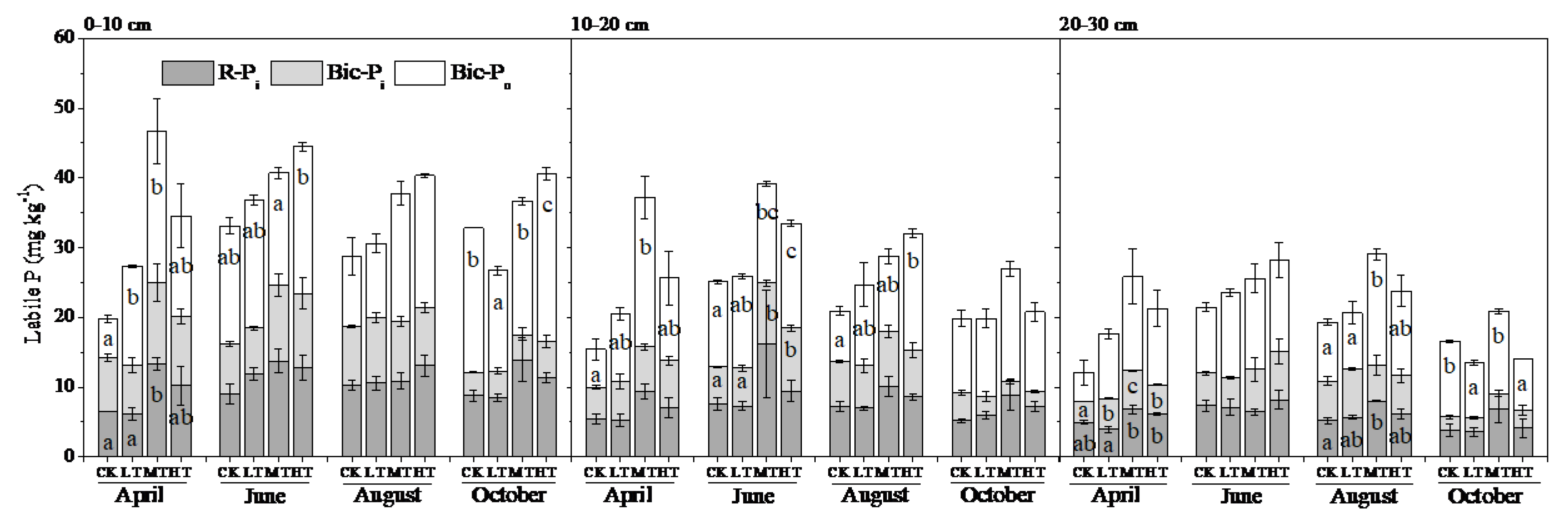
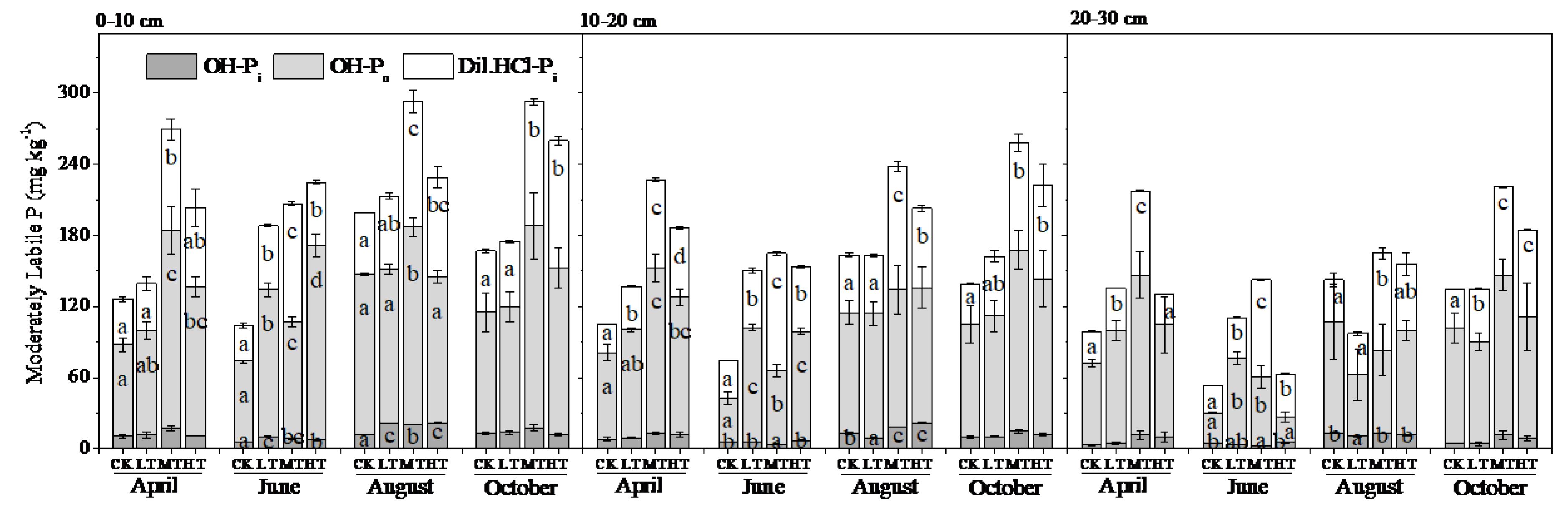
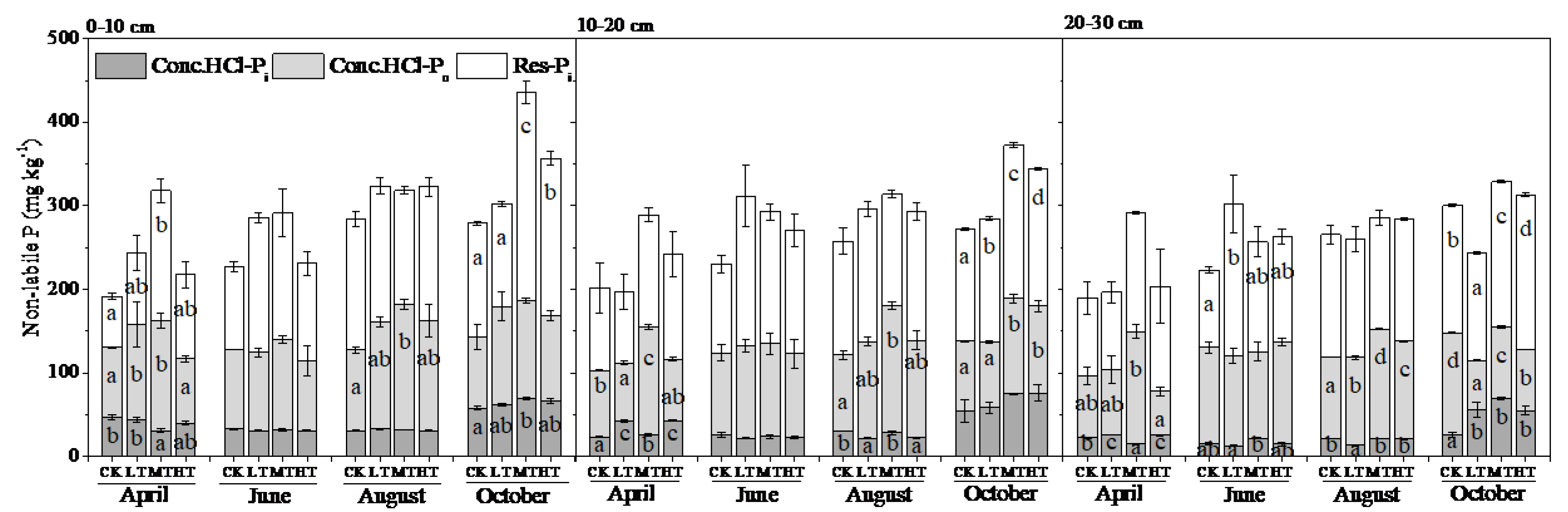
3.2. Soil P Fractions
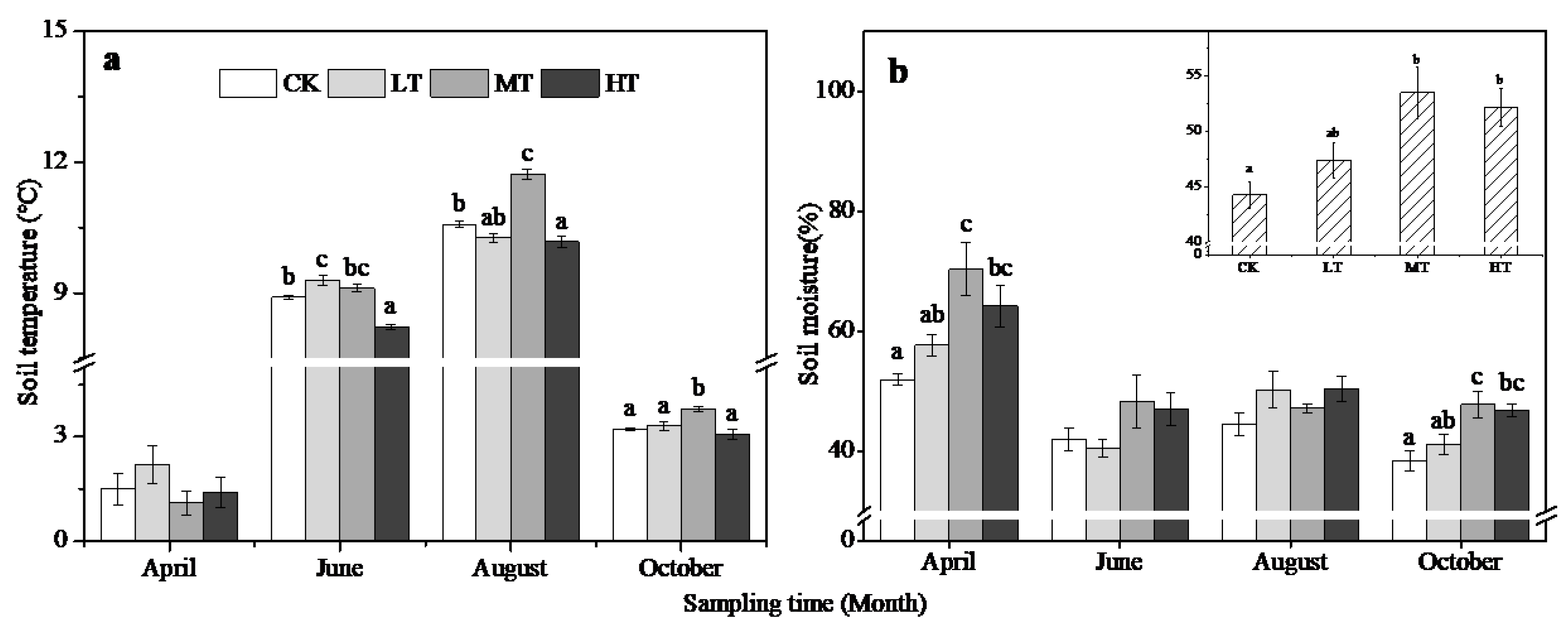
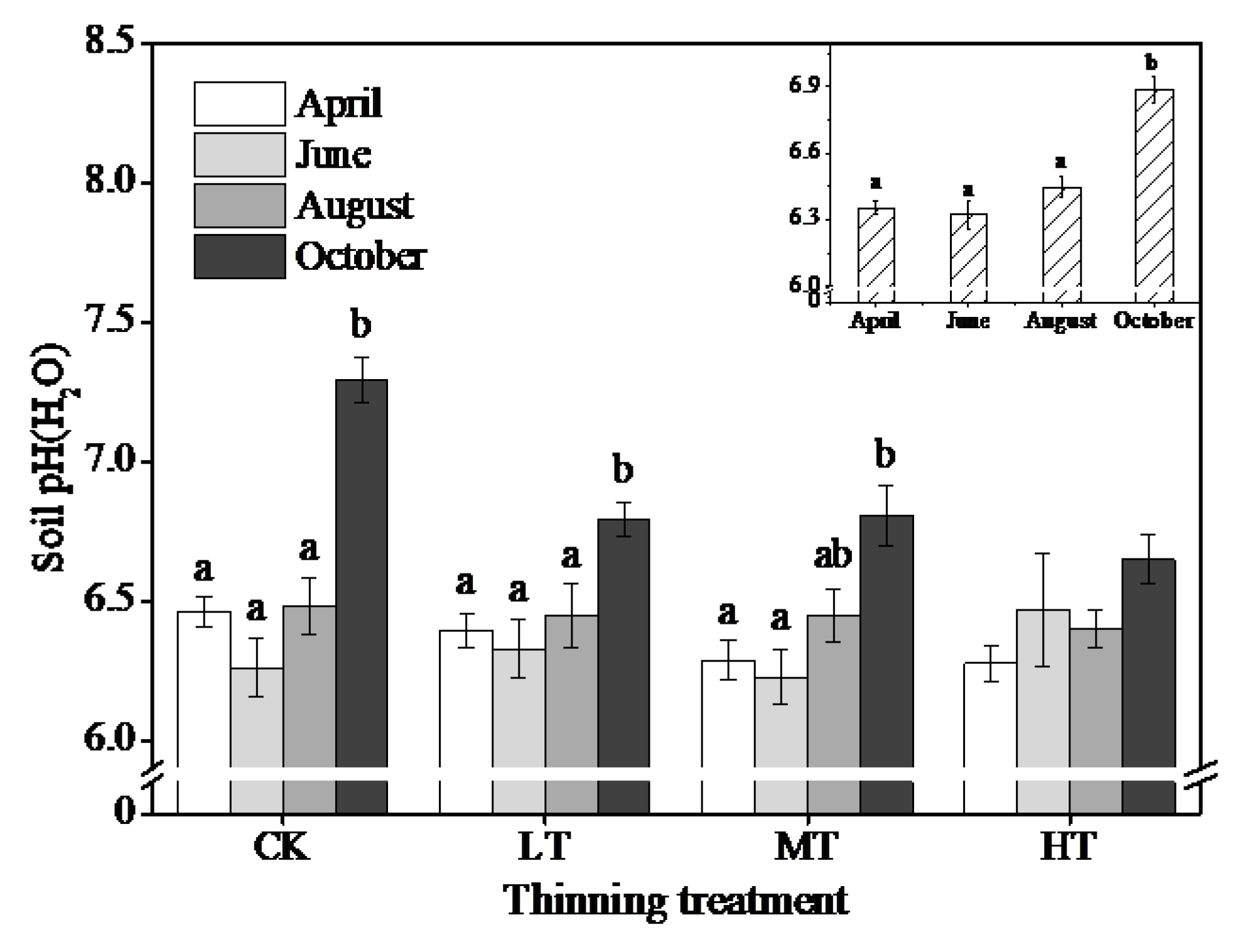
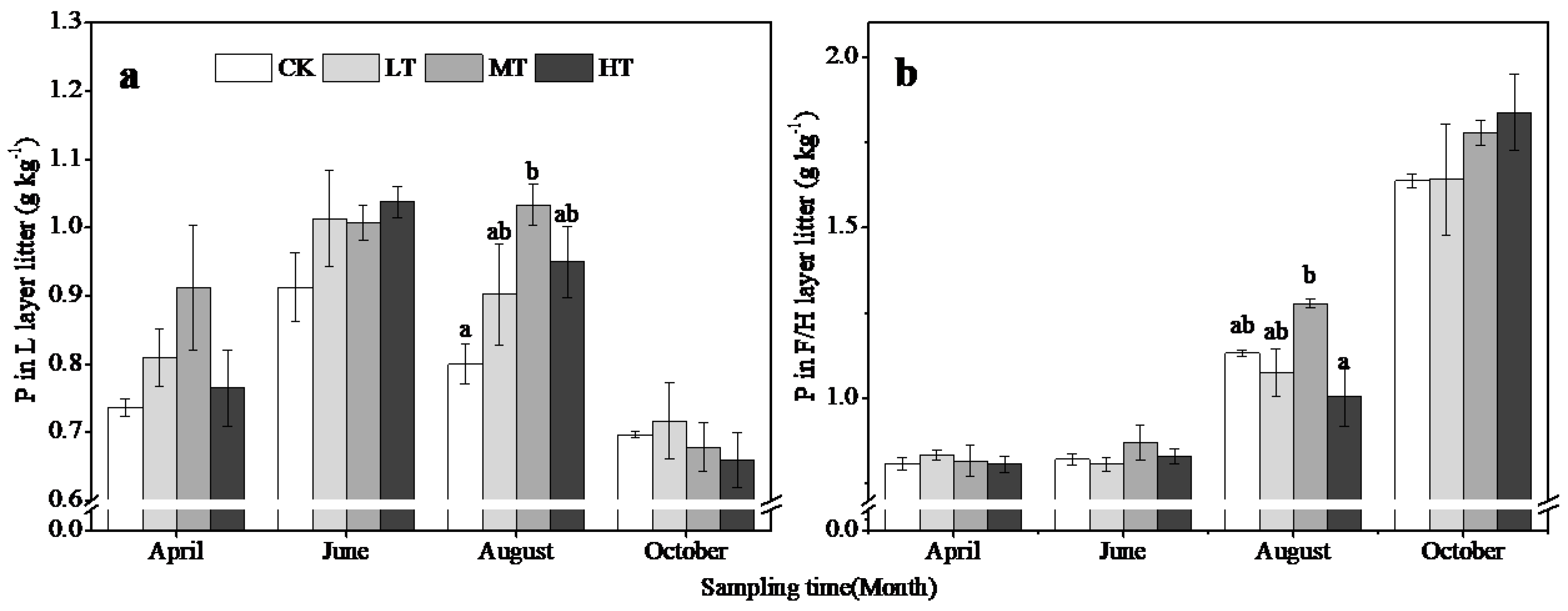
3.3. Changes in Soil and Litter Properties
3.4. Correlation between P Fractions and Soil Properties
4. Discussion
4.1. Seasonal Variation
4.2. Treatment Effects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vincent, A.G.; Schleucher, J.; Gröbner, G.; Vestergren, J.; Persson, P.; Jansson, M.; Giesler, R. Changes in organic phosphorus composition in boreal forest humus soils: The role of iron and aluminium. Biogeochemistry 2012, 108, 485–499. [Google Scholar] [CrossRef]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Mcdowell, R.W.; Stewart, I. The phosphorus composition of contrasting soils in pastoral, native and forest management in Otago, New Zealand: Sequential extraction and 31P NMR. Geoderma 2006, 130, 176–189. [Google Scholar] [CrossRef]
- Cherubin, M.R.; Franco, A.L.C.; Cerri, C.E.P.; Karlen, D.L.; Pavinato, P.S.; Rodrigues, M.; Davies, C.A.; Cerri, C.C. Phosphorus pools responses to land-use change for sugarcane expansion in weathered Brazilian soils. Geoderma 2016, 265, 27–38. [Google Scholar] [CrossRef]
- Liu, C.; Jin, Y.; Liu, C.; Tang, J.; Wang, Q.; Xu, M. Phosphorous fractions in soils of rubber-based agroforestry systems: Influence of season, management and stand age. Sci. Total Environ. 2018, 616–617, 1576–1588. [Google Scholar] [CrossRef] [PubMed]
- Stutter, M.I.; Shand, C.A.; George, T.S.; Blackwell, M.S.A.; Dixon, L.; Bol, R.; MacKay, R.L.; Richardson, A.E.; Condron, L.M.; Haygarth, P.M. Land use and soil factors affecting accumulation of phosphorus species in temperate soils. Geoderma 2015, 257–258, 29–39. [Google Scholar] [CrossRef]
- von Sperber, C.; Stallforth, R.; Preez, C.D.; Amelung, W. Changes in soil phosphorus pools during prolonged arable cropping in semiarid grasslands. Eur. J. Soil Sci. 2017, 68, 462–471. [Google Scholar] [CrossRef]
- Oberson, A.; Friesen, D.K.; Rao, I.M.; Bühler, S.; Frossard, E. Phosphorus Transformations in an Oxisol under contrasting land-use systems: The role of the soil microbial biomass. Plant Soil 2001, 237, 197–210. [Google Scholar] [CrossRef]
- Khan, K.S.; Joergensen, R.G. Response of white mustard (Sinapis alba) and the soil microbial biomass to P and Zn addition in a greenhouse pot experiment. J. Plant Nutr. Soil Sci. 2015, 178, 834–840. [Google Scholar] [CrossRef]
- Hou, E.; Chen, C.; Wen, D.; Liu, X. Phosphatase activity in relation to key litter and soil properties in mature subtropical forests in China. Sci. Total Environ. 2015, 515, 83–91. [Google Scholar] [CrossRef]
- Slazak, A.; Freese, D.; Matos, E.D.S.; Hüttl, R.F. Soil organic phosphorus fraction in pine-oak forest stands in Northeastern Germany. Geoderma 2010, 158, 156–162. [Google Scholar] [CrossRef]
- Kuehne, C.; Weiskittel, A.; Pommerening, A.; Wagner, R.G. Evaluation of 10-year temporal and spatial variability in structure and growth across contrasting commercial thinning treatments in spruce-fir forests of northern Maine, USA. Ann. For. Sci. 2018, 75, 20. [Google Scholar] [CrossRef]
- Hu, B.; Yang, B.; Pang, X.; Bao, W.; Tian, G. Responses of soil phosphorus fractions to gap size in a reforested spruce forest. Geoderma 2016, 279, 61–69. [Google Scholar] [CrossRef]
- Kim, S.; Li, G.; Han, S.H.; Kim, H.J.; Kim, C.; Lee, S.T.; Son, Y. Thinning affects microbial biomass without changing enzyme activity in the soil of Pinus densiflora Sieb. et Zucc. forests after 7 years. Ann. For. Sci. 2018, 75, 13. [Google Scholar] [CrossRef]
- Zhu, J.; Matsuzaki, T.; Lee, F.; Gonda, Y. Effect of gap size created by thinning on seedling emergency, survival and establishment in a coastal pine forest. For. Ecol. Manag. 2003, 182, 339–354. [Google Scholar] [CrossRef]
- Latif, Z.A.; Blackburn, G.A. The effects of gap size on some microclimate variables during late summer and autumn in a temperate broadleaved deciduous forest. Int. J. Biometeorol. 2010, 54, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Kang, F.; Cheng, X.; Han, H. Moderate thinning increases soil organic carbon in Larix principis-rupprechtii (Pinaceae) plantations. Geoderma 2018, 329, 118–128. [Google Scholar] [CrossRef]
- Lei, L.; Xiao, W.; Zeng, L.; Zhu, J.; Huang, Z.; Cheng, R.; Gao, S.; Li, M.H. Thinning but not understory removal increased heterotrophic respiration and total soil respiration in Pinus massoniana stands. Sci. Total Environ. 2017, 621, 1360–1369. [Google Scholar] [CrossRef]
- Netzer, F.; Thöm, C.; Celepirovic, N.; Ivankovic, M.; Alfarraj, S.; Dounavi, A.; Simon, J.; Herschbach, C.; Rennenberg, H. Drought effects on C, N, and P nutrition and the antioxidative system of beech seedlings depend on geographic origin. J. Plant Nutr. Soil Sci. 2016, 179, 136–150. [Google Scholar] [CrossRef]
- Richards, J.D.; Hart, J.L. Canopy gap dynamics and development patterns in secondary Quercus stands on the Cumberland Plateau, Alabama, USA. For. Ecol. Manag. 2011, 262, 2229–2239. [Google Scholar] [CrossRef]
- Chen, C.R.; Condron, L.M.; Xu, Z.H. Impacts of grassland afforestation with coniferous trees on soil phosphorus dynamics and associated microbial processes: A review. For. Ecol. Manag. 2008, 255, 396–409. [Google Scholar] [CrossRef]
- Magid, J.; Nielsen, N.E. Seasonal variation in organic and inorganic phosphorus fractions of temperate-climate sandy soils. Plant Soil 1992, 144, 155–165. [Google Scholar] [CrossRef]
- Frossard, E.; Condron, L.M.; Oberson, A.; Sinaj, S.; Fardeau, J.C. Processes governing phosphorus availability in temperate soils. J. Environ. Qual. 2000, 29, 15–23. [Google Scholar] [CrossRef]
- Wu, C.; Mo, Q.; Wang, H.; Zhang, Z.; Huang, G.; Ye, Q.; Zou, Q.; Kong, F.; Liu, Y.; Wang, G.G. Moso bamboo (Phyllostachys edulis (Carriere) J. Houzeau) invasion affects soil phosphorus dynamics in adjacent coniferous forests in subtropical China. Ann. For. Sci. 2018, 75, 24. [Google Scholar] [CrossRef]
- Zhao, Q.; Zeng, D.H.; Fan, Z.P.; Yu, Z.Y.; Hu, Y.L.; Zhang, J. Seasonal variations in phosphorus fractions in semiarid sandy soils under different vegetation types. For. Ecol. Manag. 2009, 258, 1376–1382. [Google Scholar] [CrossRef]
- Styles, D.; Coxon, C. Meteorological and management influences on seasonal variation in phosphorus fractions extracted from soils in western Ireland. Geoderma 2007, 142, 152–164. [Google Scholar] [CrossRef]
- Fabre, A.; Pinay, G.; Ruffinoni, C. Seasonal Changes in Inorganic and Organic Phosphorus in the Soil of a Riparian Forest. Biogeochemistry 1996, 35, 419–432. [Google Scholar] [CrossRef]
- Hooper, D.U.; Vitousek, P.M. Effects of plant composition and diversity on nutrient cycling. Ecol. Monogr. 1998, 68, 121–149. [Google Scholar] [CrossRef]
- Chen, C.R.; Condron, L.M.; Davis, M.R.; Sherlock, R.R. Seasonal changes in soil phosphorus and associated microbial properties under adjacent grassland and forest in New Zealand. For. Ecol. Manag. 2003, 177, 539–557. [Google Scholar] [CrossRef]
- Yuan, J.; Jose, S.; Hu, Z.; Pang, J.; Hou, L.; Zhang, S. Biometric and Eddy Covariance Methods for Examining the Carbon Balance of a Larix principis-rupprechtii Forest in the Qinling Mountains, China. Forests 2018, 9, 67. [Google Scholar] [CrossRef]
- Lun, F.; Liu, Y.; He, L.; Yang, L.; Liu, M.; Li, W. Life cycle research on the carbon budget of the Larix principis-rupprechtii plantation forest ecosystem in North China. J. Clean. Prod. 2018, 177, 178–186. [Google Scholar] [CrossRef]
- Cheng, X.; Kang, F.; Han, H.; Liu, H.; Zhang, Y. Effect of thinning on partitioned soil respiration in a young Pinus tabulaeformis plantation during growing season. Agr. For. Meteorol. 2015, 214–215, 473–482. [Google Scholar] [CrossRef]
- Shi, X.Z.; Yu, D.S.; Xu, S.X.; Warner, E.D.; Wang, H.J.; Sun, W.X.; Zhao, Y.C.; Gong, Z.T. Cross-reference for relating Genetic Soil Classification of China with WRB at different scales. Geoderma 2010, 155, 344–350. [Google Scholar] [CrossRef]
- Brookes, P.C.; Powlson, D.S.; Jenkinson, D.S. Measurement of microbial biomass phosphorus in soil. Soil Biol. Biochem. 1982, 14, 319–329. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Tiessen, H.; Moir, J. Characterization of available P by sequential extraction. In Soil Sampling and Methods of Analysis; Carter, M.R., Ed.; Lewis Publishers: Boca Raton, FL, USA, 1993. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Bowman, R.A.; Cole, C.V. Transformations of organic phosphorus substrates in soils as evaluated by NaHCO3 extraction. Soil Sci. 1978, 125, 49–54. [Google Scholar] [CrossRef]
- Rodrigues, M.; Pavinato, P.S.; Withers, P.J.A.; Teles, A.P.B.; Herrera, W.F.B. Legacy phosphorus and no tillage agriculture in tropical oxisols of the Brazilian savanna. Sci. Total Environ. 2016, 542, 1050–1061. [Google Scholar] [CrossRef]
- Chaneton, E.J.; Lemcoff, J.H.; Lavado, R.S. Nitrogen and Phosphorus Cycling in Grazed and Ungrazed Plots in a Temperate Subhumid Grassland in Argentina. J. Appl. Ecol. 1996, 33, 291–302. [Google Scholar] [CrossRef]
- Saggar, S.; Hedley, C.B. Estimating seasonal and annual carbon inputs, and root decomposition rates in a temperate pasture following field 14C pulse-labelling. Plant Soil 2001, 236, 91–103. [Google Scholar] [CrossRef]
- Yang, K.; Zhu, J.; Yan, Q.; Sun, O.J. Changes in soil P chemistry as affected by conversion of natural secondary forests to larch plantations. For. Ecol. Manag. 2010, 260, 422–428. [Google Scholar] [CrossRef]
- Weihrauch, C.; Opp, C. Ecologically relevant phosphorus pools in soils and their dynamics: The story so far. Geoderma 2018, 325, 183–194. [Google Scholar] [CrossRef]
- Godlinski, F.; Reiche, E.W.; Lennartz, B.; Meissner, R. Simulation of phosphorus losses from lysimeters. J. Plant Nutr. Soil Sci. 2008, 171, 621–633. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, L.; Wen, D.; Yu, K. Soil potential labile but not occluded phosphorus forms increase with forest succession. Biol. Fertility Soils 2016, 52, 41–51. [Google Scholar] [CrossRef]
- Özgül, M.; Günes, A.; Esringü, A.; Turan, M. The effects of freeze-and-thaw cycles on phosphorus availability in highland soils in Turkey. J. Plant Nutr. Soil Sci. 2012, 175, 827–839. [Google Scholar] [CrossRef]
- Whitehead, P.G.; Crossman, J. Macronutrient cycles and climate change: Key science areas and an international perspective. Sci. Total Environ. 2012, 434, 13–17. [Google Scholar] [CrossRef]
- Weintraub, S.R.; Wieder, W.R.; Cleveland, C.C.; Townsend, A.R. Organic matter inputs shift soil enzyme activity and allocation patterns in a wet tropical forest. Biogeochemistry 2013, 114, 313–326. [Google Scholar] [CrossRef]
- Gang, Q.; Yan, Q.; Zhu, J. Effects of thinning on early seed regeneration of two broadleaved tree species in larch plantations: Implication for converting pure larch plantations into larch-broadleaved mixed forests. Forestry 2015, 88, 573–585. [Google Scholar] [CrossRef]
- Johnson, A.H.; Frizano, J.; Vann, D.R. Biogeochemical implications of labile phosphorus in forest soils determined by the Hedley fractionation procedure. Oecologia 2003, 135, 487–499. [Google Scholar] [CrossRef]
- De Schrijver, A.; Vesterdal, L.; Hansen, K.; De Frenne, P.; Augusto, L.; Achat, D.L.; Staelens, J.; Baeten, L.; De Keersmaeker, L.; De Neve, S. Four decades of post-agricultural forest development have caused major redistributions of soil phosphorus fractions. Oecologia 2012, 169, 221–234. [Google Scholar] [CrossRef]
- Lang, F.; Bauhus, J.; Frossard, E.; George, E.; Kaiser, K.; Kaupenjohann, M.; Krüger, J.; Matzner, E.; Polle, A.; Prietzel, J.; et al. Phosphorus in forest ecosystems: New insights from an ecosystem nutrition perspective. J. Plant Nutr. Soil Sci. 2016, 179, 129–135. [Google Scholar] [CrossRef]
- He, Y.Q.; Zhu, Y.G.; Smith, S.E.; Smith, F.A. Interactions between soil moisture content and phosphorus supply in spring wheat plants grown in pot culture. J. Plant Nutr. 2002, 25, 913–925. [Google Scholar] [CrossRef]
- Huang, W.; Spohn, M. Effects of long-term litter manipulation on soil carbon, nitrogen, and phosphorus in a temperate deciduous forest. Soil Biol. Biochem. 2015, 83, 12–18. [Google Scholar] [CrossRef]
- Teles, A.P.B.; Rodrigues, M.; Herrera, W.F.B.; Soltangheisi, A.; Sartor, L.R.; Withers, P.J.A.; Pavinato, P.S. Do cover crops change the lability of phosphorus in a clayey subtropical soil under different phosphate fertilizers? Soil Use Manag. 2017, 33, 34–44. [Google Scholar] [CrossRef]
| Thinning | Density (Trees hm−2) | Stand Age (a) | Slope Gradient (°) | Aspect | Elevation (m) | Mean Height (m) | Mean DBH (cm) |
|---|---|---|---|---|---|---|---|
| CK | 2096 ± 37 | 35 | 25 ± 2.7 | N | 2327 ± 16 | 14.2 ± 1.3 | 15.0 ± 0.3 |
| LT | 1829 ± 8 | 35 | 25 ± 0.3 | N | 2335 ± 3 | 14.1 ± 1.2 | 14.9 ± 0.4 |
| MT | 1413 ± 7 | 35 | 23 ± 0.3 | N | 2346 ± 6 | 15.9 ± 0.6 | 16.0 ± 0.2 |
| HT | 1087 ± 5 | 35 | 22 ± 1.5 | N | 2340 ± 10 | 15.4 ± 1.3 | 16.8 ± 0.7 |
| Thinning | SOC (g kg−1) | TN (g kg−1) | C/N | MBP (mg kg−1) | AcPME (μg g−1 h−1) |
|---|---|---|---|---|---|
| CK | 39.2 ± 1.4 ab | 2.6 ± 0.1 a | 15.7 ± 0.8 | 22.6 ± 0.8 a | 435.9 ± 42.4 |
| LT | 36.6 ± 0.8 a | 2.8 ± 0.1 ab | 13.0 ± 0.3 | 32.1 ± 3.2 ab | 416.2 ± 39.3 |
| MT | 46.1 ± 1.5 c | 3.3 ± 0.2 b | 15.3 ± 0.9 | 37.0 ± 2.3 b | 510.0 ± 50.7 |
| HT | 42.8 ± 1.5 bc | 3.2 ±0.2 b | 14.6 ± 0.8 | 29.6 ± 3.7 ab | 546.6 ± 47.4 |
| Depth | Factors | df | Correlation Index | Labile P | Mod.Labile P | Non-Labile P | Total Pi | Total Po | Total Pt |
|---|---|---|---|---|---|---|---|---|---|
| 0−10 cm | Thinning | 3 | F | 19.2 | 61.3 | 19.2 | 32.0 | 26.1 | 49.9 |
| P | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||
| Season | 3 | F | 5.4 | 18.6 | 28.5 | 31.5 | 10.3 | 30.8 | |
| P | 0.004 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||
| T × S | 9 | F | 3.2 | 4.9 | 3.1 | 4.1 | 2.9 | 3.7 | |
| P | 0.008 | <0.001 | 0.009 | 0.001 | 0.012 | 0.003 | |||
| 10−20 cm | Thinning | 3 | F | 18.1 | 46.7 | 10.6 | 20.6 | 25.2 | 36.1 |
| P | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||
| Season | 3 | F | 8.9 | 19.1 | 14.5 | 14.9 | 10.6 | 16.4 | |
| P | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||
| T × S | 9 | F | 1.9 | 2.9 | 1.5 | 1.2 | 3.9 | 2.4 | |
| P | 0.09 | 0.012 | 0.19 | 0.359 | 0.002 | 0.036 | |||
| 20−30 cm | Thinning | 3 | F | 12.2 | 17.5 | 3.8 | 13.2 | 5.8 | 13.9 |
| P | <0.001 | <0.001 | 0.019 | <0.001 | 0.003 | <0.001 | |||
| Season | 3 | F | 16.8 | 20.7 | 10.6 | 14.5 | 2.0 | 10.9 | |
| P | <0.001 | <0.001 | <0.001 | <0.001 | 0.131 | <0.001 | |||
| T × S | 9 | F | 2.7 | 4.0 | 2.3 | 1.9 | 3.2 | 3.3 | |
| P | 0.02 | 0.002 | 0.042 | 0.09 | 0.007 | 0.006 |
| Depth/cm | Soil Properties | R-Pi | Bic-Po | OH-Pi | OH-Po | Dil.HCl-Pi | Total Pi | Total Po | Total Pt |
|---|---|---|---|---|---|---|---|---|---|
| 0−10 | SOC | 0.68 * | 0.69 * | 0.02 | 0.66 * | 0.66 * | 0.59 * | 0.60 * | 0.60 * |
| Moisture | 0.83 ** | 0.94 ** | 0.60 * | 0.85 ** | 0.88 ** | 0.86 ** | 0.77 ** | 0.83 ** | |
| pH | −0.13 | −0.40 | −0.23 | −0.19 | −0.36 | −0.36 | −0.27 | −0.33 | |
| Temperature | −0.22 | −0.16 | 0.36 | −0.13 | 0.09 | 0.15 | 0.07 | 0.12 | |
| MBP | 0.76 ** | 0.55 | 0.84 ** | 0.79 ** | 0.74 ** | 0.80 ** | 0.91 ** | 0.86 ** | |
| AcPME | 0.72 ** | 0.82 ** | 0.00 | 0.72 ** | 0.69 * | 0.61 * | 0.60 * | 0.61 * | |
| 10−20 | SOC | 0.80 ** | 0.91 ** | 0.70 * | 0.76 ** | 0.87 ** | 0.88 ** | 0.90 ** | 0.91 ** |
| Moisture | 0.59 * | 0.70 * | 0.69 * | 0.64 * | 0.79 ** | 0.73 ** | 0.74 ** | 0.75 ** | |
| pH | −0.15 | −0.48 | −0.20 | −0.45 | −0.33 | −0.45 | −0.37 | −0.42 | |
| 20−30 | SOC | 0.83 ** | 0.78 ** | 0.84 ** | 0.80 ** | 0.84 ** | 0.88 ** | 0.88 ** | 0.95 ** |
| Moisture | 0.58 * | 0.70 * | 0.85 ** | 0.43 | 0.64 * | 0.79 ** | 0.45 | 0.69 * | |
| pH | −0.04 | −0.61 * | −0.08 | −0.56 | −0.28 | −0.34 | −0.44 | −0.41 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, H.; Cheng, X.; Han, H.; Jing, H.; Liu, X.; Li, Z. Seasonal Variations and Thinning Effects on Soil Phosphorus Fractions in Larix principis-rupprechtii Mayr. Plantations. Forests 2019, 10, 172. https://doi.org/10.3390/f10020172
Tian H, Cheng X, Han H, Jing H, Liu X, Li Z. Seasonal Variations and Thinning Effects on Soil Phosphorus Fractions in Larix principis-rupprechtii Mayr. Plantations. Forests. 2019; 10(2):172. https://doi.org/10.3390/f10020172
Chicago/Turabian StyleTian, Huixia, Xiaoqin Cheng, Hairong Han, Hongyuan Jing, Xujun Liu, and Zuzheng Li. 2019. "Seasonal Variations and Thinning Effects on Soil Phosphorus Fractions in Larix principis-rupprechtii Mayr. Plantations" Forests 10, no. 2: 172. https://doi.org/10.3390/f10020172
APA StyleTian, H., Cheng, X., Han, H., Jing, H., Liu, X., & Li, Z. (2019). Seasonal Variations and Thinning Effects on Soil Phosphorus Fractions in Larix principis-rupprechtii Mayr. Plantations. Forests, 10(2), 172. https://doi.org/10.3390/f10020172






