Genome-Wide Analysis of the D-type Cyclin Gene Family Reveals Differential Expression Patterns and Stem Development in the Woody Plant Prunus mume
Abstract
:1. Introduction
2. Materials and Methods
2.1. Genome-Wide Scanning and Characteristic Analysis
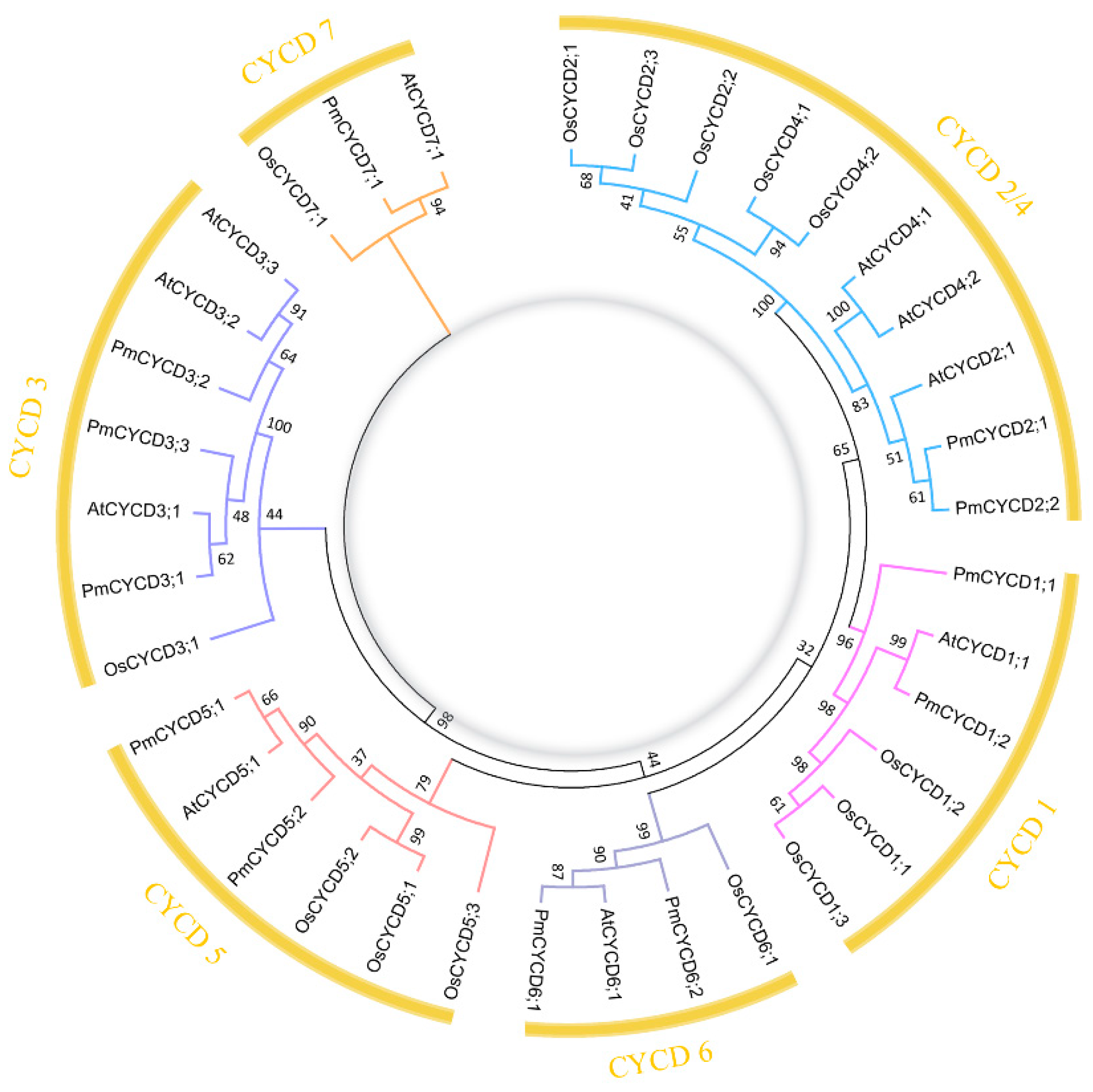
2.2. Phylogenetic Analysis
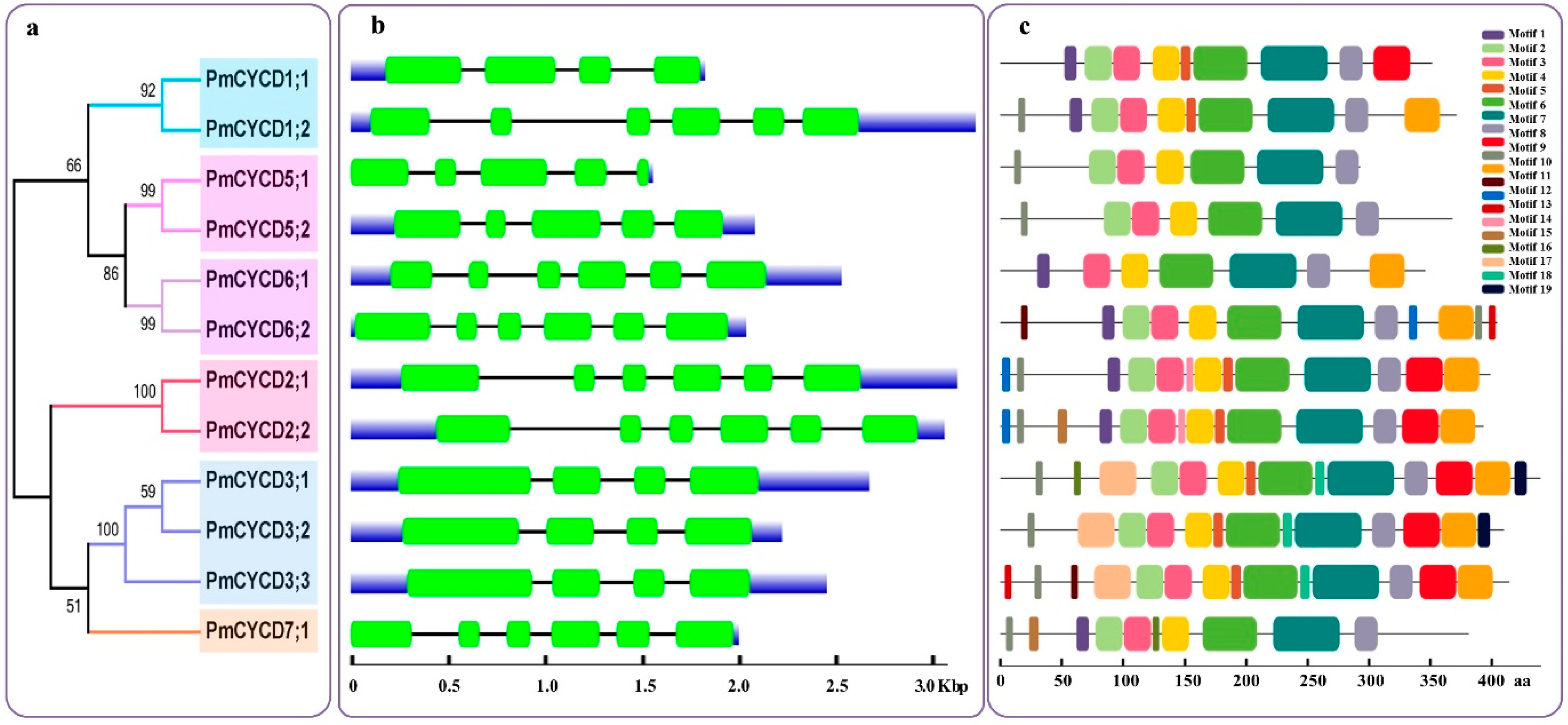
2.3. Gene Structure and Conserved Motif Analyses
2.4. Transcriptome Data Analysis
2.5. Plant, Tissues, and Phytohormone Treatment
2.6. RNA Extraction and qRT-PCR
3. Results
3.1. Identification of Putative PmCYCDs
3.2. Gene Structure and MEME Analysis
3.3. Core Conservative Region Analysis
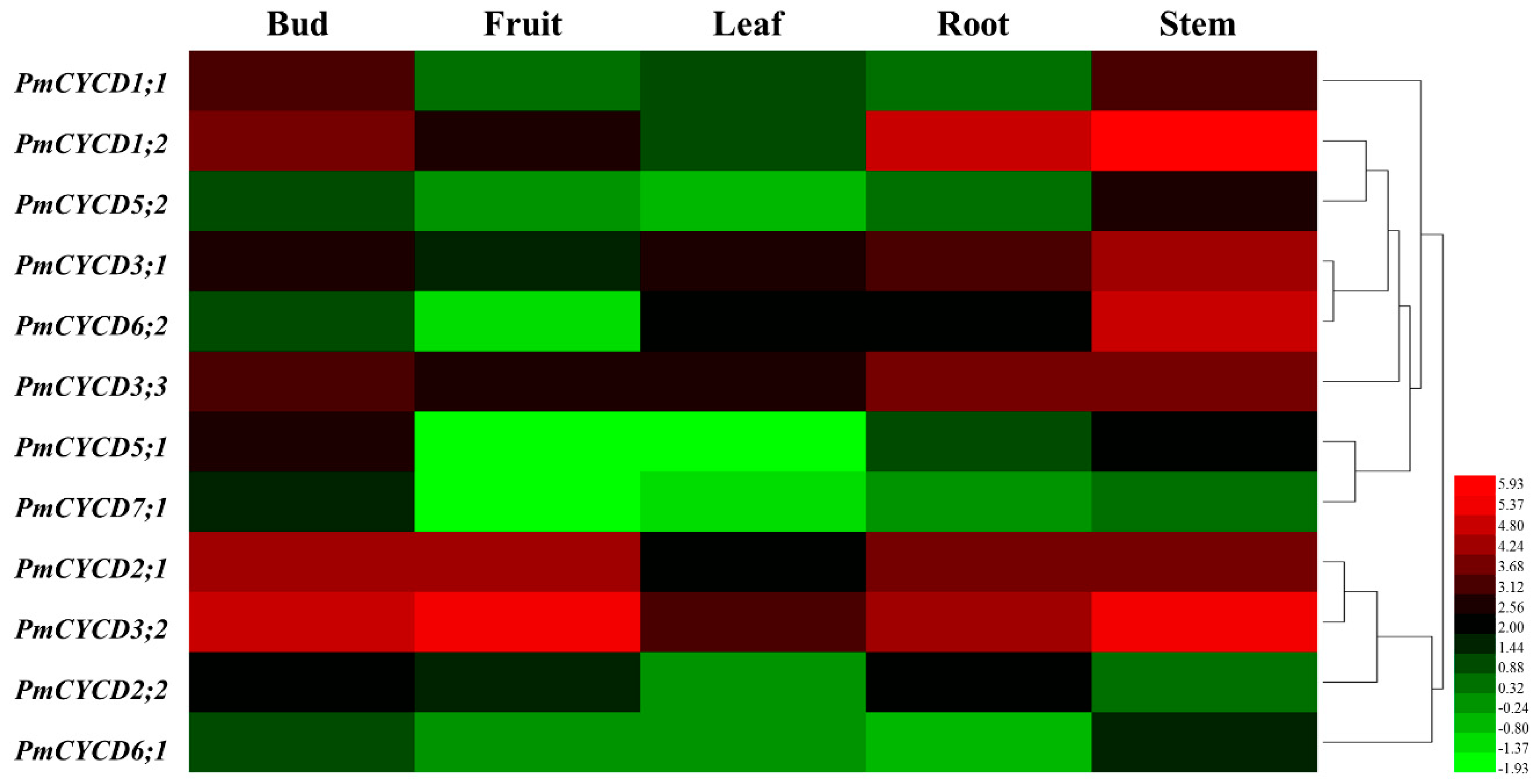
3.4. Gene Expression Analysis by Transcriptome
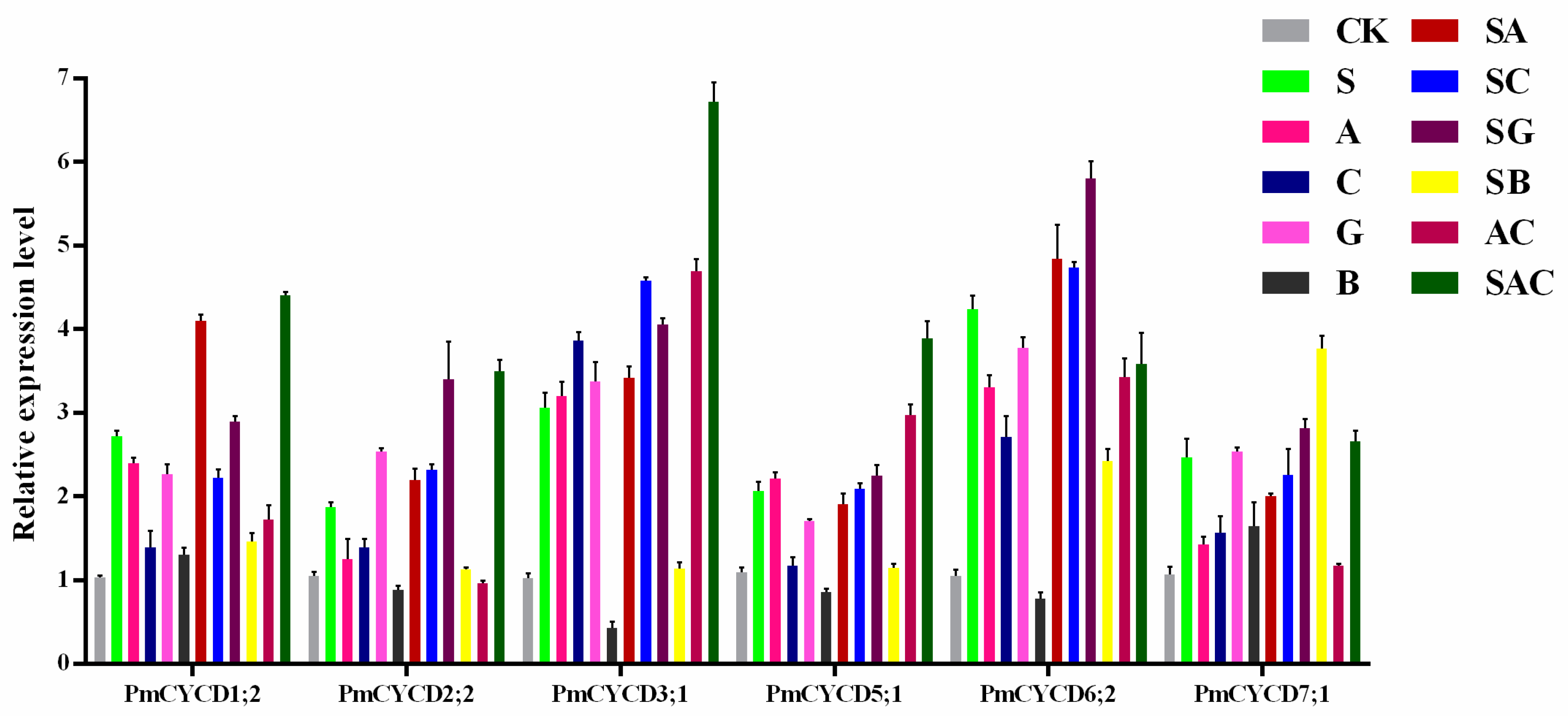
3.5. The Transcript Level of PmCYCDs under Sucrose and Phytohormone Treatments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 6-BA | 6-Benzylaminopurine |
| ABA | Abscisic acid |
| CDK | Cyclin-dependent kinase |
| CHX | Cycloheximide |
| CYCA | A-type cyclins |
| CYCD | D-type cyclins |
| DP | Dimerization partner |
| E2F | E2 promoter binding factor |
| GA | Gibberellin |
| HMMER | Biosequence analysis using profile hidden Markov models |
| ICK | Inhibitors of CDK |
| MEME | Motif-based sequence analysis tools |
| MW | Molecular weight |
| NAA | 1-Naphthylacetic acid |
| NLS | Nuclear location signal |
| pI | Theoretical isoelectric point |
| Rb | Retinoblastoma |
| RBR | Retinoblastoma-related |
| RPKM | Reads per kilobase per million |
References
- Zhao, X.; Harashima, H.; Dissmeyer, N.; Pusch, S.; Weimer, A.K.; Bramsiepe, J.; Bouyer, D.; Rademacher, S.; Nowack, M.K.; Novák, B.; et al. A General G1/S-Phase Cell-Cycle Control Module in the Flowering Plant Arabidopsis thaliana. PLoS Genet 2012, 8, e1002847. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.O. CYCLIN-DEPENDENT KINASES: Engines, Clocks, and Microprocessors. Annu. Rev. Cell Dev. Biol. 1997, 13, 261–291. [Google Scholar] [CrossRef] [PubMed]
- Nakagami, H.; Kawamura, K.; Sugisaka, K.; Sekine, M.; Shinmyo, A. Phosphorylation of Retinoblastoma-Related Protein by the Cyclin D/Cyclin-Dependent Kinase Complex Is Activated at the G1/S-Phase Transition in Tobacco. Plant Cell 2002, 14, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- Uemukai, K.; Iwakawa, H.; Kosugi, S.; De Uemukai, S.; Kato, K.; Kondorosi, E.; Murray, J.A.; Ito, M.; Shinmyo, A.; Sekine, M.; et al. Transcriptional Activation of Tobacco E2F is Repressed by Co-transfection with the Retinoblastoma-related Protein: Cyclin D Expression Overcomes this Repressor Activity. Plant Mol. Biol. 2005, 57, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Magwanga, R.O.; Lu, P.; Kirungu, J.N.; Cai, X.; Zhou, Z.; Wang, X.; Diouf, L.; Xu, Y.; Hou, Y.; Hu, Y.; et al. Whole Genome Analysis of Cyclin Dependent Kinase (CDK) Gene Family in Cotton and Functional Evaluation of the Role of CDKF4 Gene in Drought and Salt Stress Tolerance in Plants. IJMS 2018, 19, 2625. [Google Scholar] [CrossRef] [PubMed]
- Harbour, J.W.; Dean, D.C. Chromatin Remodeling and Rb Activity. Curr. Opin. Cell Biol. 2000, 12, 685–689. [Google Scholar] [CrossRef]
- Soni, R. A Family of Cyclin D Homologs from Plants Differentially Controlled by Growth Regulators and Containing the Conserved Retinoblastoma Protein Interaction Motif. Plant Cell 1995, 7, 85–103. [Google Scholar] [CrossRef]
- Reichheld, J.-P.; Chaubet, N.; Shen, W.H.; Renaudin, J.-P.; Gigot, C. Multiple A-type Cyclins Express Sequentially During the Cell Cycle in Nicotiana tabacum BY2 cells. Proc. Natl. Acad. Sci. USA 1996, 93, 13819–13824. [Google Scholar] [CrossRef]
- McKibbin, R.S.; Halford, N.G.; Francis, D. Expression of Fission Yeast cdc25 Alters the Frequency of Lateral Root Formation in Transgenic Tobacco. Plant Mol. Biol. 1998, 36, 601–612. [Google Scholar] [CrossRef]
- Umeda, M.; Umeda-Hara, C.; Yamaguchi, M.; Hashimoto, J.; Uchimiya, H. Differential Expression of Genes for Cyclin-Dependent Protein Kinases in Rice Plants. Plant Physiol. 1999, 119, 31–40. [Google Scholar] [CrossRef]
- Sornay, E.; Forzani, C.; Forero-Vargas, M.; Dewitte, W.; Murray, J.A.; Forero-Vargas, M. Activation of CYCD7;1 in the Central Cell and Early Endosperm Overcomes Cell Cycle Arrest in the Arabidopsis Female Gametophyte and Promotes Early Endosperm and Embryo Development. Plant J. 2015, 84, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Vandepoele, K.; Raes, J.; De Veylder, L.; Rouzé, P.; Rombauts, S.; Inzé, D. Genome-Wide Analysis of Core Cell Cycle Genes in Arabidopsis. Plant Cell 2002, 14, 903–916. [Google Scholar] [CrossRef] [PubMed]
- Masubelele, N.H.; Dewitte, W.; Menges, M.; Maughan, S.; Collins, C.; Huntley, R.; Nieuwland, J.; Scofield, S.; Murray, J.A.H. D-type Cyclins Activate Division in the Root Apex to Promote Seed Germination in Arabidopsis. Proc. Natl. Acad. Sci. USA 2005, 102, 15694–15699. [Google Scholar] [CrossRef] [PubMed]
- Menges, M.; Samland, A.K.; Planchais, S.; Murray, J.A. The D-Type Cyclin CYCD3;1 Is Limiting for the G1-to-S-Phase Transition in Arabidopsis. Plant Cell 2006, 18, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Cockcroft, C.E.; Boer, B.G.D.; Healy, J.M.; A Murray, J. Cyclin D Control of Growth Rate in Plants. Nature 2000, 405, 575–579. [Google Scholar]
- Gaudin, V. The Expression of D-Cyclin Genes Defines Distinct Developmental Zones in Snapdragon Apical Meristems and Is Locally Regulated by the Cycloidea Gene. Plant Physiol. 2000, 122, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Riou-Khamlichi, C.; Huntley, R.; Jacqmard, A.; Murray, J.A. Cytokinin Activation of Arabidopsis Cell Division Through a D-Type Cyclin. Science 1999, 283, 1541–1544. [Google Scholar] [CrossRef]
- Riou-Khamlichi, C.; Menges, M.; Healy, J.M.S.; Murray, J.A.H. Sugar Control of the Plant Cell Cycle: Differential Regulation of Arabidopsis D-Type Cyclin Gene Expression. Mol. Cell. Biol. 2000, 20, 4513–4521. [Google Scholar] [CrossRef]
- Lara-Núñez, A.; García-Ayala, B.B.; Garza-Aguilar, S.M.; Flores-Sánchez, J.; Sánchez-Camargo, V.A.; Bravo-Alberto, C.E.; Vázquez-Santana, S.; Vázquez-Ramos, J.M. Glucose and Sucrose Differentially Modify Cell Proliferation in Maize During Germination. Plant Physiol. Biochem. 2017, 113, 20–31. [Google Scholar] [CrossRef]
- Menges, M.; Pavesi, G.; Morandini, P.A.; Bögre, L.; Murray, J.A. Genomic Organization and Evolutionary Conservation of Plant D-type Cyclins. Plant Physiol. 2007, 145, 1558–1576. [Google Scholar] [CrossRef]
- Wang, F.; Huo, S.N.; Guo, J.; Zhang, X.S. Wheat D-type Cyclin Triae;CYCD2;1 Regulate Development of Transgenic Arabidopsis Plants. Planta 2006, 224, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.; Lowndes, L.; Regan, S.; Beardmore, T. Overexpression of CYCD1;2 in Activation-Tagged Populus tremula x Populus alba Results in Decreased Cell Size and Altered Leaf Morphology. Tree Genet. Genomes 2015, 11, 1–12. [Google Scholar] [CrossRef]
- Collins, C.; Dewitte, W.; Murray, J.A.H. D-type Cyclins Control Cell Division and Developmental Rate During Arabidopsis Seed Development. Exbot J. 2012, 63, 3571–3586. [Google Scholar] [CrossRef] [PubMed]
- Sornay, E.; Dewitte, W.; Murray, J.A.H. Seed Size Plasticity in Response to Embryonic Lethality Conferred by Ectopic CYCD Activation is Dependent on Plant Architecture. Plant Signal. Behav. 2016, 11, e1192741. [Google Scholar] [CrossRef] [PubMed]
- Ru, L.; Osorio, S.; Wang, L.; Fernie, A.R.; Patrick, J.W.; Ruan, Y.-L. Transcriptomic and Metabolomics Responses to Elevated Cell Wall Invertase Activity During Tomato Fruit Set. Exbot J. 2017, 68, 4263–4279. [Google Scholar] [CrossRef] [PubMed]
- Prunus mume Genome Home Page. Available online: https://www.ncbi.nlm.nih.gov/genome/?term=13911 (accessed on 9 February 2019).
- Pfam Database Home Page. Available online: http://pfam.xfam.org (accessed on 9 February 2019).
- HMMER3 Software Home Page. Available online: http://www.hmmer.org (accessed on 9 February 2019).
- SMART Software Home Page. Available online: http://smart.embl-heidelberg.de (accessed on 9 February 2019).
- ExPASy Software Home Page. Available online: http://www.expasy.org/tools (accessed on 9 February 2019).
- WoLF PSORT Software Home Page. Available online: https://www.genscript.com/wolf-psort.html (accessed on 9 February 2019).
- Plant-mPLoc Software Home Page. Available online: http://www.csbio.sjtu.edu.cn/bioinf/plant-multi (accessed on 9 February 2019).
- The Arabidopsis Information Resource (TAIR) Home Page. Available online: http://www.arabidopsis.org (accessed on 9 February 2019).
- Rice Genome Annotation Project Home Page. Available online: http://rice.plantbiology.msu.edu (accessed on 9 February 2019).
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X Windows Interface: Flexible Strategies for Multiple Sequence Alignment Aided by Quality Analysis Tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.-Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An Upgraded Gene Feature Visualization Eerver. Bioinformatics 2014, 31, 1296–1297. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for Motif Discovery and Eearching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- GEO DataSets Home Page. Available online: http://www.ncbi.nlm.nih.gov/geo (accessed on 9 February 2019).
- Deng, W.; Wang, Y.; Liu, Z.; Cheng, H.; Xue, Y. HemI: A Toolkit for Illustrating Heatmaps. PloS ONE 2014, 9, e111988. [Google Scholar] [CrossRef]
- RealTime qPCR Assay tool Home Page. Available online: https://sg.idtdna.com/scitools/Applications/RealTimePCR (accessed on 9 February 2019).
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Renaudin, J.-P.; Doonan, J.H.; Freeman, D.; Hashimoto, J.; Hirt, H.; Jacobs, T.; Kouchi, H.; Sauter, M.; Sorrell, D.A.; Sundaresan, V.; et al. Plant Cyclins: A Unified Nomenclature for Plant A-, B- and D-type Cyclins Based on Sequence Organization. Plant Mol. Biol. 1996, 32, 1003–1018. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Kong, H.; Sun, Y.; Zhang, X.; Zhang, W.; Altman, N.; Depamphilis, C.W.; Ma, H. Genome-Wide Analysis of the Cyclin Family in Arabidopsis and Comparative Phylogenetic Analysis of Plant Cyclin-Like Proteins. Plant Physiol. 2004, 135, 1084–1099. [Google Scholar] [CrossRef]
- NetOGlyc 4.0 Server Home Page. Available online: http://www.cbs.dtu.dk/services/NetOGlyc (accessed on 9 February 2019).
- NetNGlyc 1.0 Server Home Page. Available online: http://www.cbs.dtu.dk/services/NetNGlyc (accessed on 9 February 2019).
- Menges, M.; De Jager, S.M.; Gruissem, W.; Murray, J.A. Global Analysis of the Core Cell Cycle Regulators of Arabidopsis Identifies Novel Genes, Reveals Multiple and Highly Specific Profiles of Expression and Provides a Coherent Model for Plant Cell Cycle Control. Plant J. 2005, 41, 546–566. [Google Scholar] [CrossRef] [PubMed]
- Boruc, J.; Daele, H.V.D.; Hollunder, J.; Rombauts, S.; Mylle, E.; Hilson, P.; Inzé, D.; De Veylder, L.; Russinova, E. Functional Modules in the Arabidopsis Core Cell Cycle Binary Protein-Protein Interaction Network. Plant Cell 2010, 22, 1264–1280. [Google Scholar] [CrossRef] [PubMed]
- Sorrell, D.A.; Menges, M.; Healy, J.S.; Deveaux, Y.; Amano, C.; Su, Y.; Nakagami, H.; Shinmyo, A.; Doonan, J.H.; Sekine, M.; et al. Cell Cycle Regulation of Cyclin-Dependent Kinases in Tobacco Cultivar Bright Yellow-2 Cells. Plant Physiol. 2001, 126, 1214–1223. [Google Scholar] [CrossRef]
- Buendía-Monreal, M.; Rentería-Canett, I.; Guerrero-Andrade, O.; Bravo-Alberto, C.E.; Martínez-Castilla, L.P.; García, E.; Vázquez-Ramos, J.M.; Buendía-Monreal, M.; Rentería-Canett, I.; Guerrero-Andrade, O.; et al. The Family of Maize D-type Cyclins: Genomic Organization, Phylogeny and Expression Patterns. Physiol. Plant. 2011, 143, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Nugent, J.H.; E Alfa, C.; Young, T.; Hyams, J.S. Conserved Structural Motifs in Cyclins Identified by Sequence Analysis. J. Cell Sci. 1991, 99, 669–674. [Google Scholar] [PubMed]
- Goolsby, G.L. Cyclin G1 and Cyclin G2 Comprise a New Family of Cyclins with Contrasting Tissue-specific and Cell Cycle-regulated Expression. J. Biol. Chem. 1996, 271, 6050–6061. [Google Scholar]
- Cui, L.; Li, J.; Zhang, T.; Guo, Q.; Xu, J.; Lou, Q.; Chen, J. Identification and Expression Analysis of D-type Cyclin Genes in Early Developing Fruit of Cucumber (Cucumis sativus L.). Plant Mol. Biol. Report. 2013, 32, 209–218. [Google Scholar] [CrossRef]
- Yao, X.; Yang, H.; Zhu, Y.; Xue, J.; Wang, T.; Song, T.; Yang, Z.; Wang, S. The Canonical E2Fs Are Required for Germline Development in Arabidopsis. Front. Plant Sci. 2018, 9, 638. [Google Scholar] [CrossRef] [PubMed]
- Vandepoele, K.; Vlieghe, K.; Florquin, K.; Hennig, L.; Beemster, G.T.; Gruissem, W.; Van De Peer, Y.; Inzé, D.; De Veylder, L. Genome-Wide Identification of Potential Plant E2F Target Genes. Plant Physiol. 2005, 139, 316–328. [Google Scholar] [CrossRef]
- Coudreuse, D.; Nurse, P. Driving the Cell Cycle with a Minimal CDK Control Network. Nature 2010, 468, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Zhang, C.; Li, H.; Wei, B.; Wang, Y.; Huang, H.; Li, Y.; Yu, G.; Liu, H.; Zhang, J.; et al. Identification and Functional Analysis of the ICK gene Family in Maize. Sci. Rep. 2017, 7, 43818. [Google Scholar] [CrossRef] [PubMed]
- Rhee, Y.; Hwang, K.; Cho, S.; Lee, M.; Kil, E.-J.; Choi, S.; Hahn, B.-S.; Kim, D.; Auh, C.-K.; Lee, S. Expression Analysis of D-type Cyclin in Potato (Solanum tuberosum L.) under Different Culture Conditions. Acta Physiol. Plant 2016, 38, 1–12. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, C.; Yang, Y.; Gao, X.; Zhang, H. Antisense Expression of the Fasciclin-like Arabinogalactan Protein FLA6 gene in Populus Inhibits Expression of its Homologous Genes and Alters Stem Biomechanics and Cell Wall Composition in Transgenic trees. Exbot J. 2014, 66, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Sterken, R.; Kiekens, R.; Boruc, J.; Zhang, F.; Vercauteren, A.; Vercauteren, I.; De Smet, L.; Dhondt, S.; Inzé, D.; De Veylder, L.; et al. Combined Linkage and Association Mapping Reveals CYCD5;1 as a Quantitative Trait Gene for Endoreduplication in Arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 4678–4683. [Google Scholar] [CrossRef]
- Van Rooij, J.; Vanneste, S.; Pierik, R.; McLoughlin, F.; Gühl, K.; Van Isterdael, G.; Frank, F.M.; Beeckman, T.; Peeters, A.; Athanasius, F.M.; et al. Ethylene-mediated regulation of A2-type CYCLINs modulates hyponastic growth in Arabidopsis thaliana. Plant Physiol. 2015, 169, 194–208. [Google Scholar]
- Nagata, T.; Saitou, K. Regulation of Expression of D3-type Cyclins and ADP-Glucose Pyrophosphorylase Genes by Sugar, Cytokinin and ABA in Sweet Potato (Ipomoea batatas Lam.). Plant Prod. Sci. 2009, 12, 434–442. [Google Scholar] [CrossRef]
- Yu, P.; Eggert, K.; Von Wirén, N.; Li, C.; Hochholdinger, F. Cell Type-Specific Gene Expression Analyses by RNA Sequencing Reveal Local High Nitrate-Triggered Lateral Root Initiation in Shoot-Borne Roots of Maize by Modulating Auxin-Related Cell Cycle Regulation. Plant Physiol. 2015, 169, 690–704. [Google Scholar] [CrossRef]
- De Veylder, L.; Engler, J.D.A.; Burssens, S.; Manevski, A.; Lescure, B.; Van Montagu, M.; Engler, G.; Inzé, D. A New D-type Cyclin of Arabidopsis thaliana Expressed During Lateral Root Primordia Formation. Planta 1999, 208, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Achard, P.; Gusti, A.; Cheminant, S.; Alioua, M.; Dhondt, S.; Coppens, F.; Beemster, G.T.; Genschik, P. Gibberellin Signaling Controls Cell Proliferation Rate in Arabidopsis. Curr. Biol. 2009, 19, 1188–1193. [Google Scholar] [CrossRef] [PubMed]
- Fabian, T.; Lorbiecke, R.; Umeda, M.; Sauter, M. The Cell Cycle Genes CycA1;1 and cdc2Os-3 are Coordinately Regulated by Gibberellin in Planta. Planta 2000, 211, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Swiatek, A.; Lenjou, M.; Van Bockstaele, D.; Inzé, D.; Van Onckelen, H.; Świa̧tek, A. Differential Effect of Jasmonic Acid and Abscisic Acid on Cell Cycle Progression in Tobacco BY-2 Cells. Plant Physiol. 2002, 128, 201–211. [Google Scholar] [CrossRef]
- Burssens, S.; Engler, J.D.A.; Beeckman, T.; Richard, C.; Shaul, O.; Ferreira, P.; Van Montagu, M.; Inzé, D. Developmental Expression of the Arabidopsis thaliana CycA2;1 Gene. Planta 2000, 211, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Oakenfull, E.A.; Riou-Khamlichi, C.; Murray, A.H. Plant D–type Cyclins and The Control of G1 Progression. Philos. Trans. R. Soc. Lond. Ser. B: Biol. Sci. 2002, 357, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Forzani, C.; Aichinger, E.; Sornay, E.; Willemsen, V.; Laux, T.; Dewitte, W.; Murray, J.A. WOX5 Suppresses CYCLIN D Activity to Establish Quiescence at the Center of the Root Stem Cell Niche. Curr. Biol. 2014, 24, 1939–1944. [Google Scholar] [CrossRef]
- Hu, Y.; Bao, F.; Li, J. Promotive Effect of Brassinosteroids on Cell Division Involves a Distinct CycD3-Induction Pathway in Arabidopsis. Plant J. 2000, 24, 693–701. [Google Scholar] [CrossRef]
- Healy, J.M.S.; Menges, M.; Doonan, J.H.; Murray, J.A.H. The Arabidopsis D-type Cyclins CycD2 and CycD3 Both Interact in vivo with the PSTAIRE Cyclin-dependent Kinase Cdc2a but Are Differentially Controlled. J. Biol. Chem. 2000, 276, 7041–7047. [Google Scholar] [CrossRef]
 LxCxE,
LxCxE,  cyclin box,
cyclin box,  cyclin-N domain,
cyclin-N domain,  cyclin_C domain,
cyclin_C domain,  five non-contiguous conserved amino acids,
five non-contiguous conserved amino acids,  PEST sites,
PEST sites,  N-glycosylation sites,
N-glycosylation sites,  C-glycosylation sites.
C-glycosylation sites.
 LxCxE,
LxCxE,  cyclin box,
cyclin box,  cyclin-N domain,
cyclin-N domain,  cyclin_C domain,
cyclin_C domain,  five non-contiguous conserved amino acids,
five non-contiguous conserved amino acids,  PEST sites,
PEST sites,  N-glycosylation sites,
N-glycosylation sites,  C-glycosylation sites.
C-glycosylation sites.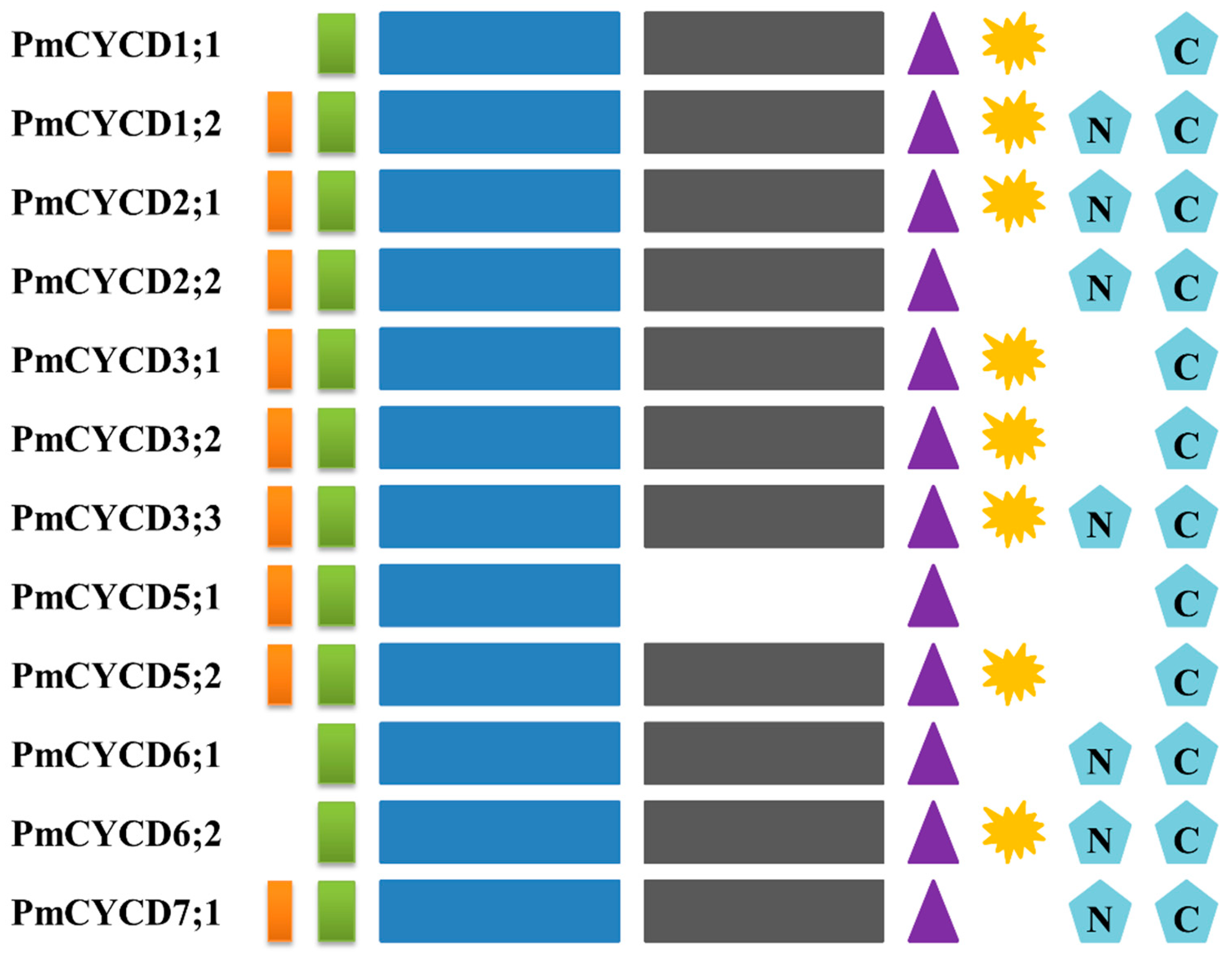

| Gene Name | Gene ID | Protein ID | Chr | Start | Stop | Strand | Length (aa) | MW (Da) | pI | Subcellular localization | Cyclin N | Cyclin C | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PmCYCD1;1 | XM_008238127.1 | XP_008236349.1 | LG6 | 6969039 | 6970390 | - | 318 | 35552 | 6.22 | Nucleus | 38-171 | 70-101 | 173-288 |
| PmCYCD1;2 | XM_008247248.1 | XP_008245470.1 | Scaffold | 18686 | 20782 | + | 336 | 37443.58 | 4.99 | Nucleus | 42-175 | 75-106 | 177-302 |
| PmCYCD2;1 | XM_008237197.2 | XP_008235419.1 | LG1 | 16974056 | 16976030 | - | 361 | 40045.52 | 5.74 | Nucleus | 71-202 | 102-133 | 204-315 |
| PmCYCD2;2 | XM_008221165.2 | XP_008219387.1 | LG2 | 5620754 | 5622822 | - | 356 | 39734.36 | 4.85 | Nucleus | 64-196 | None | 198-311 |
| PmCYCD3;1 | XM_008238346.1 | XP_008236568.1 | LG6 | 8594925 | 8596474 | + | 398 | 45630.26 | 5.2 | Nucleus | 85-219 | 119-150 | 221-338 |
| PmCYCD3;2 | XM_008240617.1 | XP_008238839.1 | LG1 | 19784269 | 19785768 | - | 371 | 42075.01 | 5.04 | Nucleus | 66-195 | None | 197-312 |
| PmCYCD3;3 | XM_008222052.2 | XP_008220274.1 | LG2 | 10446717 | 10448190 | + | 375 | 42695.66 | 5.06 | Nucleus | 79-208 | 108-139 | 210-335 |
| PmCYCD5;1 | XM_008222143.1 | XP_008220365.1 | LG2 | 10807955 | 10809234 | + | 265 | 30328.97 | 4.92 | Nucleus | 44-169 | 73-104 | None |
| PmCYCD5;2 | XM_008239987.2 | XP_008238209.1 | LG1 | 19180744 | 19182155 | - | 333 | 37822.23 | 5.6 | Nucleus | 71-182 | None | 184-297 |
| PmCYCD6;1 | XM_008231974.2 | XP_008230196.2 | LG4 | 18715637 | 18717250 | - | 313 | 35225.73 | 6.32 | Nucleus | 18-146 | None | 148-274 |
| PmCYCD6;2 | XM_008226697.1 | XP_008224919.1 | LG3 | 1476240 | 1477840 | - | 366 | 41515.4 | 6.71 | Nucleus | 66-196 | None | 198-332 |
| PmCYCD7;1 | XM_008244265.2 | XP_008242487.2 | LG1 | 20815021 | 20816000 | + | 345 | 39367.29 | 5.75 | Nucleus | 48-178 | None | 180-284 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, T.; Zhuo, X.; Li, L.; Wang, J.; Cheng, T.; Zhang, Q. Genome-Wide Analysis of the D-type Cyclin Gene Family Reveals Differential Expression Patterns and Stem Development in the Woody Plant Prunus mume. Forests 2019, 10, 147. https://doi.org/10.3390/f10020147
Zheng T, Zhuo X, Li L, Wang J, Cheng T, Zhang Q. Genome-Wide Analysis of the D-type Cyclin Gene Family Reveals Differential Expression Patterns and Stem Development in the Woody Plant Prunus mume. Forests. 2019; 10(2):147. https://doi.org/10.3390/f10020147
Chicago/Turabian StyleZheng, Tangchun, Xiaokang Zhuo, Lulu Li, Jia Wang, Tangren Cheng, and Qixiang Zhang. 2019. "Genome-Wide Analysis of the D-type Cyclin Gene Family Reveals Differential Expression Patterns and Stem Development in the Woody Plant Prunus mume" Forests 10, no. 2: 147. https://doi.org/10.3390/f10020147
APA StyleZheng, T., Zhuo, X., Li, L., Wang, J., Cheng, T., & Zhang, Q. (2019). Genome-Wide Analysis of the D-type Cyclin Gene Family Reveals Differential Expression Patterns and Stem Development in the Woody Plant Prunus mume. Forests, 10(2), 147. https://doi.org/10.3390/f10020147






