Physiological and Transcriptome Analysis of a Yellow-Green Leaf Mutant in Birch (Betula platyphylla × B. Pendula)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Measurement of Growth Traits and Pigment Content
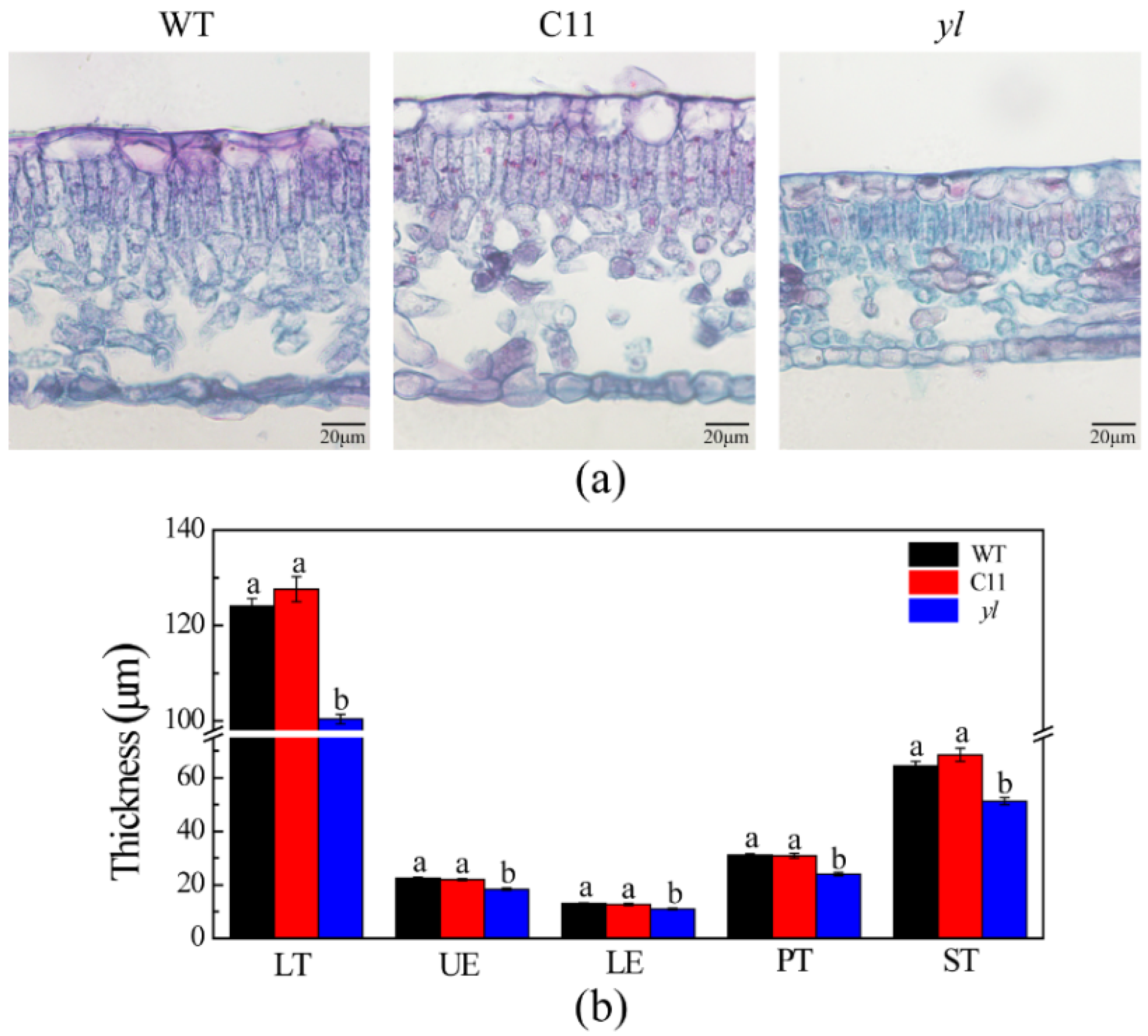
2.2.2. Light Microscopy
2.2.3. Leaf Gas-Exchange Measurement
2.2.4. RNA Extraction, Library Construction, and RNA-seq
2.2.5. Gene Annotation and DEG Analysis
2.2.6. RNA Extraction and Quantitative RT-PCR
2.2.7. Statistical Analysis
3. Results
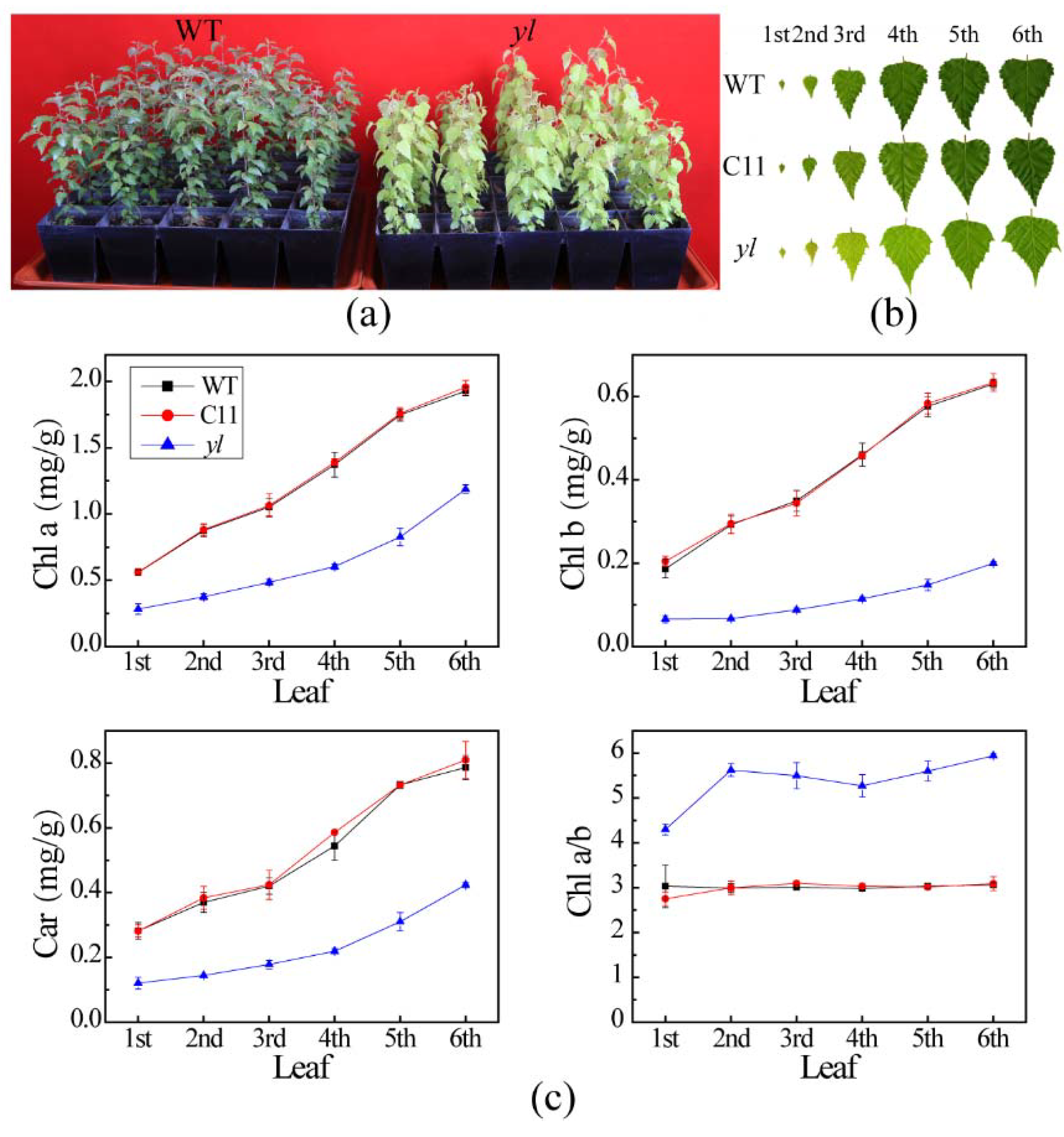
3.1. Pigment Contents and Leaf Anatomical Structure of Chl-Deficient Mutant yl
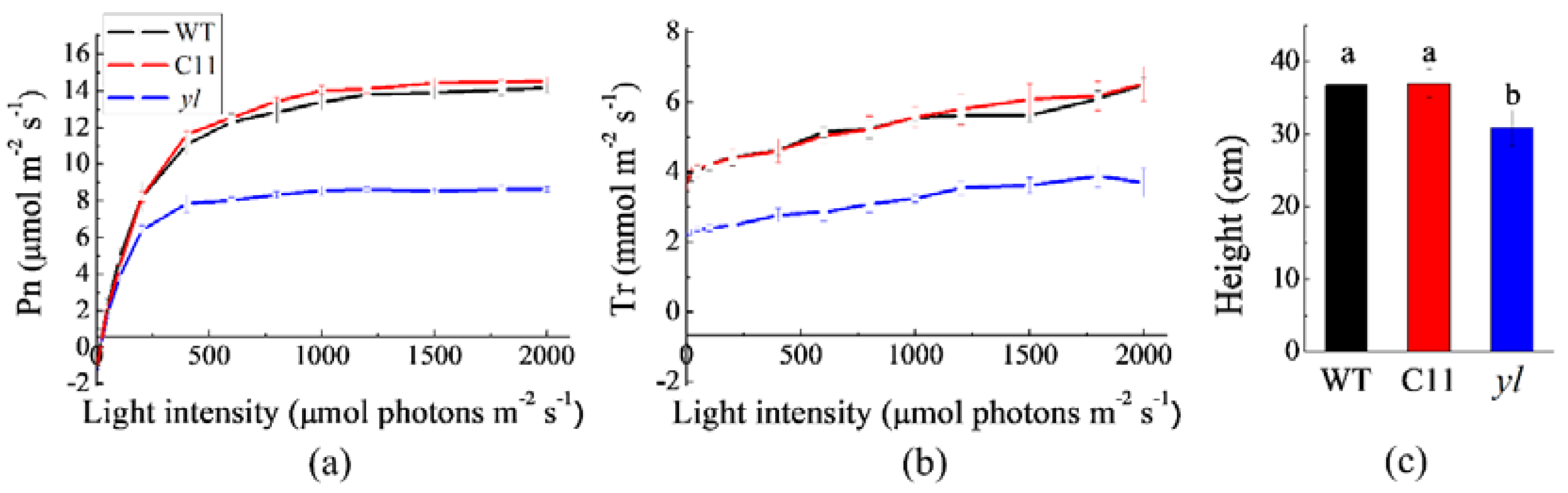
3.2. Changes in Photosynthesis and Growth
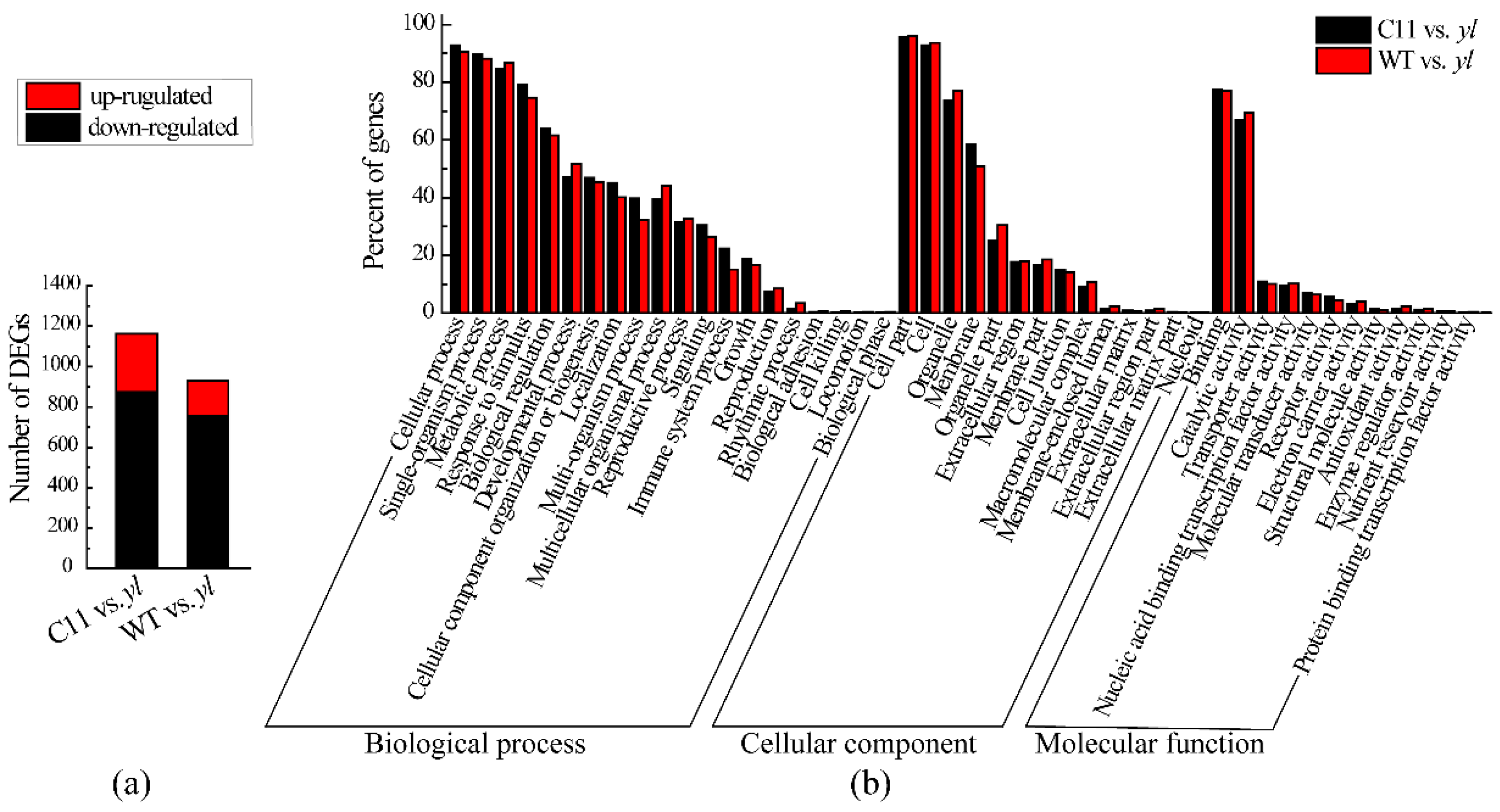
3.3. Differently Expressed Genes between yl and Control Plants
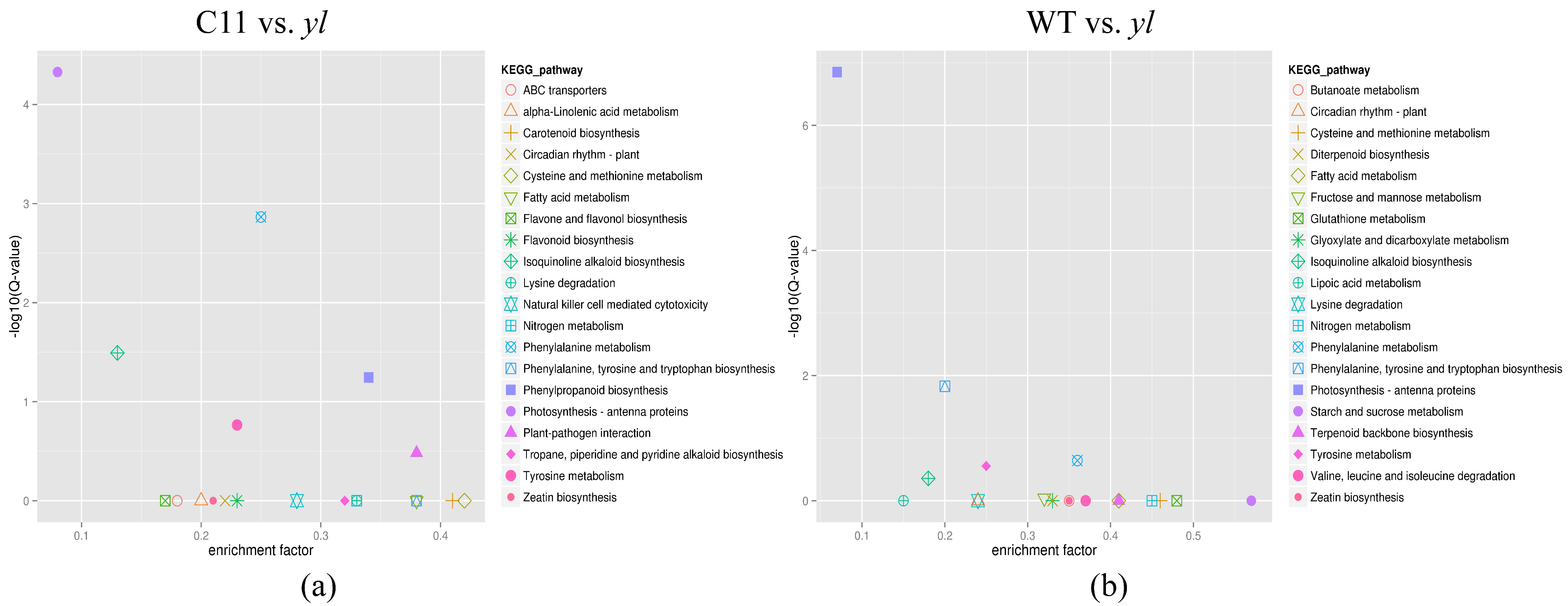
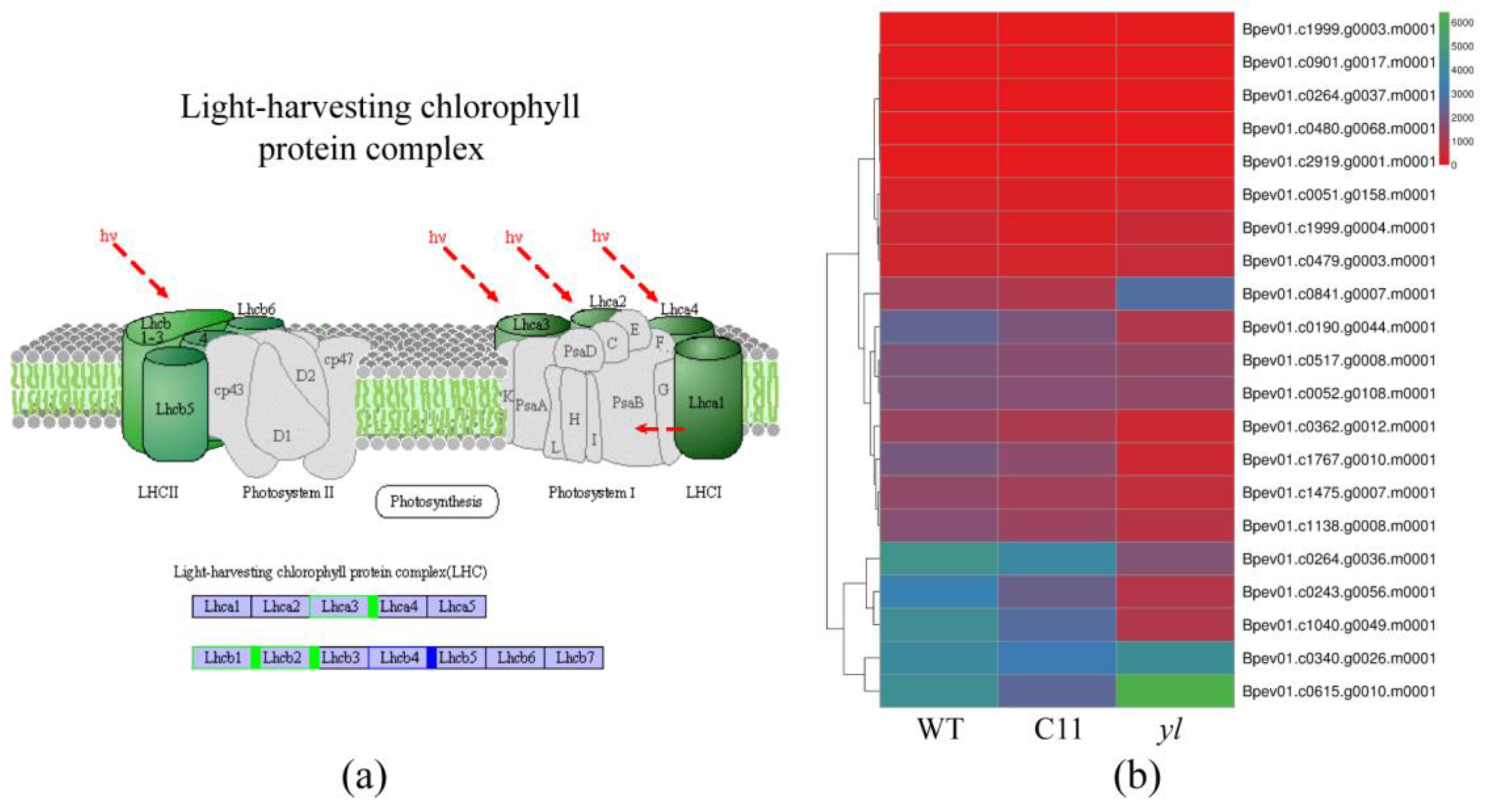
3.4. KEGG Pathway Analysis of DEGs
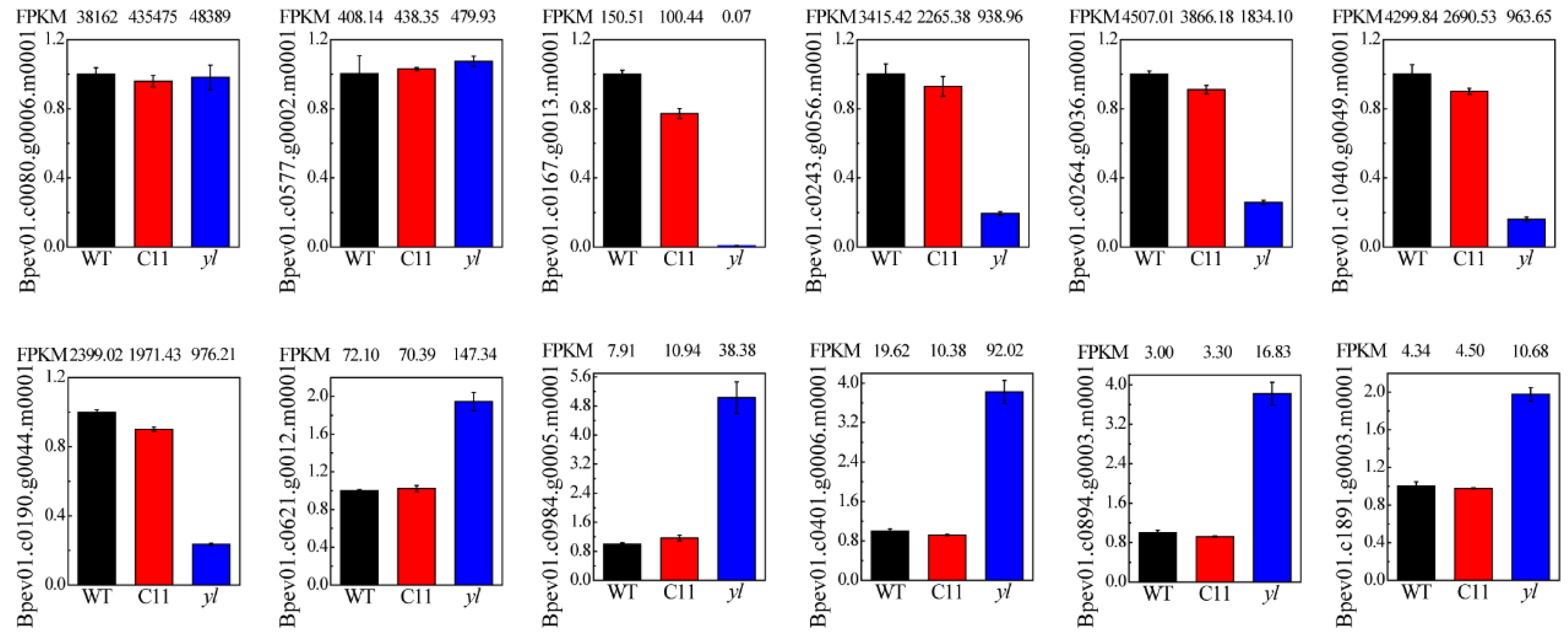
3.5. qRT-PCR Verification of RNA-seq
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zyryanova, O.A.; Terazawa, M.; Koike, T.; Zyryanov, V.I. White birch trees as resource species of Russia: Their distribution, ecophysiological features, multiple utilizations. Eurasian J. For. Res. 2010, 13, 25–40. [Google Scholar]
- Dulamsuren, C.; Hauck, M.; Bader, M.K.-F.; Osokhjargal, D.; Oyungerel, S.; Nyambayar, S.; Runge, M.; Leuschner, C. Water relations and photosynthetic performance in Larix sibirica growing in the forest-steppe ecotone of northern Mongolia. Tree Physiol. 2008, 29, 99–110. [Google Scholar] [CrossRef]
- Bekhta, P.; Hiziroglu, S.; Shepelyuk, O. Properties of plywood manufactured from compressed veneer as building material. Mater. Des. 2009, 30, 947–953. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Q.; Chen, P.; Song, F.; Guan, M.; Jin, L.; Wang, Y.; Yang, C. Four Novel Cellulose Synthase (CESA) Genes from Birch (Betula platyphylla Suk.) Involved in Primary and Secondary Cell Wall Biosynthesis. IJMS 2012, 13, 12195–12212. [Google Scholar] [CrossRef] [PubMed]
- Borrega, M.; Tolonen, L.K.; Bardot, F.; Testova, L.; Sixta, H. Potential of hot water extraction of birch wood to produce high-purity dissolving pulp after alkaline pulping. Bioresour. Technol. 2013, 135, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Rubert-Nason, K.F.; Holeski, L.M.; Couture, J.J.; Gusse, A.; Undersander, D.J.; Lindroth, R.L. Rapid phytochemical analysis of birch (Betula) and poplar (Populus) foliage by near-infrared reflectance spectroscopy. Anal. Bioanal. Chem. 2012, 405, 1333–1344. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Mu, H.; Jiang, J.; Liu, G. Transcriptomic analysis of purple leaf determination in birch. Gene 2013, 526, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.B.; Wei, R.; Chen, S.; Jiang, J.; Li, H.Y.; Huang, H.J.; Yang, G.; Wang, S.; Wei, H.R.; Liu, G.F. Functional characterization of CCR in birch (Betula platyphylla x Betula pendula) through overexpression and suppression analysis. Physiol. Plant. 2015, 154, 283–296. [Google Scholar] [CrossRef]
- Córdoba, J.; Molina-Cano, J.-L.; Martínez-Carrasco, R.; Morcuende, R.; Pérez, P. Functional and transcriptional characterization of a barley mutant with impaired photosynthesis. Plant Sci. 2016, 244, 19–30. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Yao, X.; Dong, X.; Chen, W.; Li, Z.K. Fine mapping and photosynthetic characteristics of the lower chlorophyllb1 mutant in rice (Oryza sativa L.). Plant Breed. 2015, 134, 661–667. [Google Scholar] [CrossRef]
- Zhong, X.M.; Sun, S.F.; Li, F.H.; Wang, J.; Shi, Z.S. Photosynthesis of a yellow-green mutant line in maize. Photosynthetica 2015, 53, 499–505. [Google Scholar] [CrossRef]
- Li, N.; Jia, J.; Xia, C.; Liu, X.; Kong, X. Characterization and mapping of novel chlorophyll deficient mutant genes in durum wheat. Breed. Sci. 2013, 63, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Karaca, M.; Saha, S.; Callahan, F.E.; Jenkins, J.N.; Read, J.J.; Percy, R.G. Molecular and cytological characterization of a cytoplasmic-specific mutant in pima cotton (Gossypium barbadense L.). Euphytica 2004, 139, 187–197. [Google Scholar] [CrossRef]
- Wang, L.; Yue, C.; Cao, H.; Zhou, Y.; Zeng, J.; Yang, Y.; Wang, X. Biochemical and transcriptome analyses of a novel chlorophyll-deficient chlorina tea plant cultivar. BMC Plant Biol. 2014, 14, 239. [Google Scholar] [CrossRef]
- Li, W.; Tang, S.; Zhang, S.; Shan, J.; Tang, C.; Chen, Q.; Jia, G.; Han, Y.; Zhi, H.; Diao, X. Gene mapping and functional analysis of the novel leaf color gene SiYGL1 in foxtail millet [Setaria italica (L.) P. Beauv]. Physiol. Plant. 2016, 157, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Guo, S.; Wang, Z.; Du, Q.; Xing, Y.; Zhang, T.; Shen, W.; Sang, X.; Ling, Y.; He, G. Map-based cloning and functional analysis of YGL8, which controls leaf colour in rice (Oryza sativa). BMC Plant Biol. 2016, 16, 134. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Schneeberger, K.; Ossowski, S.; Günther, T.; Bender, S.; Fitz, J.; Koenig, D.; Lanz, C.; Stegle, O.; Lippert, C.; et al. Whole-genome sequencing of multiple Arabidopsis thaliana populations. Nat. Genet. 2011, 43, 956–963. [Google Scholar] [CrossRef]
- Cai, Q.; Li, B.; Lin, F.; Huang, P.; Guo, W.; Zheng, Y. De novo sequencing and sssembly analysis of transcriptome in Pinus bungeana Zucc. ex Endl. Forests 2018, 9, 156. [Google Scholar] [CrossRef]
- Du, M.; Ding, G.; Cai, Q. The Transcriptomic Responses of Pinus massoniana to Drought Stress. Forests 2018, 9, 326. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Wang, P.; Wang, S.; Ma, L.; Li, L.; Yang, R.; Ma, Y.; Wang, Q. Comprehensive transcriptome analysis discovers novel candidate genes related to leaf color in a Lagerstroemia indica yellow leaf mutant. Genes Genom. 2015, 37, 851–863. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Chang, S.; Puryear, J.; Cairney, J. A simple and efficient method for isolating RNA from pine trees. Plant Mol. Biol. Report. 1993, 11, 113–116. [Google Scholar] [CrossRef]
- Salojärvi, J.; Smolander, O.-P.; Nieminen, K.; Rajaraman, S.; Safronov, O.; Safdari, P.; Lamminmäki, A.; Immanen, J.; Lan, T.; Tanskanen, J.; et al. Genome sequencing and population genomic analyses provide insights into the adaptive landscape of silver birch. Nat. Genet. 2017, 49, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Gingeras, T.R. Comment on “TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions” by Kim et al. Bioinformatics 2013, 14, 000851. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wang, S.; Jiang, J.; Liu, G.; Li, H.; Chen, S.; Xu, H. Overexpression of BpAP1 induces early flowering and produces dwarfism in Betula platyphylla x Betula pendula. Physiol. Plant. 2014, 151, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Sun, X.; Li, C.; Huan, R.; Sun, C.; Wang, Y.; Xiao, F.; Wang, Q.; Chen, P.; Ma, F.; et al. Map-based cloning and characterization of the novel yellow-green leaf gene ys83 in rice (Oryza sativa). Plant Physiol. Biochem. 2017, 111, 1–9. [Google Scholar] [CrossRef]
- Dong, H.; Fei, G.-L.; Wu, C.-Y.; Wu, F.-Q.; Sun, Y.-Y.; Chen, M.-J.; Ren, Y.-L.; Zhou, K.-N.; Cheng, Z.-J.; Wang, J.-L.; et al. A rice virescent-yellow Leaf mutant reveals new insights into the role and sssembly of plastid caseinolytic protease in higher plants. Plant Physiol. 2013, 162, 1867–1880. [Google Scholar] [CrossRef]
- Tanaka, R.; Koshino-Kimura, Y.; Sawa, S.; Ishiguro, S.; Okada, K.; Tanaka, A. Overexpression of chlorophyllide an oxygenase (CAO) enlarges the antenna size of photosystem II in Arabidopsis thaliana. Plant J. 2002, 26, 365–373. [Google Scholar] [CrossRef]
- Highkin, H.R.; Boardman, N.K.; Goodchild, D.J. Photosynthetic Studies on a Pea-mutant Deficient in Chlorophyll. Plant Physiol. 1969, 44, 1310–1320. [Google Scholar] [CrossRef]
- Polle, J.E.W.; Benemann, J.R.; Tanaka, A.; Melis, A. Photosynthetic apparatus organization and function in the wild type and a chlorophyll b -less mutant of Chlamydomonas reinhardtii. Dependence on carbon source. Planta 2000, 211, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.; Wentworth, M.; Walters, R.G.; Howard, C.A.; Ruban, A.; Horton, P.; Jansson, S. Absence of the Lhcb1 and Lhcb2 proteins of the light-harvesting complex of photosystem II—Effects on photosynthesis, grana stacking and fitness. Plant J. 2003, 35, 350–361. [Google Scholar] [PubMed]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Li, X.P.; Niyogi, K.K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Sukhov, V.; Surova, L.; Sherstneva, O.; Katicheva, L.; Vodeneev, V. Variation potential influence on photosynthetic cyclic electron flow in pea. Front. Plant Sci. 2015, 5, 766. [Google Scholar] [CrossRef] [PubMed]
- Sukhova, E.; Mudrilov, M.; Vodeneev, V.; Sukhov, V. Influence of the variation potential on photosynthetic flows of light energy and electrons in pea. Photosynth. Res. 2017, 136, 215–228. [Google Scholar] [CrossRef]
- Cheng, J.; Fan, P.; Liang, Z.; Wang, Y.; Niu, N.; Li, W.; Li, S. Accumulation of End Products in Source Leaves Affects Photosynthetic Rate in Peach via Alteration of Stomatal Conductance and Photosynthetic Efficiency. J. Amer. Soc. Hort. Sci. 2009, 134, 667–676. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, W.; Liu, G.; Yang, C.; Sun, Y.; Wu, W.; Cao, S.; Wang, C.; Hai, G.; Wang, Z.; et al. The Conserved Endoribonuclease YbeY Is Required for Chloroplast Ribosomal RNA Processing in Arabidopsis. Plant Physiol. 2015, 168, 205–221. [Google Scholar] [CrossRef]
- Zhou, X.-S.; Wu, D.-X.; Shen, S.-Q.; Sun, J.-W.; Shu, Q.-Y. High photosynthetic efficiency of a rice (Oryza sativa L.) xantha mutant. Photosynthetica 2006, 44, 316–319. [Google Scholar] [CrossRef]
- Hudson, D.; Guevara, D.R.; Hand, A.J.; Xu, Z.; Hao, L.; Chen, X.; Zhu, T.; Bi, Y.-M.; Rothstein, S.J. Rice Cytokinin GATA Transcription Factor1 Regulates Chloroplast Development and Plant Architecture. Plant Physiol. 2013, 162, 132–144. [Google Scholar] [CrossRef]
- Sjögren, L.L.; Macdonald, T.M.; Sutinen, S.; Clarke, A.K. Inactivation of the clpC1 Gene Encoding a Chloroplast Hsp100 Molecular Chaperone Causes Growth Retardation, Leaf Chlorosis, Lower Photosynthetic Activity, and a Specific Reduction in Photosystem Content. Plant Physiol. 2004, 136, 4114–4126. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.-G.; Shi, Y.-F.; Xu, X.; Wei, Y.-L.; Wang, H.-M.; Zhang, X.-B.; Wu, J.-L. Oryza sativa Chloroplast Signal Recognition Particle 43 (OscpSRP43) Is Required for Chloroplast Development and Photosynthesis. PLoS ONE 2015, 10, e0143249. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Gruber, M.Y.; Khachatourians, G.G.; Zhou, R.; Epp, D.J.; Hegedus, D.D.; Parkin, I.A.P.; Welsch, R.; Hannoufa, A. Arabidopsis cpSRP54 regulates carotenoid accumulation in Arabidopsis and Brassica napus. J. Exp. Bot. 2012, 63, 5189–5202. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.M.; Li, C.M.; Ma, X.Z.; Wang, Y.; Sun, C.H.; Huang, R.; Zhong, P.; Gao, Z.Y.; Chen, D.; Xu, Z.J.; et al. GRY79 encoding a putative metallo-beta-lactamase-trihelix chimera is involved in chloroplast development at early seedling stage of rice. Plant Cell Rep. 2015, 34, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, K.; Ito, Y.; Serizawa, A.; Kurata, N. OsHAP3genes regulate chloroplast biogenesis in rice. Plant J. 2003, 36, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Perez-Ruiz, J.M. Rice NTRC Is a High-Efficiency Redox System for Chloroplast Protection against Oxidative Damage. Plant Cell 2006, 18, 2356–2368. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, M.; Liang, N.; Yan, H.; Wei, Y.; Xu, X.; Liu, J.; Xu, Z.; Chen, F.; Wu, G.; et al. Molecular cloning and function analysis of the stay green gene in rice. Plant J. 2007, 52, 197–209. [Google Scholar] [CrossRef]
- Nott, A.; Jung, H.-S.; Koussevitzky, S.; Chory, J. Plastid-to-nucleus retrograde signaling. Annu. Rev. Plant Biol. 2006, 57, 739–759. [Google Scholar] [CrossRef]
- Rossini, L.; Cribb, L.; Martin, D.J.; Langdale, J.A. The Maize Golden2 Gene Defines a Novel Class of Transcriptional Regulators in Plants. Plant Cell 2001, 13, 1231. [Google Scholar] [CrossRef]
- Yasumura, Y.; Moylan, E.C.; Langdale, J.A. A Conserved Transcription Factor Mediates Nuclear Control of Organelle Biogenesis in Anciently Diverged Land Plants. Plant Cell 2005, 17, 1894–1907. [Google Scholar] [CrossRef]
- Wang, P.; Korkaric, M.; Waters, M.T.; Capper, R.G.; Saunders, N.J.; Langdale, J.A. GLK transcription factors coordinate expression of the photosynthetic apparatus in Arabidopsis. Plant Cell 2009, 21, 1109–1128. [Google Scholar]

| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| Bpev01.c0080.g0006.m0001 | GGCTCAATCCAGCATGGTTGC | CCACACCTGCATGCATTGCAC |
| Bpev01.c0577.g0002.m0001 | CGTTCATGGTGGACCTGAGCC | AGCTAAAGTGAGGGACTTTGTCGAT |
| Bpev01.c0167.g0013.m0001 | CACAACATAGCCAGCCACCTTC | GTCGGTGCTACCCAAGGACTC |
| Bpev01.c0243.g0056.m0001 | CAACGGACGTTATGCCATGTTGG | TCCCAGCCGGCGGAATTAC |
| Bpev01.c0264.g0036.m0001 | CGGTGAGGCCGTGTGGTT | CGCCCATCAGGATGACCTGT |
| Bpev01.c1040.g0049.m0001 | CCCGAGACATTTGCCAAGAACC | GCCTTGAACCAGACTGCCTCTC |
| Bpev01.c0190.g0044.m0001 | CTTGGCCGGCGATGTGATC | GCCAACATGGCCCACCTC |
| Bpev01.c0621.g0012.m0001 | GCAATAGGCCTTGCCTCCTTCATAG | CGAGTACCCGTCTTCTCATTCGC |
| Bpev01.c0984.g0005.m0001 | GGGAGACTAAGGTACAAGCAGTGG | CCAGCTGCTCAATTGCTTCAGAG |
| Bpev01.c0401.g0006.m0001 | GGGAAGGCAAGGCTAGTGCAG | GCCTTCAACAGCAAGGCAAGT |
| Bpev01.c0894.g0003.m0001 | CCTCCAACAGGGAGTGGCAAC | CTGTCAATCATCCCAGAACAGCTTG |
| Bpev01.c1891.g0003.m0001 | GGCGTTCCGTATATGAGCCTCTTC | GGCTACTGCTGTTTTACCGGTCT |
| Correlation | Gs in WT (mol m−2 s−1) | Tr in WT (mmol m−2 s−1) | Gs in C11 (mol m−2 s−1) | Tr in C11 (mmol m−2 s−1) | Gs in yl (mol m−2 s−1) | Tr in yl (mmol m−2 s−1) |
|---|---|---|---|---|---|---|
| Tr in WT (mmol m−2 s−1) | 0.987 ** | - | - | - | - | - |
| Pn in WT (μmol m−2 s−1) | 0.860 ** | 0.915 ** | - | - | - | - |
| Tr in C11 (mmol m−2 s−1) | - | - | 0.986 ** | - | - | - |
| Pn in C11 (μmol m−2 s−1) | - | - | 0.856 ** | 0.895 ** | - | - |
| Tr in yl (mmol m−2 s−1) | - | - | - | - | 0.991 ** | - |
| Pn in yl (μmol m−2 s−1) | - | - | - | - | 0.773 ** | 0.815 ** |
| Gene | C11 and WT vs. yl |
|---|---|
| Bpev01.c0080.g0006.m0001 | normal |
| Bpev01.c0577.g0002.m0001 | normal |
| Bpev01.c0167.g0013.m0001 | down-regulated |
| Bpev01.c0243.g0056.m0001 | down-regulated |
| Bpev01.c0264.g0036.m0001 | down-regulated |
| Bpev01.c1040.g0049.m0001 | down-regulated |
| Bpev01.c0190.g0044.m0001 | down-regulated |
| Bpev01.c0621.g0012.m0001 | up-regulated |
| Bpev01.c0984.g0005.m0001 | up-regulated |
| Bpev01.c0401.g0006.m0001 | up-regulated |
| Bpev01.c0894.g0003.m0001 | up-regulated |
| Bpev01.c1891.g0003.m0001 | up-regulated |
| Gene | ID | Annotation | C11 and WT vs. yl | Mutant phenotype |
|---|---|---|---|---|
| Cga1 | Bpev01.c0051.g0036.m0001 | Cytokinin-responsive gata transcription factor1 | normal | yellow leaf |
| ClpC1 | Bpev01.c1202.g0038.m0001 | Chaperone protein ClpC | normal | chlorotic leaf |
| cpSRP43 | Bpev01.c1171.g0012.m0001 | Signal recognition particle 43 kDa protein | normal | yellow-green leaf |
| cpSRP54 | Bpev01.c1238.g0002.m0001 | Chloroplast signal recognition particle subunit | normal | yellow-green leaf |
| GRY79 | Bpev01.c0787.g0006.m0001 | Metallo-beta-lactamase | normal | yellow turn green |
| HAP3A | Bpev01.c0288.g0025.m0001 | Nuclear transcription factor Y | normal | pale-green leaf |
| NTRC | Bpev01.c0029.g0129.m0001 | NADPH-dependent thioredoxin reductase C | normal | light-green leaf |
| SGR | Bpev01.c0717.g0015.m0001 | Protein stay-green | normal | stay-green |
| YbeY | Bpev01.c1489.g0003.m0001 | YbeY endoribonuclease | normal | pale-green leaf |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gang, H.; Liu, G.; Chen, S.; Jiang, J. Physiological and Transcriptome Analysis of a Yellow-Green Leaf Mutant in Birch (Betula platyphylla × B. Pendula). Forests 2019, 10, 120. https://doi.org/10.3390/f10020120
Gang H, Liu G, Chen S, Jiang J. Physiological and Transcriptome Analysis of a Yellow-Green Leaf Mutant in Birch (Betula platyphylla × B. Pendula). Forests. 2019; 10(2):120. https://doi.org/10.3390/f10020120
Chicago/Turabian StyleGang, Huixin, Guifeng Liu, Su Chen, and Jing Jiang. 2019. "Physiological and Transcriptome Analysis of a Yellow-Green Leaf Mutant in Birch (Betula platyphylla × B. Pendula)" Forests 10, no. 2: 120. https://doi.org/10.3390/f10020120
APA StyleGang, H., Liu, G., Chen, S., & Jiang, J. (2019). Physiological and Transcriptome Analysis of a Yellow-Green Leaf Mutant in Birch (Betula platyphylla × B. Pendula). Forests, 10(2), 120. https://doi.org/10.3390/f10020120





