Herbaceous Vegetation Responses to Gap Size within Natural Disturbance-Based Silvicultural Systems in Northeastern Minnesota, USA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Study Design & Field Procedures
2.3. Statistical Analyses
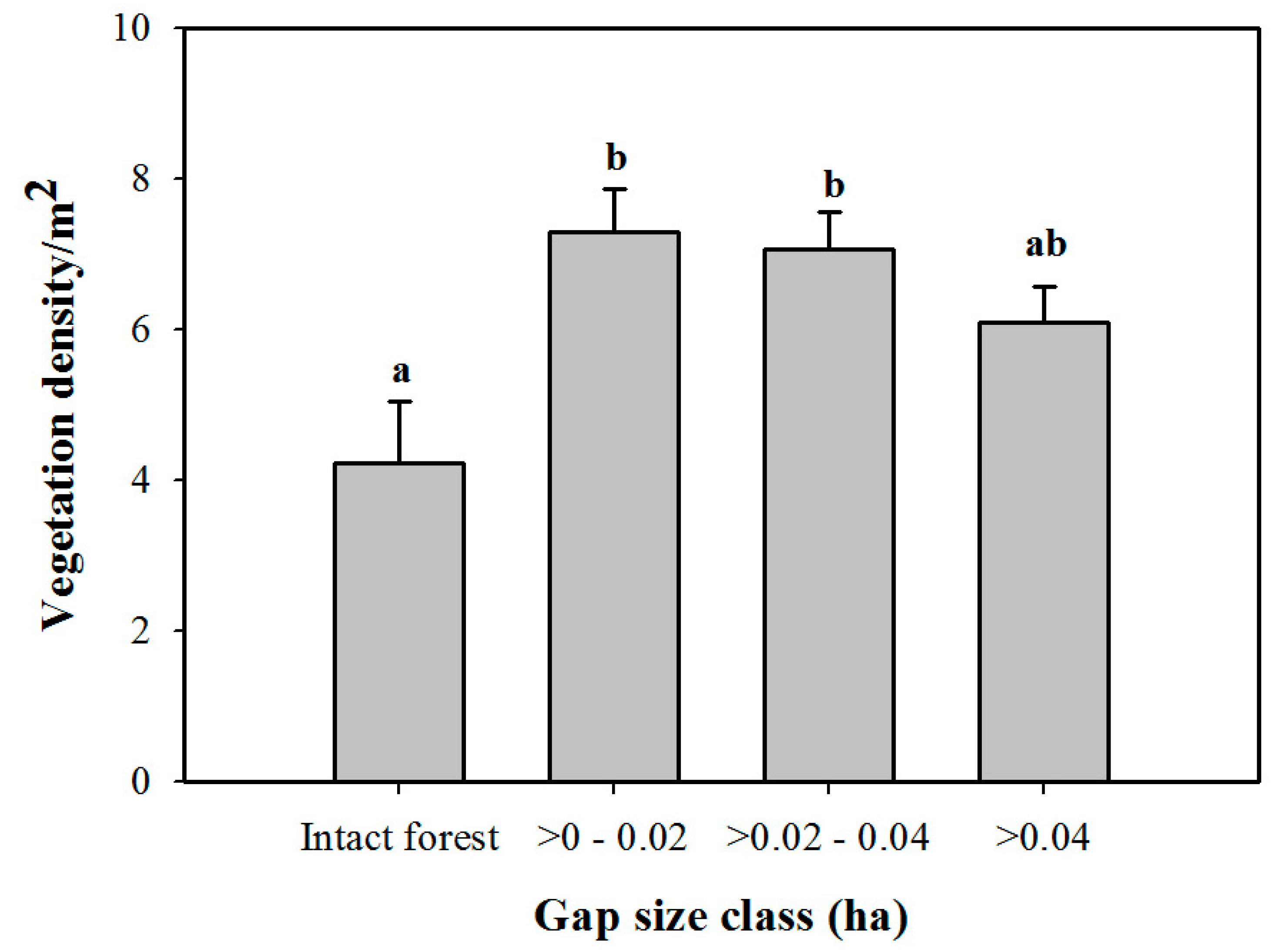
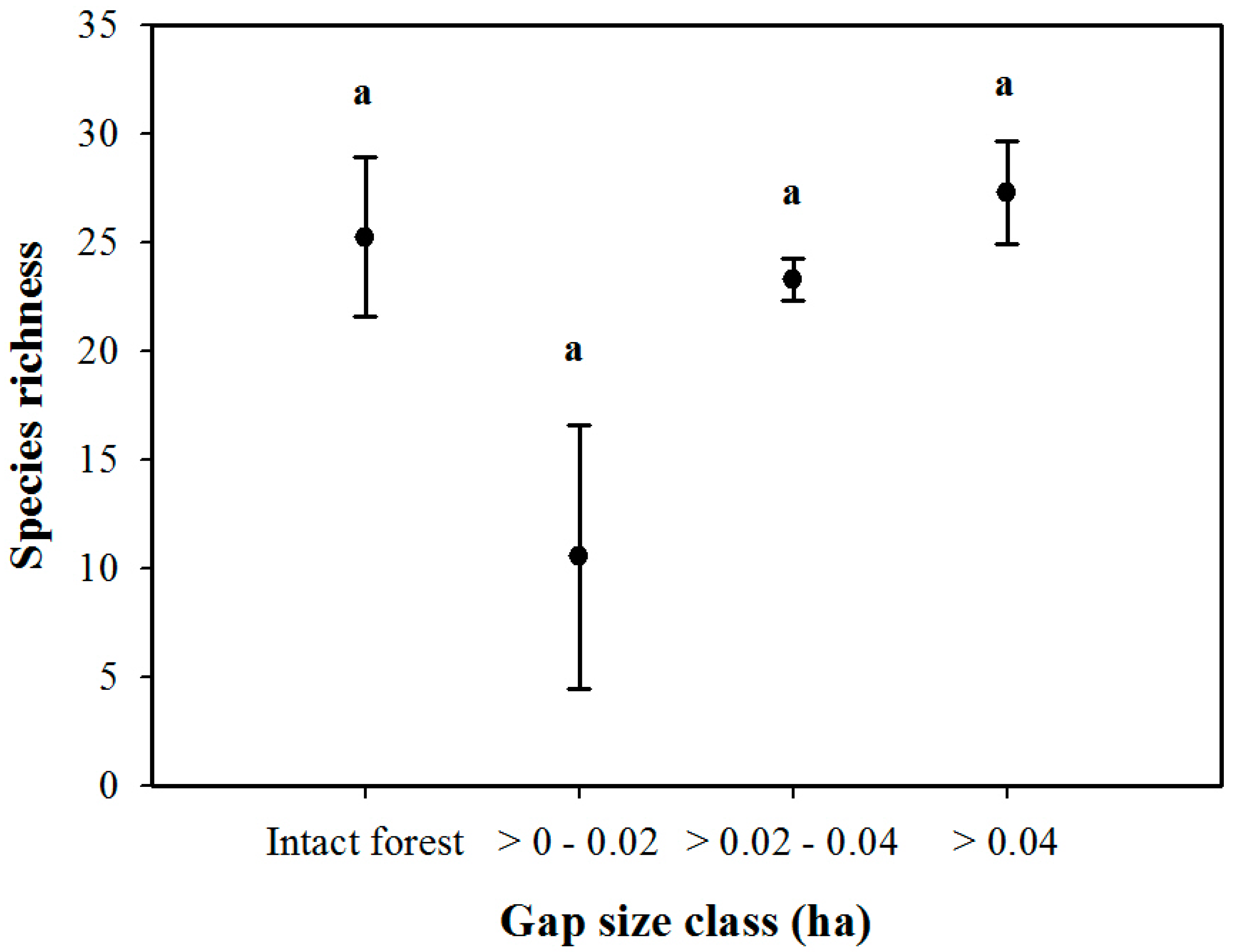
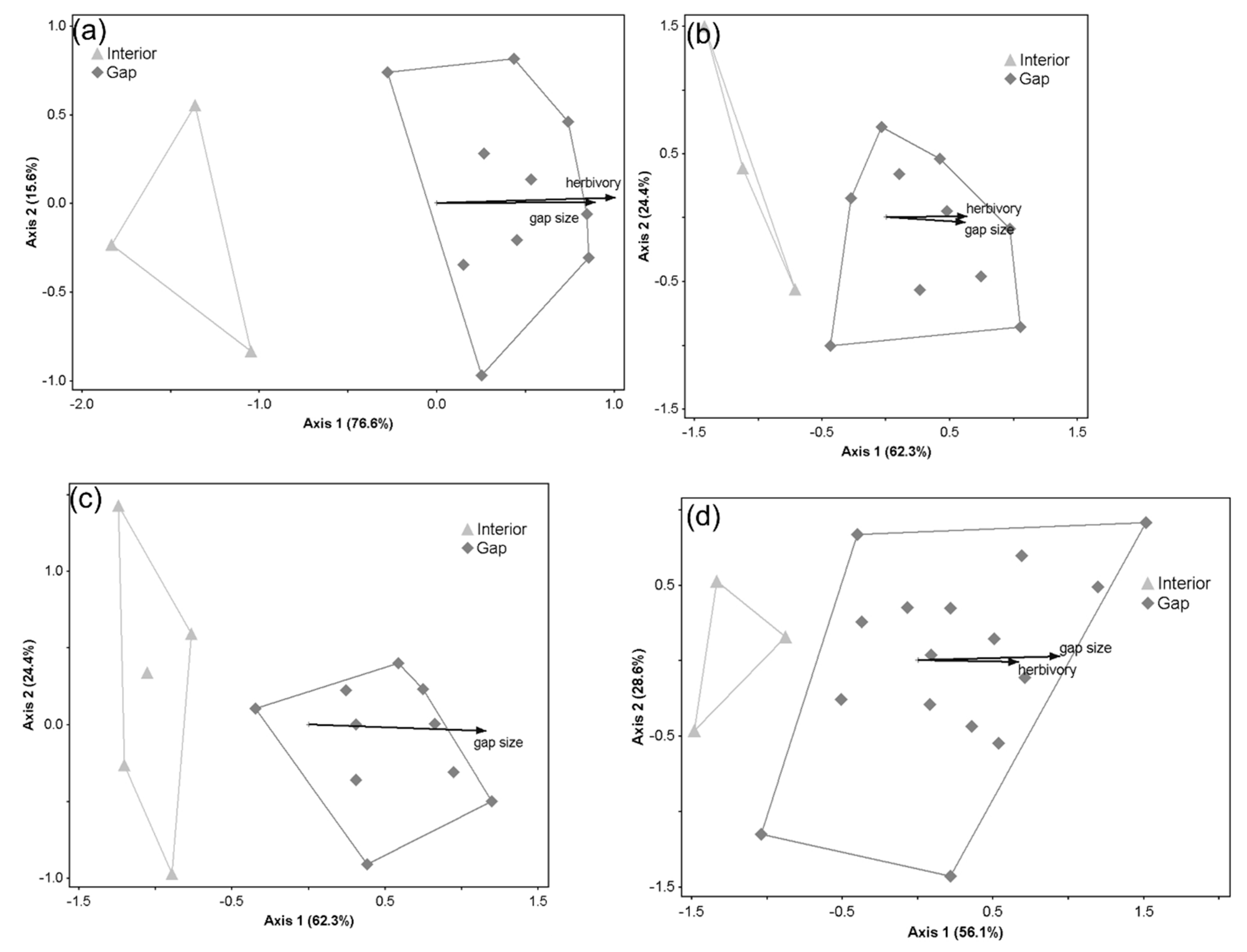
3. Results
3.1. Density and Diversity of Understory Vegetation
3.2. Patterns in Understory Plant Composition across Gap Conditions
4. Discussion
Understory Vegetation Responses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Whigham, D.F. Ecology of woodland herbs in temperate deciduous forests. Annu. Rev. Ecol. Syst. 2004, 35, 583–621. [Google Scholar] [CrossRef]
- Roberts, M.R.; Gilliam, F.S. Patterns and mechanisms of plant diversity in forested ecosystems: Implications for forest management. Ecol. Appl. 1995, 5, 969–977. [Google Scholar] [CrossRef]
- Hunter, M.L. Maintaining Biodiversity in Forest Ecosystems; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Goldberg, D.E.; Gross, K.L. Disturbance regimes of midsuccessional old fields. Ecology 1988, 69, 1677–1688. [Google Scholar] [CrossRef]
- Sousa, W.P. Intertidal mosaics: Patch size, propagule availability, and spatially variable patterns of succession. Ecology 1984, 65, 1918–1935. [Google Scholar] [CrossRef]
- White, P.S.; Pickett, S.T.A. Natural Disturbance and Patch Dynamics: An Introduction; Academic: New York, NY, USA, 1985. [Google Scholar]
- Fries, C.; Johansson, O.; Pettersson, B.; Simonsson, P. Silvicultural models to maintain and restore natural stand structures in Swedish boreal forests. For. Ecol. Manag. 1997, 94, 89–103. [Google Scholar] [CrossRef]
- Seymour, R.S.; White, A.S.; de Maynadier, P.G. Natural disturbance regimes in northeastern North America—Evaluating silvicultural systems using natural scales and frequencies. For. Ecol. Manag. 2002, 155, 357–367. [Google Scholar] [CrossRef]
- Schumann, M.E.; White, A.S.; Witham, J.W. The effects of harvest-created gaps on plant species diversity, composition, and abundance in a Maine oak-pine forest. For. Ecol. Manag. 2003, 176, 543–561. [Google Scholar] [CrossRef]
- Smith, K.J.; Keeton, W.S.; Twery, M.J.; Tobi, D.R. Understory plant responses to uneven-aged forestry alternatives in northern hardwoodconifer forests. Can. J. For. Res. 2008, 38, 1303–1318. [Google Scholar] [CrossRef]
- Falk, K.J.; Burke, D.M.; Elliott, K.A.; Holmes, S.B. Effects of single-tree and group selection harvesting on the diversity and abundance of spring forest herbs in deciduous forests in southwestern Ontario. For. Ecol. Manag. 2008, 255, 2486–2494. [Google Scholar] [CrossRef]
- Frelich, L.E.; Lorimer, C.G. Natural disturbance regimes in hemlock-hardwood forests of the upper Great-Lakes region. Ecol. Monogr. 1991, 61, 145–164. [Google Scholar] [CrossRef]
- Zhang, Q.; Pregitzer, K.S.; Reed, D.D. Catastrophic disturbance in the presettlement forests of the Upper Peninsula of Michigan. Can. J. For. Res. 1999, 29, 106–114. [Google Scholar] [CrossRef]
- Webb, S.L.; Scanga, S.E. Windstorm disturbance without patch dynamics: Twelve years of change in a Minnesota forest. Ecology 2001, 82, 893–897. [Google Scholar] [CrossRef]
- Schulte, L.A.; Mladenoff, D.J. Severe wind and fire regimes in northern forests: Historical variability at the regional scale. Ecology 2005, 86, 431–445. [Google Scholar] [CrossRef]
- Bazzaz, F.A. The physiological ecology of plant succession. Annu. Rev. Ecol. Syst. 1979, 10, 351–371. [Google Scholar] [CrossRef]
- Sipe, T.W.; Bazzaz, F.A. Gap Partitioning Among Maples (Acer) in Central New England: Survival and Growth. Ecology 1995, 76, 1587–1602. [Google Scholar] [CrossRef]
- Busing, R.T.; White, P.S. Species diversity and small-scale disturbance in an old-growth temperate forest: A consideration of gap partitioning concepts. Oikos 1997, 78, 562–568. [Google Scholar] [CrossRef]
- Beatty, S.W. Influence of microtopography and canopy species on spatial patterns of forest understory plants. Ecology 1984, 65, 1406–1419. [Google Scholar] [CrossRef]
- Zenner, E.K.; Kabrick, J.M.; Jensen, R.G.; Peck, J.L.E.; Grabner, J.K. Responses of ground flora to a gradient of harvest intensity in the Missouri Ozarks. For. Ecol. Manag. 2006, 222, 326–334. [Google Scholar] [CrossRef]
- Peterson, C.J.; Carson, W.P.; McCarthy, B.C.; Pickett, S.T.A. Microsite variation and soil dynamics within newly created treefall pits and mounds. Oikos 1990, 58, 39–46. [Google Scholar] [CrossRef]
- Naaf, T.; Wulf, M. Effects of gap size, light and herbivory on the herb layer vegetation in European beech forest gaps. For. Ecol. Manag. 2007, 244, 141–149. [Google Scholar] [CrossRef]
- Anderson, K.L.; Leopold, D.J. The role of canopy gaps in maintaining vascular plant diversity at a forested wetland in New York State. J. Torrey Bot. Soc. 2002, 129, 238–250. [Google Scholar] [CrossRef]
- Goldblum, D. The effects of treefall gaps on understory vegetation in New York State. J. Veg. Sci. 1997, 8, 125–132. [Google Scholar] [CrossRef]
- Royo, A.A.; Collins, R.; Adams, M.B.; Kirschbaum, C.; Carson, W.P. Pervasive interactions between ungulate browsers and disturbance regimes promote temperate forest herbaceous diversity. Ecology 2010, 91, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Kern, C.C.; Montgomery, R.A.; Reich, P.B.; Strong, T.F. Harvest-created canopy gaps increase species and functional trait diversity of the forest ground-layer communiiy. For. Sci. 2014, 60, 335–344. [Google Scholar] [CrossRef]
- Metzger, F.; Schultz, J. Understory response to 50 years of management of a northern hardwood forest in upper Michigan. Am. Midl. Nat. 1984, 112, 209–223. [Google Scholar] [CrossRef]
- Shields, J.M.; Webster, C.R. Ground-layer response to group selection with legacy-tree retention in a managed northern hardwood forest. Can. J. For. Res. 2007, 37, 1797–1807. [Google Scholar] [CrossRef]
- Schoonmaker, P.; McKee, A. Species composition and diversity during secondary succession of coniferous forests in the western Cascade Mountains of Oregon. For. Sci. 1988, 34, 960–979. [Google Scholar]
- Kern, C.C.; Montgomery, R.A.; Reich, P.B.; Strong, T.F. Canopy gap size influences niche partitioning of the ground-layer plant community in a northern temperate forest. J. Plant Ecol. 2013, 6, 101–112. [Google Scholar] [CrossRef]
- Kern, C.C.; Burton, J.I.; Raymond, P.; D’Amato, A.W.; Keeton, W.S.; Royo, A.A.; Walters, M.B.; Webster, C.R.; Willis, J.L. Challenges facing gap-based silviculture and possible solutions for mesic northern forests in North America. For. Int. J. For. Res. 2017, 90, 4–17. [Google Scholar] [CrossRef]
- Holling, C.S. Resilience and stability of ecological systems. Annu. Rev. Ecol. Syst. 1973, 4, 1–23. [Google Scholar] [CrossRef]
- Bauhus, J.; Puettmann, K.; Messier, C. Silviculture for old-growth attributes. For. Ecol. Manag. 2009, 258, 525–537. [Google Scholar] [CrossRef]
- Webster, C.R.; Dickinson, Y.L.; Burton, J.I.; Frelich, L.E.; Jenkins, M.A.; Kern, C.C.; Raymond, P.; Saunders, M.R.; Walters, M.B.; Willis, J.L. Promoting and maintaining diversity in contemporary hardwood forests: Confronting contemporary drivers of change and the loss of ecological memory. For. Ecol. Manag. 2018, 421, 98–108. [Google Scholar] [CrossRef]
- Fahey, R.T.; Alveshere, B.C.; Burton, J.I.; D’Amato, A.W.; Dickinson, Y.L.; Keeton, W.S.; Kern, C.C.; Larson, A.J.; Palik, B.J.; Puettmann, K.J. Shifting conceptions of complexity in forest management and silviculture. For. Ecol. Manag. 2018, 421, 59–71. [Google Scholar] [CrossRef]
- Moore, M.R.; Vankat, J.L. Responses of the herb layer to the gap dynamics of a mature beech-maple forest. Am. Midl. Nat. 1986, 115, 336–347. [Google Scholar] [CrossRef]
- Hobbs, H.C.; Goebel, J.E. Geologic Map of Minnesota, Quarternary Geology (Scale 1:500,000); Minnesota Geologcial Survey, University of Minnesota: Minneapolis, MN, USA, 1982. [Google Scholar]
- Donoso, P.J.; Nyland, R.D. Interference to hardwood regeneration in northeastern North America: The effects of raspberries (Rubus spp.) following clearcutting and shelterwood methods. North. J. Appl. For. 2006, 23, 288–296. [Google Scholar]
- Kern, C.C.; D’Amato, A.W.; Strong, T.F. Diversifying the composition and structure of managed, late-successional forests with harvest gaps: What is the optimal gap size? For. Ecol. Manag. 2013, 304, 110–120. [Google Scholar] [CrossRef]
- SAS Institute. SAS Version 9.1; SAS Institute: Cary, NC, USA, 2008. [Google Scholar]
- McCune, B.; Grace, J. MRPP (Multi-Response Permutation Procedures) and Related Techniques; MjM Software Design: Gleneden Beach, OR, USA, 2002; pp. 188–197. [Google Scholar]
- McCune, B.; Mefford, M. PC-ORD: Multivariate Analysis of Ecological Data; Version 4 for Windows; [User’s Guide]; MjM Software Design: Gleneden Beach, OR, USA, 1999. [Google Scholar]
- Collins, B.S.; Pickett, S.T.A. Demographic responses of herb layer species to experimental canopy gaps in a northern hardwoods forest. J. Ecol. 1988, 76, 437–450. [Google Scholar] [CrossRef]
- Crow, T.R.; Buckley, D.S.; Nauertz, E.A.; Zasada, J.C. Effects of management on the composition and structure of northern hardwood forests in Upper Michigan. For. Sci. 2002, 48, 129–145. [Google Scholar]
- Scheller, R.M.; Mladenoff, D.J. Understory species patterns and diversity in old-growth and managed northern hardwood forests. Ecol. Appl. 2002, 12, 1329–1343. [Google Scholar] [CrossRef]
- Bolton, N.W.; D’Amato, A.W. Regeneration responses to gap size and coarse woody debris within natural disturbance-based silvicultural systems in northeastern Minnesota, USA. For. Ecol. Manag. 2011, 262, 1215–1222. [Google Scholar] [CrossRef]
- USDA National Plant Data Team. The PLANTS Database; National Plant Data Team: Greensboro, NC, USA, 2015. Available online: http://plants.usda.gov (accessed on 15 May 2011).
- Sumners, W.H.; Archibold, O.W. Exotic plant species in the southern boreal forest of Saskatchewan. For. Ecol. Manag. 2007, 251, 156–163. [Google Scholar] [CrossRef]
- Berger, A.L.; Puettmann, K.J.; Host, G.E. Harvesting impacts on soil and understory vegetation: The influence of season of harvest and within-site disturbance patterns on clear-cut aspen stands in Minnesota. Can. J. For. Res. 2004, 34, 2159–2168. [Google Scholar] [CrossRef]
- Buckley, D.S.; Crow, T.R.; Nauertz, E.A.; Schulz, K.E. Influence of skid trails and haul roads on understory plant richness and composition in managed forest landscapes in Upper Michigan, USA. For. Ecol. Manag. 2003, 175, 509–520. [Google Scholar] [CrossRef]
- Heimann, B.; Cussans, G.W. The importance of seeds and sexual reproduction in the population biology of Cirsium arvense-a literature review. Weed Res. 1996, 36, 493–503. [Google Scholar] [CrossRef]
- Wilson, R.G., Jr. Germination and seedling development of Canada thistle (Cirsium arvense). Weed Sci. 1979, 27, 146–151. [Google Scholar]
- Mladenoff, D.J. The relationship of the soil seed bank and understory vegetation in old-growth northern hardwood-hemlock treefall gaps. Can. J. Bot. 1990, 68, 2714–2721. [Google Scholar] [CrossRef]
- Ehrenfeld, J.G. Understory response to canopy gaps of varying size in a mature oak forest. Bull. Torrey Bot. Club 1980, 107, 29–41. [Google Scholar] [CrossRef]
- Anderson, M.C. Studies of the Woodland Light Climate: II. Seasonal Variation in the Light Climate. J. Ecol. 1964, 52, 643–663. [Google Scholar] [CrossRef]
- Davison, S.E.; Forman, R.T.T. Herb and shrub dynamics in a mature oak forest: A thirty-year study. Bull. Torrey Bot. Club 1982, 109, 64–73. [Google Scholar] [CrossRef]
- Meier, A.J.; Bratton, S.P.; Duffy, D.C. Possible ecological mechanisms for loss of vernal-herb diversity in logged eastern deciduous forests. Ecol. Appl. 1995, 5, 935–946. [Google Scholar] [CrossRef]
- Whitney, G.G. The productivity and carbohydrate economy of a developing stand of Rubus idaeus. Can. J. Bot. 1982, 60, 2697–2703. [Google Scholar] [CrossRef]
- Hibbs, D.E. Gap dynamics in a hemlock-hardwood forest. Can. J. For. Res. 1982, 12, 538–544. [Google Scholar] [CrossRef]
- Webster, C.R.; Lorimer, C.G. Minimum opening sizes for canopy recruitment of midtolerant tree species: A retrospective approach. Ecol. Appl. 2005, 15, 1245–1262. [Google Scholar] [CrossRef]
- Fahey, R.T.; Puettmann, K.J. Patterns in spatial extent of gap influence on understory plant communities. For. Ecol. Manag. 2008, 255, 2801–2810. [Google Scholar] [CrossRef]
- Burton, J.I.; Zenner, E.K.; Frelich, L.E.; Cornett, M.W. Patterns of plant community structure within and among primary and second-growth northern hardwood forest stands. For. Ecol. Manag. 2009, 258, 2556–2568. [Google Scholar] [CrossRef]
| Site | Lat/Long | Harvest Year | Elevation (m) | Aspect | Slope | Soils | Canopy Composition † (% Basal Area) | Number of Gaps | Gap Size Range (ha) |
|---|---|---|---|---|---|---|---|---|---|
| Big Pine (BP) | (47.47, −91.15) | 2003 | 487 | 162° | 8% | Loam | Acer saccharum Marshall: 84% Betula alleghaniensis Britt.: 3% Betula papyrifera Marshall: 3% Thuja occidentalis L.: 9% | 10 | (0.024–0.066) |
| Birch Cut (BC) | (47.45, −91.19) | 2002 | 455 | 119° | 6% | Loam | Acer saccharum: 89% Betula alleghaniensis: 3% Acer rubrum L.: 3% Picea glauca (Moench) Voss: 3% Thuja occidentalis: 3% | 10 | (0.021–0.071) |
| Power Line (PL) | (47.34, −91.20) | 2003 | 385 | 142° | 10% | Fine loam | Acer saccharum: 74% Fraxinus nigra Marshall: 24% Populus grandidentata Michaux: 2% | 10 | (0.011–0.069) |
| Schoolhouse (SH) | (47.46, −91.20) | 2002 | 478 | 327° | 2% | Loam | Acer saccharum: 63% Betula alleghaniensis: 15% Picea glauca: 15% Thuja occidentalis: 7% | 16 | (0.008–0.050) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolton, N.W.; D’Amato, A.W. Herbaceous Vegetation Responses to Gap Size within Natural Disturbance-Based Silvicultural Systems in Northeastern Minnesota, USA. Forests 2019, 10, 111. https://doi.org/10.3390/f10020111
Bolton NW, D’Amato AW. Herbaceous Vegetation Responses to Gap Size within Natural Disturbance-Based Silvicultural Systems in Northeastern Minnesota, USA. Forests. 2019; 10(2):111. https://doi.org/10.3390/f10020111
Chicago/Turabian StyleBolton, Nicholas W., and Anthony W. D’Amato. 2019. "Herbaceous Vegetation Responses to Gap Size within Natural Disturbance-Based Silvicultural Systems in Northeastern Minnesota, USA" Forests 10, no. 2: 111. https://doi.org/10.3390/f10020111
APA StyleBolton, N. W., & D’Amato, A. W. (2019). Herbaceous Vegetation Responses to Gap Size within Natural Disturbance-Based Silvicultural Systems in Northeastern Minnesota, USA. Forests, 10(2), 111. https://doi.org/10.3390/f10020111





