Pine Pitch Canker (PPC): Pathways of Pathogen Spread and Preventive Measures
Abstract
1. Introduction
2. Biology and Ecology of the Pathogen
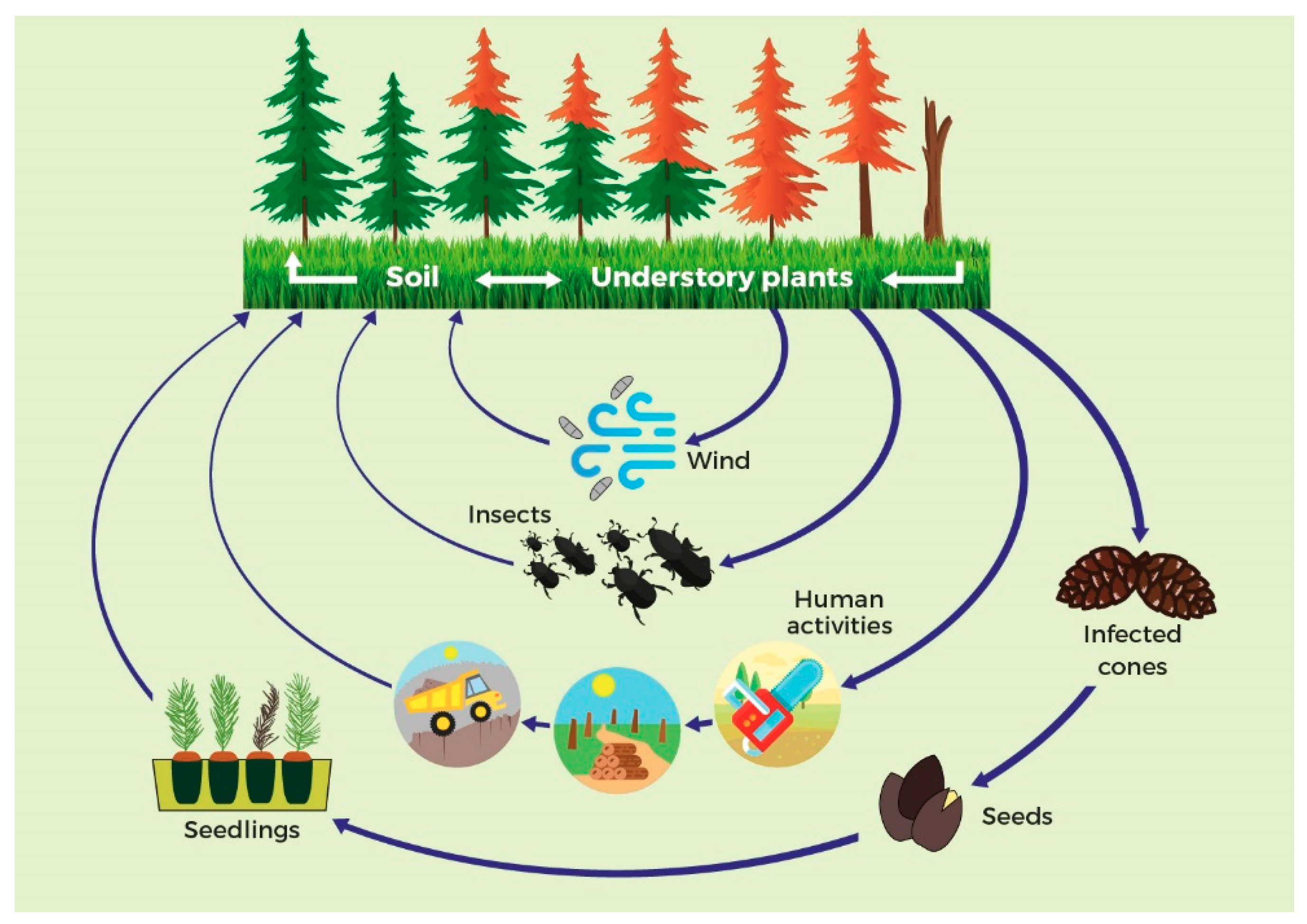
3. Pathways of Spread of the PPC Pathogen
3.1. Natural Spread
3.1.1. Weather Conditions
3.1.2. Disease Vectoring
3.2. Human-Assisted Spread
3.2.1. Seeds and Plants for Planting
3.2.2. Wood and Bark
3.2.3. Dispersion Via Soil
3.2.4. Other Pathways of Spread
4. Pathway-Specific Preventive Measures
4.1. Measures to Suppress Natural Spread
4.1.1. Species Selection and Diversity
4.1.2. Biocontrol Strategies
4.1.3. Management of Insect Vectors
4.2. Measures to Prevent Human-Assisted Spread
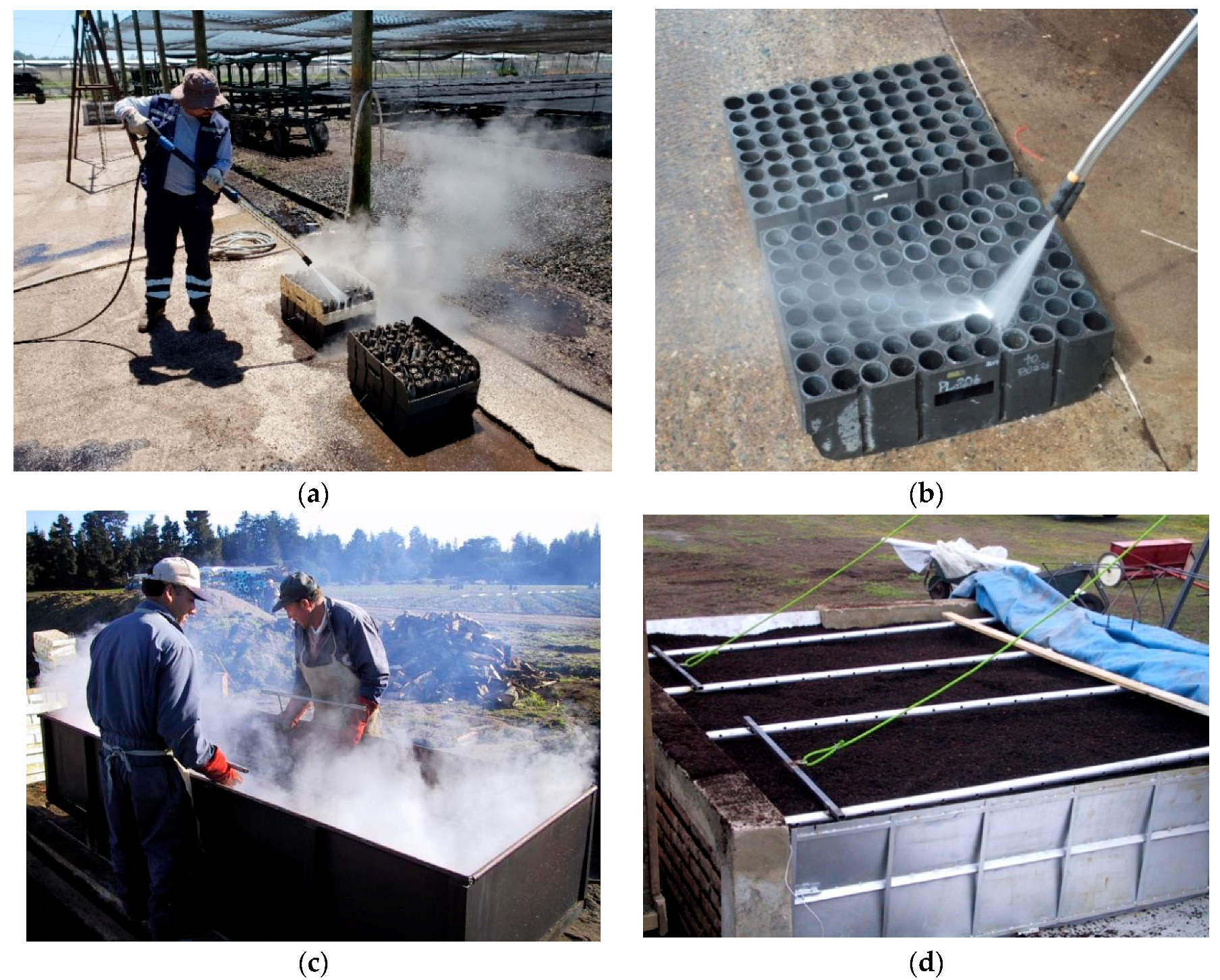
4.2.1. Good Nursery Practices
4.2.2. Early and Accurate Detection of the Pathogen
4.2.3. Preventive Silvicultural Treatments
4.2.4. Legislation Based on the Current Specific Knowledge
5. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hulme, P.E. Trade, transport and trouble: Managing invasive species pathways in an era of globalization. J. Appl. Ecol. 2009, 46, 10–18. [Google Scholar] [CrossRef]
- Santini, A.; Liebhold, A.; Migliorini, D.; Woodward, S. Tracing the role of human civilization in the globalization of plant pathogens. ISME J. 2018, 12, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Stenlid, J.; Oliva, J.; Boberg, J.B.; Hopkins, A.J.M. Emerging Diseases in European Forest Ecosystems and Responses in Society. Forests 2011, 2, 486–504. [Google Scholar] [CrossRef]
- Santini, A.; Ghelardini, L.; De Pace, C.; Desprez-Loustau, M.L.; Capretti, P.; Chandelier, A.; Cech, T.; Chira, D.; Diamandis, S.; Gaitniekis, T.; et al. Biogeographical patterns and determinants of invasion by forest pathogens in Europe. New Phytol. 2013, 197, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Brasier, C.M.; Gibbs, J.N. Origin of the Dutch elm disease epidemic in Britain. Nature 1973, 242, 607–609. [Google Scholar] [CrossRef]
- Anagnostakis, S.L. Chestnut Blight: The Classical Problem of an Introduced Pathogen. Mycologia 1987, 79, 23. [Google Scholar] [CrossRef]
- Wikler, K.; Gordon, T. An initial assessment of genetic relationships among populations of Fusarium circinatum in different parts of the world. Can. J. Bot. 2000, 78, 709–717. [Google Scholar]
- Drenkhan, R.; (Estonian University of Life Sciences, Tartu, Estonia); Martín-García, J.; (University of Valladolid, Palencia, Spain). Personal Communication, 2019.
- Correll, J.C.; Gordon, T.R.; McCain, A.H.; Fox, J.W.; Koehler, C.S.; Wood, D.L.; Schultz, M.E. Pitch Canker Disease in California: Pathogenicity, Distribution, and Canker Development on Monterey Pine (Pinus radiata). Plant Dis. 1991, 75, 676–682. [Google Scholar] [CrossRef]
- Aegerter, B.J.; Gordon, T.R. Rates of pitch canker induced seedling mortality among Pinus radiata families varying in levels of genetic resistance to Gibberella circinata (anamorph Fusarium circinatum). For. Ecol. Manag. 2006, 235, 14–17. [Google Scholar] [CrossRef]
- Sakamoto, J.M.; Gordon, T.R. Factors influencing infection of mechanical wounds by Fusarium circinatum on Monterey pines (Pinus radiata). Plant Pathol. 2006, 55, 130–136. [Google Scholar] [CrossRef]
- Storer, A.J.; Gordon, T.R.; Dallara, P.L.; Wood, D.L. Pitch canker kills pines, spreads to new species and regions. Calif. Agric. 1994, 48, 9–13. [Google Scholar]
- Aegerter, B.J.; Gordon, T.R.; Storer, A.J.; Wood, D.L. Pitch Canker: A Technical Review; University of California Agriculture and Natural Resources: Oakland, CA, USA, 2003; p. 13. [Google Scholar]
- Wingfield, M.J.; Hammerbacher, A.; Ganley, R.J.; Steenkamp, E.T.; Gordon, T.R.; Wingfield, B.D.; Coutinho, T.A. Pitch canker caused by Fusarium circinatum—A growing threat to pine plantations and forests worldwide. Australas. Plant Pathol. 2008, 37, 319–334. [Google Scholar] [CrossRef]
- Martínez-Álvarez, P.; Pando, V.; Diez, J.J. Alternative species to replace Monterey pine plantations affected by pitch canker caused by Fusarium circinatum in northern Spain. Plant Pathol. 2014, 63, 1086–1094. [Google Scholar] [CrossRef]
- OEPP/EPPO. Gibberella circinata. EPPO Bull. 2009, 39, 298–309. [Google Scholar] [CrossRef]
- Miller, T.; Bramlett, D.L. Damage to reproductive structures of slash pine by two seed-borne pathogens: Diplodia gossypina and Fusarium moniliforme var. subglutinans. In Flowering and Seed Development in Trees: A symposium; Bonner, F., Ed.; USDA: New Orleans, LA, USA, 1979; pp. 347–355. [Google Scholar]
- Barrows-Broaddus, J.B. Colonization of Cones and Seed of Loblolly Pine Following Inoculation with Fusarium subglutinans. Plant Dis. 1990, 74, 1002–1005. [Google Scholar] [CrossRef]
- Storer, A.J.; Gordon, T.R.; Clark, S.L. Association of the pitch canker fungus, Fusarium subglutinans f.sp. pini with Monterey pine seeds and seedlings in California. Plant Pathol. 1998, 47, 649–656. [Google Scholar] [CrossRef]
- Evira-Recuenco, M.; Iturritxa, E.; Raposo, R. Impact of seed transmission on the infection and development of pitch canker disease in Pinus radiata. Forests 2015, 6, 3353–3368. [Google Scholar] [CrossRef]
- Viljoen, A.; Wingfield, M.J.; Marasas, W.F.O. First Report of Fusarium subglutinans f. sp. pini on Pine Seedlings in South Africa. Plant Dis. 1994, 78, 309–312. [Google Scholar] [CrossRef]
- Martín-Rodrigues, N.; Sanchez-Zabala, J.; Salcedo, I.; Majada, J.; González-Murua, C.; Duñabeitia, M.K. New insights into radiata pine seedling root infection by Fusarium circinatum. Plant Pathol. 2015, 64, 1336–1348. [Google Scholar] [CrossRef]
- Swett, C.L.; Kirkpatrick, S.C.; Gordon, T.R. Evidence for a Hemibiotrophic Association of the Pitch Canker Pathogen Fusarium circinatum with Pinus radiata. Plant Dis. 2016, 100, 79–84. [Google Scholar] [CrossRef]
- Hepting, G.H.; Roth, E.R. Pitch canker, a new disease of some southern pines. J. For. 1946, 44, 742–744. [Google Scholar]
- Dwinell, L.D.; Phelps, W.R. Pitch Canker of Slash Pine in Florida. J. For. 1977, 75, 488–489. [Google Scholar] [CrossRef]
- Barrows-Broaddus, J.B.; Dwinell, L.D. Branch dieback and cone and seed infection caused by Fusarium moniliforme var. subglutinans in a loblolly pine seed orchard in South Carolina. Phytopathology 1985, 75, 1104–1108. [Google Scholar]
- Gordon, T.R.; Wikler, K.R.; Clark, S.L.; Okamoto, D.; Storer, A.J.; Bonello, P.; Gordon, T.R.; Wikler, K.R.; Clark, S.L.; Okamoto, D.; et al. Resistance to pitch canker disease, caused by Fusarium subglutinans f.sp. pini in Monterey pine (Pinus radiata). Plant Pathol. 1998, 47, 706–711. [Google Scholar]
- Gordon, T.R.; Storer, A.J.J.; Wood, D.L. The pitch canker epidemic in California. Plant Dis. 2001, 85, 1128–1139. [Google Scholar] [CrossRef]
- Gordon, T.R.; Kirkpatrick, S.C.; Petersen, J.C.; Friel, C.J. Potential diversity in vegetative compatibility groupings in the California population of Gibberella circinata. Mycol. Res. 2006, 110, 936–940. [Google Scholar] [CrossRef]
- Erbilgin, N.; Ritokova, G.; Gordon, T.R.; Wood, D.L.; Storer, A.J. Temporal variation in contamination of pine engraver beetles with Fusarium circinatum in native Monterey pine forests in California. Plant Pathol. 2008, 57, 1103–1108. [Google Scholar] [CrossRef]
- Dwinell, D. Global Distribution of the Pitch Canker Fungus. In Proceedings of the IMPACT Monterey Workshop, Monterey, CA, USA, 30 November–3 December 1999; Devey, M., Matheson, A., Gordon, T., Eds.; CSIRO Forestry and Forest Products, Kingston AC: Monterey, CA, USA, 1999; pp. 54–57. [Google Scholar]
- Landeras, E.; García, P.; Fernández, M.; Braña, M.; Pérez-Sierra, A.; León, M.; Abad-Campos, P.; Armengol, J. Outbreak of Pitch Canker Caused by Fusarium circinatum on Pinus spp. in Northern Spain. Plant Dis. 2005, 89, 1015. [Google Scholar] [CrossRef]
- Bragança, H.; Diogo, E.; Moniz, F.; Amaro, P. First Report of Pitch Canker on Pines Caused by Fusarium circinatum in Portugal. Plant Dis. 2009, 93, 1079. [Google Scholar] [CrossRef]
- Carlucci, A.; Colatruglio, L.; Frisullo, S. First report of pitch canker caused by Fusarium circinatum on Pinus halepensis and P. pinea in Apulia (Southern Italy). Plant Dis. 2007, 91, 1683. [Google Scholar] [CrossRef]
- EPPO. First Report of Gibberella circinata in France; EPPO: Paris, France, 2006; Volume 5. [Google Scholar]
- Cook, D.C.; Matheson, A.C. An estimate of the potential economic impact of pine pitch canker in Australia. Aust. For. 2008, 71, 107–112. [Google Scholar] [CrossRef]
- Carrasco, A.; Sanfuentes, E.; Durán, Á.; Valenzuela, S.; Carrasco, A.; Sanfuentes, E.; Durán, Á.; Valenzuela, S. Cancro resinoso del pino: ¿una amenaza potencial para las plantaciones de Pinus radiata en Chile? Gayana Bot. 2016, 73, 369–380. [Google Scholar] [CrossRef]
- Perrings, C.; Williamson, M.; Barbier, E.B.; Delfino, D.; Dalmazzone, S.; Shogren, J.; Simmons, P.; Watkinson, A. Biological Invasion Risks and the Public Good: An Economic Perspective. Conserv. Ecol. 2002, 6. [Google Scholar] [CrossRef]
- MAPAMA. Real Decreto 637/2006, de 26 de mayo, por el que se establece el programa nacional de erradicación y control del hongo Fusarium circinatum Niremberg et O’donnell. BOE 2006, 137, 22069–22073. [Google Scholar]
- MAPAMA. Real Decreto 65/2010, de 29 de enero, por el que se Modifica el Real Decreto 637/2006, de 26 de Mayo, por el que se Establece el Programa Nacional de Erradicación y Control del Hongo de las Coníferas “Fusarium circinatum” Niremberg et O´Donnell. BOE 2010, 44, 16157–16159. [Google Scholar]
- Branco, M.; Brockerhoff, E.G.; Castagneyrol, B.; Orazio, C.; Jactel, H. Host range expansion of native insects to exotic trees increases with area of introduction and the presence of congeneric native trees. J. Appl. Ecol. 2015, 52, 69–77. [Google Scholar] [CrossRef]
- Burgess, T.I.; Wingfield, M.J. Pathogens on the Move: A 100-Year Global Experiment with Planted Eucalypts. Bioscience 2017, 67, 14–25. [Google Scholar] [CrossRef]
- O’Donnell, K.; Cigelnik, E.; Nirenberg, H.I. Molecular Systematics and Phylogeography of the Gibberella fujikuroi Species Complex. Mycologia 1998, 90, 465. [Google Scholar] [CrossRef]
- Geiser, D.M.; Aoki, T.; Bacon, C.W.; Baker, S.E.; Bhattacharyya, M.K.; Brandt, M.E.; Brown, D.W.; Burgess, L.W.; Chulze, S.; Coleman, J.J.; et al. One Fungus, One Name: Defining the Genus Fusarium in a Scientifically Robust Way That Preserves Longstanding Use. Phytopathology 2013, 103, 400–408. [Google Scholar] [CrossRef]
- Herron, D.A.; Wingfield, M.J.; Wingfield, B.D.; Rodas, C.A.; Marincowitz, S.; Steenkamp, E.T. Novel taxa in the Fusarium fujikuroi species complex from Pinus spp. Stud. Mycol. 2015, 131–150. [Google Scholar] [CrossRef]
- Berbegal, M.; Pérez-Sierra, A.; Armengol, J.; Grünwald, N.J. Evidence for Multiple Introductions and Clonality in Spanish Populations of Fusarium circinatum. Phytopathology 2013, 103, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Britz, H.; Couhnho, T.A.; Gordon, T.R.; Wingfield, M.J. Characterisation of the pitch canker fungus, Fusarium circinatum, from Mexico. S. Afr. J. Bot. 2001, 67, 609–614. [Google Scholar] [CrossRef]
- Pérez-Sierra, A.; Landeras, E.; León, M.; Berbegal, M.; García-Jiménez, J.; Armengol, J. Characterization of Fusarium circinatum from Pinus spp. in northern Spain. Mycol. Res. 2007, 111, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Steenkamp, E.; Britz, H.; Coutinho, T.; Wingfield, B.; Marasas, W.; Wingfield, M. Molecular characterization of Fusarium subglutinans associated with mango malformation. Mol. Plant Pathol. 2000, 1, 187–193. [Google Scholar] [CrossRef]
- Viljoen, A.; Wingfield, M.J.; Gordon, T.R.; Marasas, W.F.O. Genotypic diversity in a South African population of the pitch canker fungus Fusarium subglutinans f.sp. pini. Plant Pathol. 1997, 46, 590–593. [Google Scholar] [CrossRef]
- Britz, H.; Wingfield, M.J.; Coutinho, T.A.; Marasas, W.F.O.; Leslie, J.F. Female fertility and mating type distribution in a south african population of Fusarium subglutinans f. sp. pini. Appl. Environ. Microbiol. 1998, 64, 2094–2095. [Google Scholar]
- Gordon, T.R.; Storer, A.J.; Okamoto, D. Population structure of the pitch canker pathogen, Fusarium subglutinans f. sp. pini, in California. Mycol. Res. 1996, 100, 850–854. [Google Scholar] [CrossRef]
- Britz, H.; Coutinho, T.A.; Wingfield, M.J.; Marasas, W.F.O.; Gordon, T.R.; Leslie, J.F. Fusarium subglutinans f. sp. pini Represents a Distinct Mating Population in the Gibberella fujikuroi Species Complex. Appl. Environ. Microbiol. 1999, 65, 1198–1201. [Google Scholar]
- Mcdonald, B.A.; Linde, C. Pathogen Population Genetics, Evolutionary Potential, and Durable Resistence. Annu. Rev. Phytopathol. 2002, 40, 349–379. [Google Scholar] [CrossRef]
- Gordon, T.R. Pitch canker disease of pines. Phytopathology 2006, 96, 657–659. [Google Scholar] [CrossRef]
- Barrows-Broaddus, J.; Dwinell, L.D. Histopathology of Fusarium moniliforme var. subglutinans in four species of southern pines. Phytopathology 1983, 73, 882–889. [Google Scholar]
- Swett, C.L.; Reynolds, G.J.; Gordon, T.R. Infection without wounding and symptomless shoot colonization of Pinus radiata by Fusarium circinatum, the cause of pitch canker. For. Pathol. 2018, 48, e12422. [Google Scholar] [CrossRef]
- Martín-Rodrigues, N.; Espinel, S.; Sanchez-Zabala, J.; Ortíz, A.; González-Murua, C.; Duñabeitia, M. Spatial and temporal dynamics of the colonization of Pinus radiata by Fusarium circinatum, of conidiophora development in the pith and of traumatic resin duct formation. New Phytol. 2013, 198, 1215–1227. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Adalia, E.J.; Flores-Pacheco, J.A.; Martínez-Álvarez, P.; Martín-García, J.; Fernández, M.; Diez, J.J. Effect of mycoviruses on the virulence of Fusarium circinatum and laccase activity. Physiol. Mol. Plant Pathol. 2016, 94, 8–15. [Google Scholar] [CrossRef]
- Gordon, T.R. Biology and management of Gibberella circinata, the cause of pitch canker in pines. In Control of Fusarium Diseases; Álves-Santos, F.M., Diez, J.J., Eds.; Research Sign Post: Kerala, India, 2011; pp. 217–232. [Google Scholar]
- Hernandez-Escribano, L.; Iturritxa, E.; Aragonés, A.; Mesanza, N.; Berbegal, M.; Raposo, R.; Elvira-Recuenco, M. Root Infection of Canker Pathogens, Fusarium circinatum and Diplodia sapinea, in Asymptomatic Trees in Pinus radiata and Pinus pinaster Plantations. Forests 2018, 9, 128. [Google Scholar] [CrossRef]
- Gordon, T.R.; Reynolds, G.J. Plasticity in plant-microbe interactions: A perspective based on the pitch canker pathosystem. Phytoparasitica 2017, 45, 1–8. [Google Scholar] [CrossRef]
- Swett, C.L.; Porter, B.; Fourie, G.; Steenkamp, E.T.; Gordon, T.R.; Wingfield, M.J. Association of the pitch canker pathogen Fusarium circinatum with grass hosts in commercial pine production areas of South Africa. South For. 2014, 76, 161–166. [Google Scholar] [CrossRef]
- Hernandez-Escribano, L.; Iturritxa, E.; Elvira-Recuenco, M.; Berbegal, M.; Campos, J.A.; Renobales, G.; Garcia, I.; Raposo, R. Herbaceous plants in the understory of a pitch canker-affected Pinus radiata plantation are endophytically infected with Fusarium circinatum. Fungal Ecol. 2018, 32, 65–71. [Google Scholar] [CrossRef]
- Swett, C.L.; Gordon, T.R. First Report of Grass Species (Poaceae) as Naturally Occurring Hosts of the Pine Pathogen Gibberella circinata. Plant Dis. 2012, 96, 908. [Google Scholar] [CrossRef]
- Gordon, T.R. Pitch Canker. In Infectious Forest Diseases; Gonthier, P., Nicolotti, G., Eds.; CABI: Wallingford, UK, 2013; pp. 376–391. [Google Scholar]
- Bezos, D.; Lomba, J.M.; Martinez-Alvarez, P.; Fernandez, M.; Diez, J.J. Effects of pruning in Monterrey pine plantations affected by Fusarium circinatum. For. Syst. 2012, 21, 481–488. [Google Scholar] [CrossRef]
- Blank, L.; Martín-García, J.; Bezos, D.; Vettraino, A.; Krasnov, H.; Lomba, J.; Fernández, M.; Diez, J.; Blank, L.; Martín-García, J.; et al. Factors Affecting the Distribution of Pine Pitch Canker in Northern Spain. Forests 2019, 10, 305. [Google Scholar] [CrossRef]
- Serra-Varela, J.M.; Alia, R.; Portoles, J.; Gonzalo, J.; Solino, M.; Grivet, D.; Raposo, R. Incorporating exposure to pitch canker disease to support management decisions of Pinus pinaster Ait. in the face of climate change. PLoS ONE 2017, 12, e0171549. [Google Scholar] [CrossRef] [PubMed]
- Watt, M.S.; Ganley, R.J.; Kriticos, D.J.; Manning, L.K. Dothistroma needle blight and pitch canker: The current and future potential distribution of two important diseases of Pinus species. Can. J. For. Res. 2011, 41, 412–424. [Google Scholar] [CrossRef]
- Möykkynen, T.; Capretti, P.; Pukkala, T. Modelling the potential spread of Fusarium circinatum, the causal agent of pitch canker in Europe. Ann. For. Sci. 2015, 72, 169–181. [Google Scholar] [CrossRef]
- Dvořák, M.; Janoš, P.; Botella, L.; Rotková, G.; Zas, R. Spore dispersal patterns of Fusarium circinatum on an infested monterey pine forest in North-Western Spain. Forests 2017, 8, 432. [Google Scholar] [CrossRef]
- Garbelotto, M.; Smith, T.; Schweigkofler, W. Variation in rates of spore deposition of Fusarium circinatum, the causal agent of pine pitch canker, over a 12-month-period at two locations in Northern California. Phytopathology 2008, 98, 137–143. [Google Scholar] [CrossRef]
- Enebak, S.A.; Stanosz, G.R. Responses of conifer species of the Great Lakes region of North America to inoculation with the pitch canker pathogen Fusarium circinatum. For. Pathol. 2003, 33, 333–338. [Google Scholar] [CrossRef]
- Mitchell, R.G.; Wingfield, M.J.; Hodge, G.R.; Steenkamp, E.T.; Coutinho, T.A. Selection of Pinus spp. in South Africa for tolerance to infection by the pitch canker fungus. New For. 2012, 43, 473–489. [Google Scholar] [CrossRef]
- Vivas, M.; Zas, R.; Solla, A. Screening of maritime pine (Pinus pinaster) for resistance to Fusarium circinatum, the causal agent of pitch canker disease. Forestry 2012, 85, 185–192. [Google Scholar] [CrossRef]
- Elvira-Recuenco, M.; Iturritxa, E.; Majada, J.; Alia, R.; Raposo, R. Adaptive Potential of Maritime Pine (Pinus pinaster) Populations to the Emerging Pitch Canker Pathogen, Fusarium circinatum. PLoS ONE 2014, 9, e114971. [Google Scholar] [CrossRef]
- Iturritxa, E.; Ganley, R.J.; Raposo, R.; García-Serna, I.; Mesanza, N.; Kirkpatrick, S.C.; Gordon, T.R. Resistance levels of Spanish conifers against Fusarium circinatum and Diplodia pinea. For. Pathol. 2013, 43, 488–495. [Google Scholar] [CrossRef]
- EPPO. EPPO Reporting Service No. 8; EPPO: Paris, France, 2019. [Google Scholar]
- González-Penalta, B.; Pintos-Varela, C.; Mansilla, J.P.; Aguín, O.; Pérez, R. Presencia de especies de Fusarium sobre semillas de Pinus spp. en Galicia. Soc. Española Cienc. For. 2008, 26, 149–154. [Google Scholar]
- Coutinho, T.A.; Steenkamp, E.T.; Mongwaketsi, K.; Wilmot, M.; Wingfield, M.J. First outbreak of pitch canker in a South African pine plantation. Australas. Plant Pathol. 2007, 36, 256–261. [Google Scholar] [CrossRef]
- Ganley, R.J.; Watt, M.S.; Manning, L.; Iturritxa, E. A global climatic risk assessment of pitch canker disease. Can. J. For. Res. 2009, 39, 2246–2256. [Google Scholar] [CrossRef]
- Deacon, J.W. Fungal Biology; Blackwell Pub: Malden, MA, USA, 2006. [Google Scholar]
- Inman, A.R.; Kirkpatrick, S.C.; Gordon, T.R.; Shaw, A.V. Limiting effects of low temperature on growth and spore germination in Gibberella circinata, the cause of pitch canker in pine species. Plant Dis. 2008, 92, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, B.; Berry, P.; Moulia, B. Review: Wind impacts on plant growth, mechanics and damage. Plant Sci. 2016, 245, 94–118. [Google Scholar] [CrossRef]
- Romon, P.; Carlos Iturrondobeitia, J.; Gibson, K.; Lindgren, B.S.; Goldarazena, A. Quantitative association of bark beetles with pitch canker fungus and effects of verbenone on their semiochemical communication in monterey pine forests in Northern Spain. Environ. Entomol. 2007, 36, 743–750. [Google Scholar] [CrossRef]
- Bezos, D.; Martinez-Alvarez, P.; Diez, J.J.; Fernandez, M.M. The pine shoot beetle Tomicus piniperda as a plausible vector of Fusarium circinatum in northern Spain. Ann. For. Sci. 2015, 72, 1079–1088. [Google Scholar] [CrossRef]
- Bezos, D.; Martínez-Álvarez, P.; Sanz-Ros, A.; Martín-García, J.; Fernandez, M.; Diez, J.; Bezos, D.; Martínez-Álvarez, P.; Sanz-Ros, A.V.; Martín-García, J.; et al. Fungal Communities Associated with Bark Beetles in Pinus radiata Plantations in Northern Spain Affected by Pine Pitch Canker, with Special Focus on Fusarium Species. Forests 2018, 9, 698. [Google Scholar] [CrossRef]
- Lombardero, M.J.; Solla, A.; Ayres, M.P. Pine defenses against the pitch canker disease are modulated by a native insect newly associated with the invasive fungus. For. Ecol. Manag. 2019, 437, 253–262. [Google Scholar] [CrossRef]
- Bezos, D.; Martinez-Alvarez, P.; Fernandez, M.; Diez, J.J. Epidemiology and management of pine pitch canker disease in Europe—A review. Balt. For. 2017, 23, 279–293. [Google Scholar]
- Webber, J.F. Experimental studies on factors influencing the transmission of Dutch elm disease. Investig. Agrar. Sist. Recur. For. 2004, 13, 197–205. [Google Scholar]
- Haack, R.A.; Lawrence, R.K.; Heaton, G.C. Seasonal Shoot-Feeding by Tomicus piniperda (Coleoptera: Scolytidae) in Michigan. Great Lakes Entomol. 2000, 33, 10. [Google Scholar]
- Wood, D.L.; Koerber, T.W.; Scharpf, R.F.; Storer, A.J. Pests of the Native California Conifers; University of California Press: Berkeley, CA, USA, 2003. [Google Scholar]
- Storer, A.J.; Wood, D.L.; Gordon, T.R. Twig beetles, Pityophthorus spp. (Coleoptera: Scolytidae), as vectors of the pitch canker pathogen in California. Can. Entomol. 2004, 136, 685–693. [Google Scholar] [CrossRef]
- Sakamoto, J.M.; Gordon, T.R.; Storer, A.J.; Wood, D.L. The role of Pityophthorus spp. as vectors of pitch canker affecting Pinus radiata. Can. Entomol. 2007, 139, 864–871. [Google Scholar] [CrossRef]
- Siegert, N.W.; McCullough, D.G. Preference of Tomicus piniperda (Coleoptera: Scolytidae) parent adults and shoot-feeding progeny adults for three pine species. Can. Entomol. 2001, 133, 343–353. [Google Scholar] [CrossRef]
- Bonello, P.; McNee, W.R.; Storer, A.J.; Wood, D.L.; Gordon, T.R. The role of olfactory stimuli in the location of weakened hosts by twig-infesting Pityophthoros spp. Ecol. Entomol. 2001, 26, 8–15. [Google Scholar] [CrossRef]
- Fernández-Fernández, M.; Naves, P.; Musolin, D.L.; Selikhovkin, A.V.; Cleary, M.; Chira, D.; Paraschiv, M.; Gordon, T.; Solla, A.; Papazova-Anakieva, I.; et al. Pine Pitch Canker and Insects: Regional Risks, Environmental Regulation, and Practical Management Options. Forests 2019, 10, 649. [Google Scholar] [CrossRef]
- Fernández-Fernández, M.; Naves, P.; Witzell, J.; Musolin, D.L.; Selikhovkin, A.V.; Paraschiv, M.; Chira, D.; Martínez-Álvarez, P.; Martín-García, J.; Muñoz-Adalia, E.J.; et al. Pine Pitch Canker and Insects: Relationships and Implications for Disease Spread in Europe. Forests 2019, 10, 627. [Google Scholar] [CrossRef]
- Brockerhoff, E.; Dick, M.; Ganley, R.; Roques, A.; Storer, A. Role of insect vectors in epidemiology and invasion risk of Fusarium circinatum, and risk assessment of biological control of invasive Pinus contorta. Biol. Invasions 2016, 18, 1177–1190. [Google Scholar] [CrossRef]
- Eschen, R.; Douma, J.C.; Grégoire, J.; Mayer, F.; Rigaux, L.; Potting, R.P.J. A risk categorisation and analysis of the geographic and temporal dynamics of the European import of plants for planting. Biol. Invasions 2017, 19, 3243–3257. [Google Scholar] [CrossRef]
- Eschen, R.; Britton, K.; Brockerhoff, E.; Burgess, T.; Dalley, V.; Epanchin-Niell, R.S.; Gupta, K.; Hardy, G.; Huang, Y.; Kenis, M.; et al. International variation in phytosanitary legislation and regulations governing importation of plants for planting. Environ. Sci. Policy 2015, 51, 228–237. [Google Scholar] [CrossRef]
- Carey, W.A.; Oak, S.W.; Enebak, S.A. Pitch canker ratings of longleaf pine clones correlate with Fusarium circinatum infestation of seeds and seedling mortality in containers. For. Pathol. 2005, 35, 205–212. [Google Scholar] [CrossRef]
- Storer, A.J.; Wood, D.L.; Wikler, K.R.; Gordon, T.R. Association between a native spittlebug (Homoptera: Cercopidae) on Monterey pine and an introduced tree pathogen which causes Pitch Canker disease. Can. Entomol. 1998, 130, 783–792. [Google Scholar] [CrossRef]
- IPPC. Implmentation Review and Support System: The Internet Trade (e-Commerce) in Plants; Food and Agriculture Organization: Rome, Italy, 2012. [Google Scholar]
- Dwinell, L.D.; Barrows-Broaddus, J.B.; Kuhlman, G. Pitch canker: A disease complex of southern pines. Plant Dis. 1985, 69, 270–276. [Google Scholar] [CrossRef]
- Anderson, R.L.; Belcher, E.; Miller, T. Occurrence of internal seed fungi in slash pine seed produced in seed orchards in the United States. In Proceedings of the International Seed Testing Association Congress 20th, Ottawa, ON, Canada, 17–25 June 1983; p. 10. [Google Scholar]
- Swett, C.; Gordon, T. Colonization of corn (Zea mays) by the pitch canker pathogen, Fusarium circinatum: Insights into the evolutionary history of a pine pathogen. Phytopathology 2009, 99, 126–127. [Google Scholar]
- Vettraino, A.; Potting, R.; Raposo, R. EU Legislation on Forest Plant Health: An Overview with a Focus on Fusarium circinatum. Forests 2018, 9, 568. [Google Scholar] [CrossRef]
- Burgess, T.; Wingfield, M.J.J. Quarantine is important in restricting the spread of exotic seed-borne tree pathogens in the southern hemisphere. Int. For. Rev. 2002, 4, 56–65. [Google Scholar] [CrossRef]
- Martin-Garcia, J.; Lukacevicova, A.; Flores-Pacheco, A.; Diez, J.J.; Dvorak, M. Evaluation of the Susceptibility of Several Czech Conifer Provenances to Fusarium circinatum. Forests 2018, 9, 72. [Google Scholar] [CrossRef]
- EFSA. Risk assessment of Gibberella circinata for the EU territory and identification and evaluation of risk management options. EFSA J. 2010, 8, 1620. [Google Scholar] [CrossRef]
- Kopinga, J.; Moraal, L.G.; Verwer, C.C.; Clerkx, A.P.P.M. Phytosanitary Risks of Wood Chips; Alterra: Wageningen, The Netherlands, 2010. [Google Scholar]
- Serrano, Y.; Iturritxa, E.; Elvira-Recuenco, M.; Raposo, R. Survival of Fusarium circinatum in soil and Pinus radiata needle and branch segments. Plant Pathol. 2017, 66, 934–940. [Google Scholar] [CrossRef]
- McNee, W.R.; Wood, D.L.; Storer, A.J.; Gordon, T.R. Incidence of the pitch canker pathogen and associated insects in intact and chipped Monterey pine branches. Can. Entomol. 2002, 134, 47–58. [Google Scholar] [CrossRef]
- Gordon, T.R.; Wood, D.L.; Storer, A.J. Survival of Fusarium circinatum and Its Insect Vectors in Recently Cut Pitch Canker Infected Trees; California Department of Forestry and Fire Protection: Sacramento, CA, USA, 2000. [Google Scholar]
- Biosecurity Australia. Technical Justification for Australia’s Requirement for Wood Packaging Material to be Bark Free; Biosecurity Australia: Canberra, Australia, 2006; p. 123.
- Dwinell, L.D.; Barrows-Broaddus, J.B. Recovery of the pine pitch canker fungus (Fusarium moniliforme subglutinans) from pine (Pinus taeda) plantation and seed orchard soil. Phytopathol. News 1978, 12, 207. [Google Scholar]
- Barrows-Broaddus, J.B.; Kerr, T.J. Inhibition of Fusarium moniliforme var. subglutinans, the causal agent of pine pitch canker, by the soil bacterium Arthrobacter sp. Can. J. Microbiol. 1981, 27, 20–27. [Google Scholar]
- Morris, A.R.; Fourie, G.; Greyling, I.; Steenkamp, E.T.; Jones, N.B. Re-use of seedling containers and Fusarium circinatum association with asymptomatic Pinus patula planting stock. South For. 2014, 76, 177–187. [Google Scholar] [CrossRef]
- Dick, M. Pine pitch canker—The threat to New Zealand. N. Z. J. For. 1998, 42, 30–34. [Google Scholar]
- Gadgil, P.; Dick, M.; Simpson, J.; Bejakovich, D.; Ross, M.; Bain, J.; Wylie, R.; Horgan, G. Management Plan: Response to an Incursion of Pine Pitch Canker in Australia or New Zealand; Forest Health Committee on behalf of the Forestry and Forest Products Committee: Savannah, GA, USA, 2003.
- The Council of the European Union. Regulation EU 2016/2031 of the European Parlament of the Council of 26 October 2016. OJ 2016, 317, 4–104. [Google Scholar]
- Wikler, K.; Storer, A.J.; Newman, W.; Gordon, T.R.; Wood, D.L. The dynamics of an introduced pathogen in a native Monterey pine (Pinus radiata) forest. For. Ecol. Manag. 2003, 179, 209–221. [Google Scholar] [CrossRef]
- Ferchaw, V.A.L.; Goldsworthy, E.; Pinkerton, J.; Yun, D.I.; Lund, U.J.; Mark, W.; Valkonen, S.; Piirto, D.D. Management strategies for pitch canker infected Año Nuevo stands of Monterey pine. For. Ecol. Manag. 2013, 308, 101–115. [Google Scholar] [CrossRef]
- Runion, G.B.; Bruck, R.I. The influence of half sib family and tree spacing on incidence of pitch canker in a loblolly pine plantation in eastern North Carolina. Phytopathology 1986, 76, 1113. [Google Scholar]
- Blakeslee, G.M.; Jokela, E.J.; Hollis, C.H.; Wilson, D.S.; Lante, W.D.; Allen, J.E. Pitch Canker in Young Loblolly Pines: Influence of Precommercial Thinning and Fertilization on Disease Incidence and Severity. South J. Appl. For. 1999, 23, 139–143. [Google Scholar] [CrossRef]
- Blakeslee, G.M.; Lante, W.D.; Allen, J.E. Influence of periodic water stress on pitch canker disease in resistant and susceptible slash pine families. Phytopathology 1982, 82, 1096. [Google Scholar]
- Gordon, T.R.; Swett, C.L.; Wingfield, M.J. Management of Fusarium diseases affecting conifers. Crop Prot. 2015, 73, 28–39. [Google Scholar] [CrossRef]
- Fraedrich, B.R.; Witcher, W. Influence of fertilization on pitch canker development on three southern pine species. Plant Dis. 1982, 66, 938–940. [Google Scholar] [CrossRef]
- Jokela, E.J.; Allen, H.L.; McFee, W.W. Fertilization of Southern Pines at Establishment. In Forest Regeneration Manual. Forestry Sciences; Duryea, M.L., Dougherty, P.M., Eds.; Springer: Dordrecht, The Netherlands, 1991; pp. 263–277. [Google Scholar]
- Fisher, R.F.; Garbett, W.S.; Underhill, E.M. Effects of fertilization on healthy and pitch-canker infected pines. South J. Appl. For. 1981, 5, 77–79. [Google Scholar] [CrossRef]
- Lopez-Zamora, I.; Bliss, C.; Jokela, E.J.; Comerford, N.B.; Grunwald, S.; Barnard, E.; Vasquez, G.M. Spatial relationships between nitrogen status and pitch canker disease in slash pine planted adjacent to a poultry operation. Environ. Pollut. 2007, 147, 101–111. [Google Scholar] [CrossRef]
- Phelps, W.R.; Chellman, C.W. Evaluation of “Pitch Canker” in Florida Slash Pine Plantations and Seed Orchards; US Department of Agriculture, Forest Service, State & Private Forestry: Atlanta, GA, USA, 1976. [Google Scholar]
- Vivas, M.; Vrhovnik, M.; Solla, A. Fertilización de plántulas de Pinus pinaster y su efecto en la susceptibilidad a Fusarium circinatum. In Proceedings of the 5th Spanish Forestry Congress, Ávila, Spain, 21–25 September 2009; pp. 2–10. [Google Scholar]
- Shackleton, R.T.; Adriaens, T.; Brundu, G.; Dehnen-Schmutz, K.; Estévez, G.R.A.; Fried, J.; Larson, B.M.H.; Liu, S.; Marchante, E.; et al. Stakeholder engagement in the study and management of invasive alien species. J. Environ. Manag. 2019, 229, 88–101. [Google Scholar] [CrossRef]
- EU. COST Action FP1406 PINESTRENGTH. Available online: http://www.pinestrength.eu/ (accessed on 10 October 2019).
- Aitken, S.N.; Bemmels, J.B. Time to get moving: Assisted gene flow of forest trees. Evol. Appl. 2016, 9, 271–290. [Google Scholar] [CrossRef]
- Montwé, D.; Isaac-Renton, M.; Hamann, A.; Spiecker, H. Drought tolerance and growth in populations of a wide-ranging tree species indicate climate change risks for the boreal north. Glob. Chang. Biol. 2016, 22, 806–815. [Google Scholar] [CrossRef]
- Showalter, D.N.; Raffa, K.F.; Sniezko, R.A.; Herms, D.A.; Liebhold, A.M.; Smith, J.A.; Bonello, P. Strategic development of tree resistance against forest pathogen and insect invasions in defense-free space. Front. Ecol. Evol. 2018, 6. [Google Scholar] [CrossRef]
- Davydenko, K.; Nowakowska, J.A.; Kaluski, T.; Gawlak, M.; Sadowska, K.; Martín-García, J.; Diez, J.J.; Okorski, A.; Oszako, T. A Comparative Study of the Pathogenicity of Fusarium circinatum and other Fusarium Species in Polish Provenances of Pinus sylvestris L. Forests 2018, 9, 560. [Google Scholar] [CrossRef]
- Martin-Garcia, J.; Paraschiv, M.; Asdrubal Flores-Pacheco, J.; Chira, D.; Javier Diez, J.; Fernandez, M. Susceptibility of Several Northeastern Conifers to Fusarium circinatum and Strategies for Biocontrol. Forests 2017, 8, 318. [Google Scholar] [CrossRef]
- Martín-García, J.; Zas, R.; Solla, A.; Woodward, S.; Hantula, J.; Vainio, E.J.; Mullett, M.; Morales-Rodríguez, C.; Vannini, A.; Martínez-Álvarez, P.; et al. Environmentally-friendly methods for controlling pine pitch canker. Plant Pathol. 2019. [Google Scholar] [CrossRef]
- Koskella, B.; Meaden, S.; Crowther, W.J.; Leimu, R.; Metcalf, C.J.E. A signature of tree health? Shifts in the microbiome and the ecological drivers of horse chestnut bleeding canker disease. New Phytol. 2017, 215, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Gopal, M.; Gupta, A.; Thomas, G.V. Bespoke microbiome therapy to manage plant diseases. Front. Microbiol. 2013, 4, 355. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.K.; Dai, C.C.; Liu, X.Z. Mechanisms of fungal endophytes in plant protection against pathogens. Afr. J. Microbiol. Res. 2010, 4, 1346–1351. [Google Scholar] [CrossRef]
- Blumenstein, K.; Albrectsen, B.R.; Martín, J.A.; Hultberg, M.; Sieber, T.N.; Helander, M.; Witzell, J. Nutritional niche overlap potentiates the use of endophytes in biocontrol of a tree disease. BioControl 2015, 60, 655–667. [Google Scholar] [CrossRef]
- Alabouvette, C.; Olivain, C.; Migheli, Q.; Steinberg, C. Microbiological control of soil-borne phytopathogenic fungi with special emphasis on wilt-inducing Fusarium oxysporum. New Phytol. 2009, 184, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Giordano, L.; Gonthier, P.; Varese, G.C.; Miserere, L.; Nicolotti, G. Mycobiota inhabiting sapwood of healthy and declining Scots pine (Pinus sylvestris L.) trees in the Alps. Fungal Divers. 2009, 38, 69–83. [Google Scholar]
- Lehto, T.; Zwiazek, J.J. Ectomycorrhizas and water relations of trees: A review. Mycorrhiza 2011, 21, 71–90. [Google Scholar] [CrossRef]
- Gonthier, P.; Giordano, L.; Zampieri, E.; Lione, G.; Vizzini, A.; Colpaert, J.; Balestrini, R. An ectomycorrhizal symbiosis differently affects host susceptibility to two congeneric fungal pathogens. Fungal Ecol. 2019, 39, 250–256. [Google Scholar] [CrossRef]
- Hu, J.; Lin, X.; Wang, J.; Shen, W.; Wu, S.; Peng, S.; Mao, T. Arbuscular Mycorrhizal Fungal Inoculation Enhances Suppression of Cucumber Fusarium Wilt in Greenhouse Soils. Pedosphere 2010, 20, 586–593. [Google Scholar] [CrossRef]
- Eke, P.; Chatue Chatue, G.; Wakam, L.N.; Kouipou, R.M.T.; Fokou, P.V.T.; Boyom, F.F. Mycorrhiza consortia suppress the fusarium root rot (Fusarium solani f. sp. phaseoli) in common bean (Phaseolus vulgaris L.). Biol. Control 2016, 103, 240–250. [Google Scholar] [CrossRef]
- Torres-Vila, L.M.; Zugasti, C.; De-Juan, J.M.; Oliva, M.J.; Montero, C.; Mendiola, F.J.; Conejo, Y.; Sánchez, Á.; Fernández, F.; Ponce, F.; et al. Mark-recapture of Monochamus galloprovincialis with semiochemical-baited traps: Population density, attraction distance, flight behaviour and mass trapping efficiency. Forestry 2015, 88, 224–236. [Google Scholar] [CrossRef]
- The Council of the European Union. Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009 establishing a framework for Community action to achieve the sustainable use of pesticides. OJ 2009, 309, 71–86. [Google Scholar]
- Wermelinger, B. Ecology and management of the spruce bark beetle Ips typographus—A review of recent research. For. Ecol. Manag. 2004, 202, 67–82. [Google Scholar] [CrossRef]
- Göthlin, E.; Schroeder, L.M.; Lindelöw, A. Attacks by Ips typographus and Pityogenes chalcographus on Windthrown Spruces (Picea abies) During the Two Years Following a Storm Felling. Scand. J. For. Res. 2000, 15, 542–549. [Google Scholar] [CrossRef]
- Walmsley, J.D.; Godbold, D.L. Stump Harvesting for Bioenergy—A Review of the Environmental Impacts. Forestry 2010, 83, 17–38. [Google Scholar] [CrossRef]
- The Council of the European Union. Council Directive 2000/29/EC of 8 May 2000 on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community. OJ 2000, 169, 1–112. [Google Scholar]
- Berbegal, M.; Landeras, E.; Sánchez, D.; Abad-Campos, P.; Pérez-Sierra, A.; Armengol, J. Evaluation of Pinus radiata seed treatments to control Fusarium circinatum: Effects on seed emergence and disease incidence. For. Pathol. 2015, 45, 525–533. [Google Scholar] [CrossRef]
- Ramsfield, T.D.; Dobbie, K.; Dick, M.A.; Ball, R.D. Polymerase chain reaction-based detection of Fusarium circinatum, the causal agent of pitch canker disease. Mol. Ecol. Resour. 2008, 8, 1270–1273. [Google Scholar] [CrossRef] [PubMed]
- Agustí-Brisach, C.; Pérez-Sierra, A.; Armengol, J.; García-Jiménez, J.; Berbegal, M. Efficacy of hot water treatment to reduce the incidence of Fusarium circinatum on Pinus radiata seeds. Forestry 2012, 85, 629–635. [Google Scholar] [CrossRef]
- Vivas, M.; Zas, R.; Sampedro, L.; Solla, A. Environmental Maternal Effects Mediate the Resistance of Maritime Pine to Biotic Stress. PLoS ONE 2013, 8, e70148. [Google Scholar] [CrossRef] [PubMed]
- Iturritxa, E.; Trask, T.; Mesanza, N.; Raposo, R.; Elvira-Recuenco, M.; Patten, C.L. Biocontrol of Fusarium circinatum Infection of Young Pinus radiata Trees. Forests 2017, 8, 32. [Google Scholar] [CrossRef]
- Martinez-Alvarez, P.; Arcadio Fernandez-Gonzalez, R.; Vicente Sanz-Ros, A.; Pando, V.; Javier Diez, J. Two fungal endophytes reduce the severity of pitch canker disease in Pinus radiata seedlings. Biol. Control 2016, 94, 1–10. [Google Scholar] [CrossRef]
- Cazorla, F.M.; Mercado-Blanco, J. Biological control of tree and woody plant diseases: An impossible task? BioControl 2016, 61, 233–242. [Google Scholar] [CrossRef]
- Moraga-Suazo, P.; Opazo, A.; Zaldúa, S.; González, G.; Sanfuentes, E. Evaluation of Trichoderma spp. and Clonostachys spp. Strains to Control Fusarium circinatum in Pinus radiata Seedlings. Chil. J. Agric. Res. 2011, 71, 412–417. [Google Scholar] [CrossRef]
- Iturritxa, E.; Ganley, R.J.; Wright, J.; Heppe, E.; Steenkamp, E.T.; Gordon, T.R.; Wingfield, M.J. A genetically homogenous population of Fusarium circinatum causes pitch canker of Pinus radiata in the Basque Country, Spain. Fungal Biol. 2011, 115, 288–295. [Google Scholar] [CrossRef]
- Amaral, J.; Pinto, G.; Flores-Pacheco, J.A.; Díez-Casero, J.J.; Cerqueira, A.; Monteiro, P.; Gómez-Cadenas, A.; Alves, A.; Martín-García, J. Effect of Trichoderma viride pre-inoculation in pine species with different levels of susceptibility to Fusarium circinatum: Physiological and hormonal responses. Plant Pathol. 2019. [Google Scholar] [CrossRef]
- Ghelardini, L.; Pepori, A.L.; Luchi, N.; Capretti, P.; Santini, A. Drivers of emerging fungal diseases of forest trees. For. Ecol. Manag. 2016, 381, 235–246. [Google Scholar] [CrossRef]
- Sharifi, R.; Ryu, C.-M. Biogenic Volatile Compounds for Plant Disease Diagnosis and Health Improvement. Plant Pathol. J. 2018, 34, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Ioos, R.; Aloi, F.; Piškur, B.; Guinet, C.; Mullett, M.; Berbegal, M.; Bragança, H.; Cacciola, S.O.; Oskay, F.; Cornejo, C.; et al. Transferability of PCR-based diagnostic protocols: An international collaborative case study assessing protocols targeting the quarantine pine pathogen Fusarium circinatum. Sci. Rep. 2019, 9, 8195. [Google Scholar] [CrossRef] [PubMed]
- Schweigkofler, W.; O’Donnell, K.; Garbelotto, M. Detection and quantification of airborne conidia of Fusarium circinatum, the causal agent of pine pitch canker, from two California sites by using a real-time PCR approach combined with a simple spore trapping method. Appl. Environ. Microbiol. 2004, 70, 3512–3520. [Google Scholar] [CrossRef] [PubMed]
- Fourie, G.; Wingfield, M.J.; Wingfield, B.D.; Jones, N.B.; Morris, A.R.; Steenkamp, E.T. Culture-independent detection and quantification of Fusarium circinatum in a pine-producing seedling nursery. South For. J. For. Sci. 2014, 76, 137–143. [Google Scholar] [CrossRef]
- Dreaden, T.J.; Smith, J.A.; Barnard, E.L.; Blakeslee, G. Development and evaluation of a real-time PCR seed lot screening method for Fusarium circinatum, causal agent of pitch canker disease. For. Pathol. 2012, 42, 405–411. [Google Scholar] [CrossRef]
- Ioos, R.; Fourrier, C.; Iancu, G.; Gordon, T.R. Sensitive Detection of Fusarium circinatum in Pine Seed by Combining an Enrichment Procedure with a Real-Time Polymerase Chain Reaction Using Dual-Labeled Probe Chemistry. Phytopathology 2009, 99, 582–590. [Google Scholar] [CrossRef]
- Lamarche, J.; Potvin, A.; Pelletier, G.; Stewart, D.; Feau, N.; Alayon, D.I.O.; Dale, A.L.; Coelho, A.; Uzunovic, A.; Bilodeau, G.J.; et al. Molecular Detection of 10 of the Most Unwanted Alien Forest Pathogens in Canada Using Real-Time PCR. PLoS ONE 2015, 10, e0134265. [Google Scholar] [CrossRef]
- Luchi, N.; Pepori, A.L.; Bartolini, P.; Ioos, R.; Santini, A. Duplex real-time PCR assay for the simultaneous detection of Caliciopsis pinea and Fusarium circinatum in pine samples. Appl. Microbiol. Biotechnol. 2018, 102, 7135–7146. [Google Scholar] [CrossRef]
- Fourrier, C.; Antoine, S.; Piou, D.; Ioos, R. Rapid detection of Fusarium circinatum propagules on trapped pine beetles. For. Pathol. 2015, 45, 324–330. [Google Scholar] [CrossRef]
- Blakeslee, G.M.; Rockwood, D.L. Variation in resistanceto pitch canker in slash and loblolly pines. In Current and Potential Impacts of Pitch Canker in Radiata Pine, Proceedings of the IMPACT Monterey Workshop, Monterey, CA, USA, 30 November–3 December 1998; Devey, M.E., Matheson, A.C., Gordon, T.R., Eds.; CSIRO: Canberra, Australia, 1999; pp. 35–39. [Google Scholar]
- Schmidt, R.A.; Wilkinson, R.C.; Moses, C.S.; Broerman, F.S. Drought and Weevils Associated with Severe Incidence of Pitch Canker in Volusia County, Florida; Institute of Food and Agricultural Sciences, University of Florida: Gainesville, FL, USA, 1976; Volume 76, pp. 1–4. [Google Scholar]
- Owen, D.; Adams, D. Impact of pitch canker on ornamental Monterey pines in Santa Cruz County, California, U.S., 1987–2000. J. Arboric. 2001, 27, 198–304. [Google Scholar]
- Gordon, T.R.; Kirkpatrick, S.C.; Aegerter, B.J.; Fisher, A.J.; Storer, A.J.; Wood, D.L. Evidence for the occurrence of induced resistance to pitch canker, caused by Gibberella circinata (anamorph Fusarium circinatum), in populations of Pinus radiata. For. Pathol. 2011, 41, 227–232. [Google Scholar] [CrossRef]
- EPPO. EPPO Standards PM 10/6 (1)-Heat Treatment of Wood to Control Insects and Wood-Borne Nematodes; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; Volume 39. [Google Scholar]
- Hantula, J.; Müller, M.M.; Uusivuori, J. International plant trade associated risks: Laissez-faire or novel solutions. Environ. Sci. Policy 2014, 37, 158–160. [Google Scholar] [CrossRef]
- IUFRO. The Montesclaros Declaration. In Proceedings of the IUFRO the Global Network for Forest Science and Cooperation, Cantabria, Spain, 23–27 May 2011. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamora-Ballesteros, C.; Diez, J.J.; Martín-García, J.; Witzell, J.; Solla, A.; Ahumada, R.; Capretti, P.; Cleary, M.; Drenkhan, R.; Dvořák, M.; et al. Pine Pitch Canker (PPC): Pathways of Pathogen Spread and Preventive Measures. Forests 2019, 10, 1158. https://doi.org/10.3390/f10121158
Zamora-Ballesteros C, Diez JJ, Martín-García J, Witzell J, Solla A, Ahumada R, Capretti P, Cleary M, Drenkhan R, Dvořák M, et al. Pine Pitch Canker (PPC): Pathways of Pathogen Spread and Preventive Measures. Forests. 2019; 10(12):1158. https://doi.org/10.3390/f10121158
Chicago/Turabian StyleZamora-Ballesteros, Cristina, Julio J. Diez, Jorge Martín-García, Johanna Witzell, Alejandro Solla, Rodrigo Ahumada, Paolo Capretti, Michelle Cleary, Rein Drenkhan, Miloň Dvořák, and et al. 2019. "Pine Pitch Canker (PPC): Pathways of Pathogen Spread and Preventive Measures" Forests 10, no. 12: 1158. https://doi.org/10.3390/f10121158
APA StyleZamora-Ballesteros, C., Diez, J. J., Martín-García, J., Witzell, J., Solla, A., Ahumada, R., Capretti, P., Cleary, M., Drenkhan, R., Dvořák, M., Elvira-Recuenco, M., Fernández-Fernández, M., Ghelardini, L., Gonthier, P., Hernández-Escribano, L., Ioos, R., Markovskaja, S., Martínez-Álvarez, P., Muñoz-Adalia, E. J., ... Hantula, J. (2019). Pine Pitch Canker (PPC): Pathways of Pathogen Spread and Preventive Measures. Forests, 10(12), 1158. https://doi.org/10.3390/f10121158










