Lidar-Derived Tree Crown Parameters: Are They New Variables Explaining Local Birch (Betula sp.) Pollen Concentrations?
Abstract
1. Introduction
2. Materials and Methods
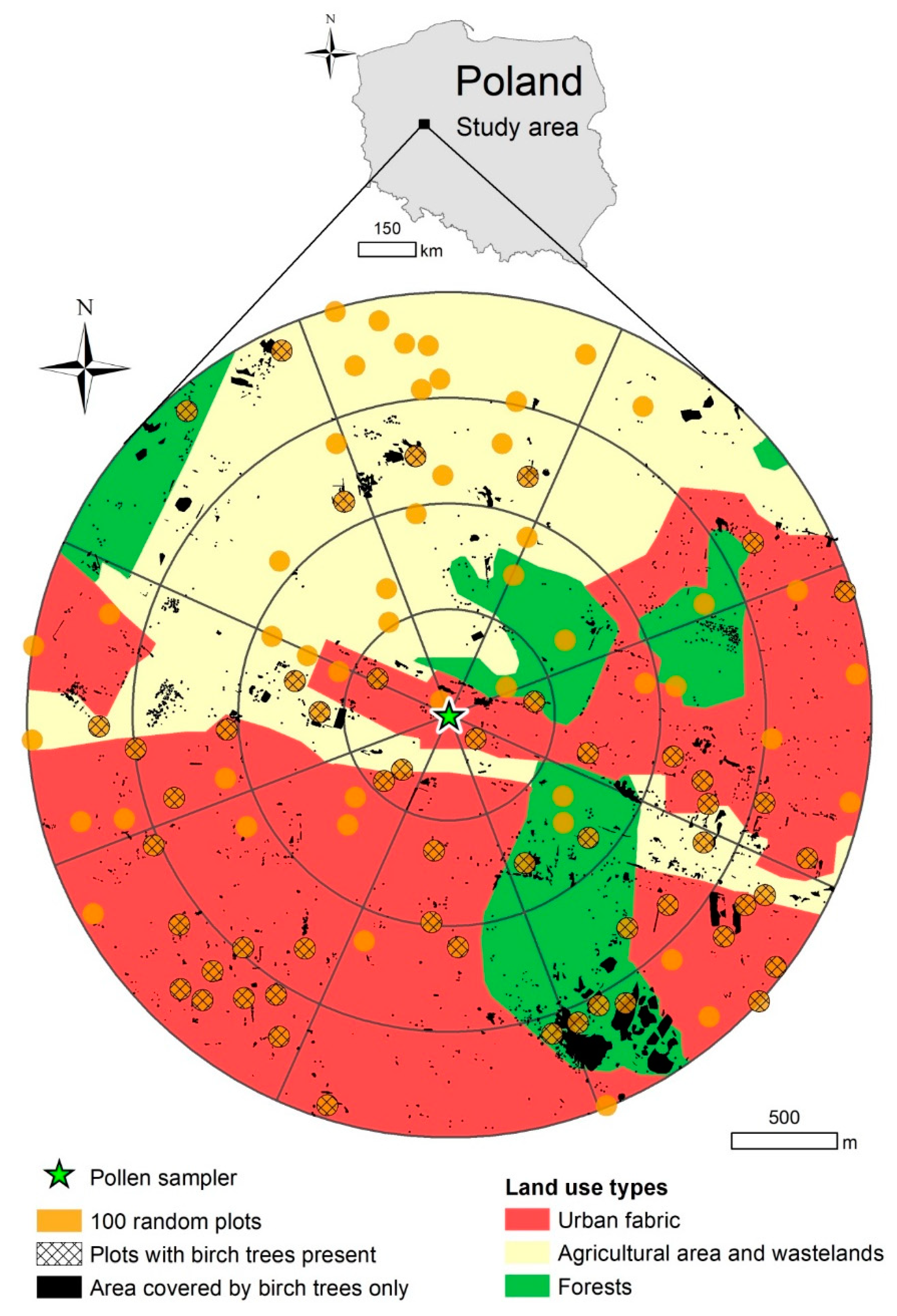
2.1. Area and Object of Study
2.2. Pollen and Meteorological Data
2.3. Birch Inventory
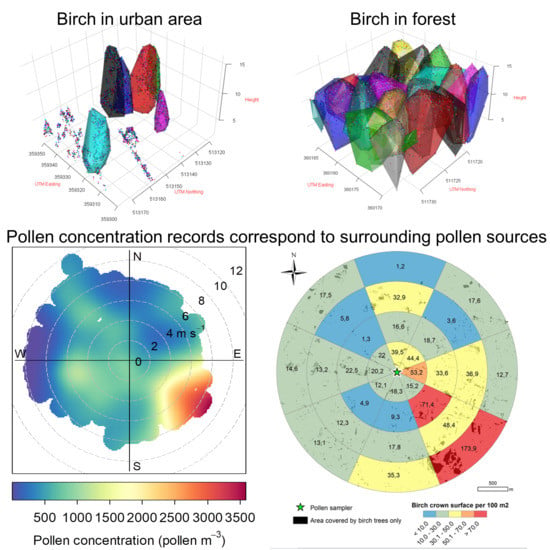
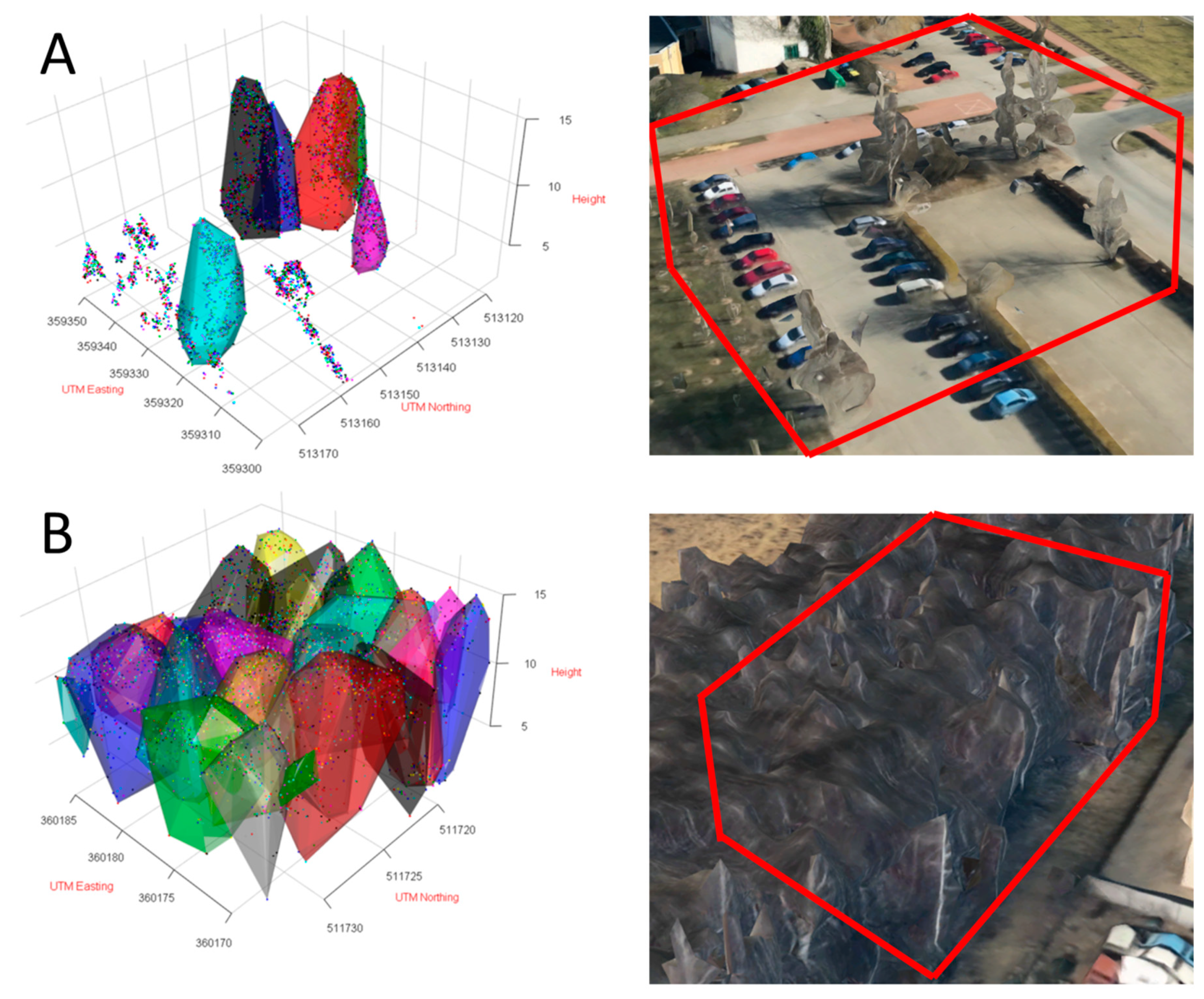
2.4. Point-Cloud Data
2.4.1. Technical Details
2.4.2. Processing of the Point Clouds
2.5. Statistical Analysis
3. Results
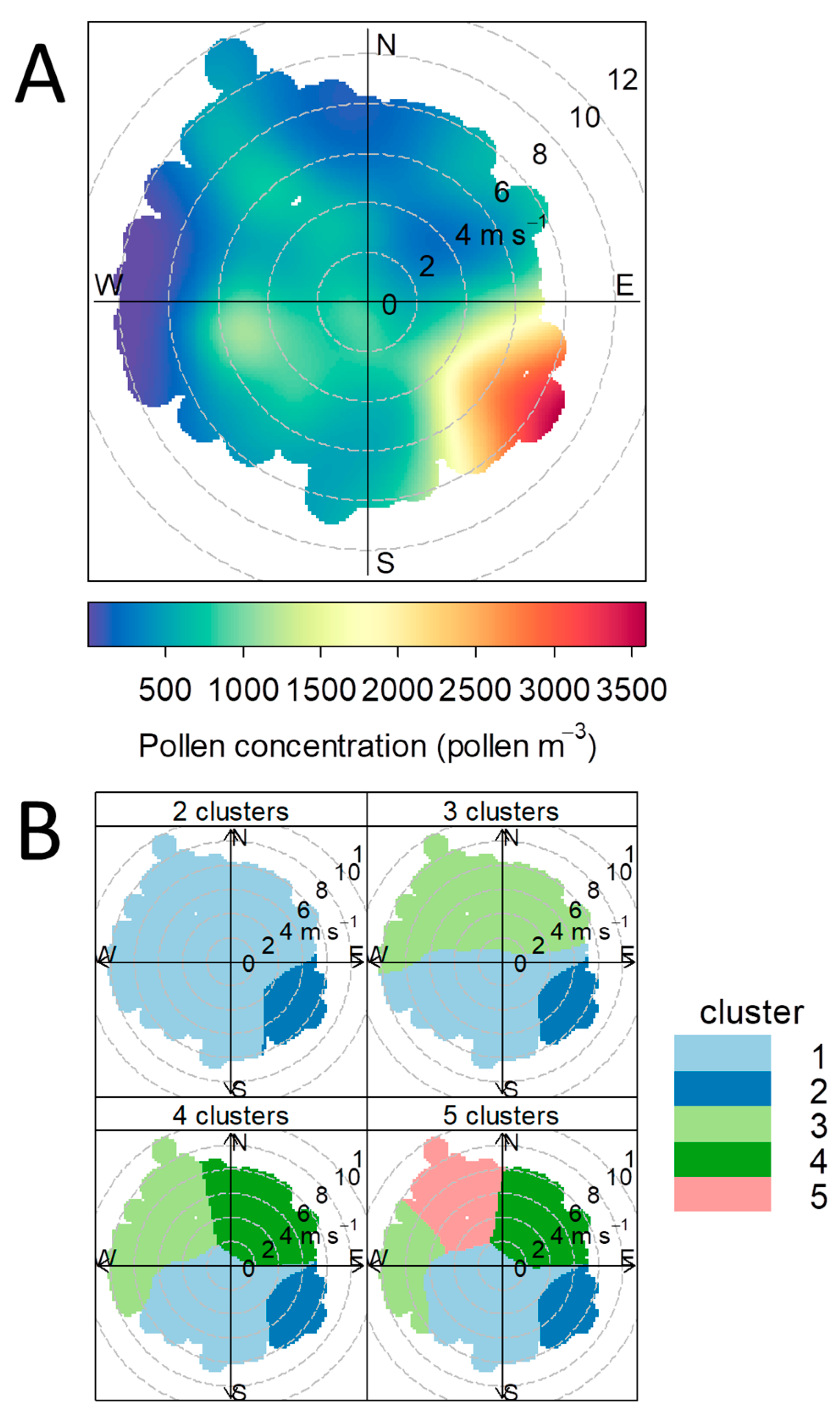
3.1. Pollen Influx
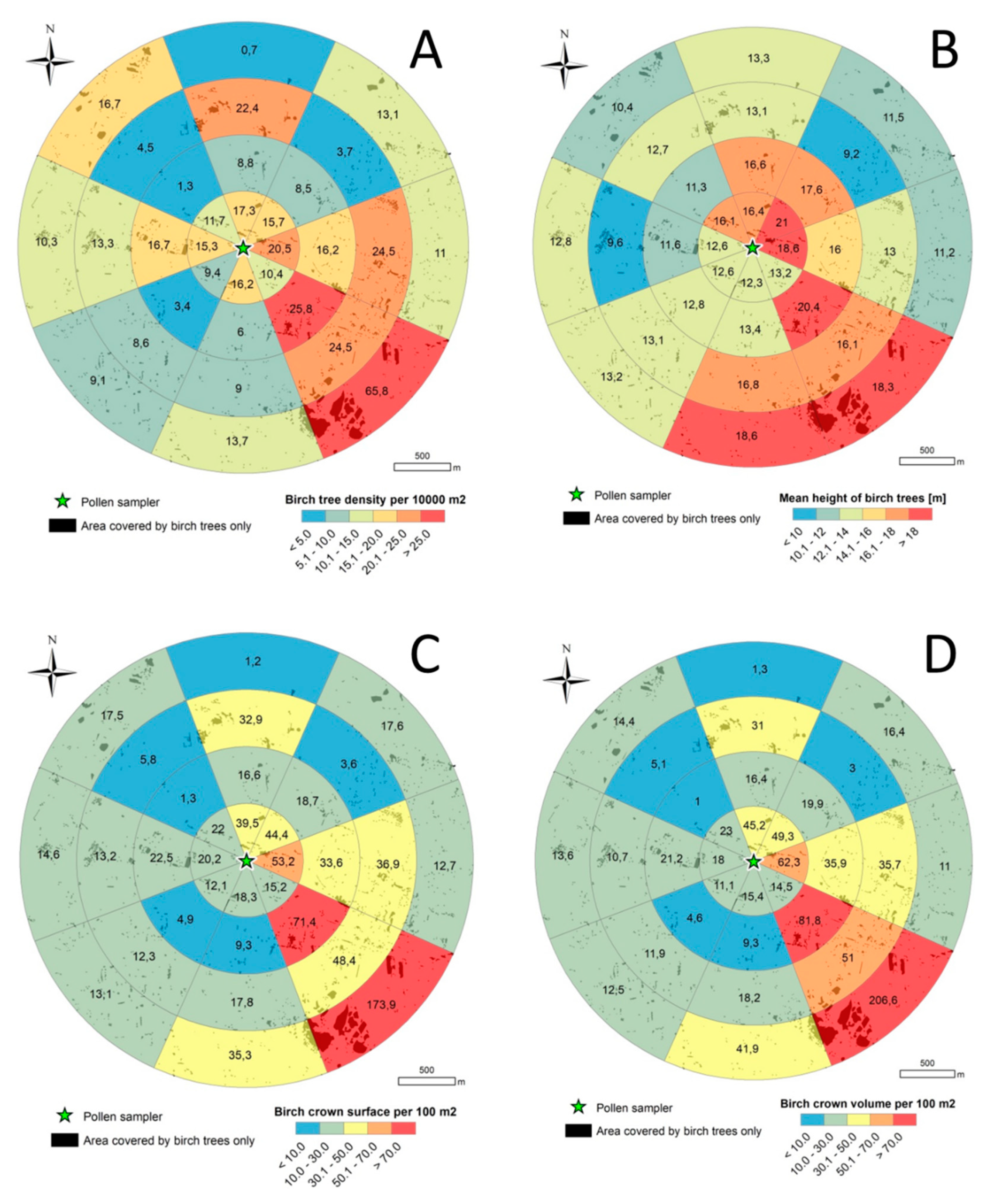
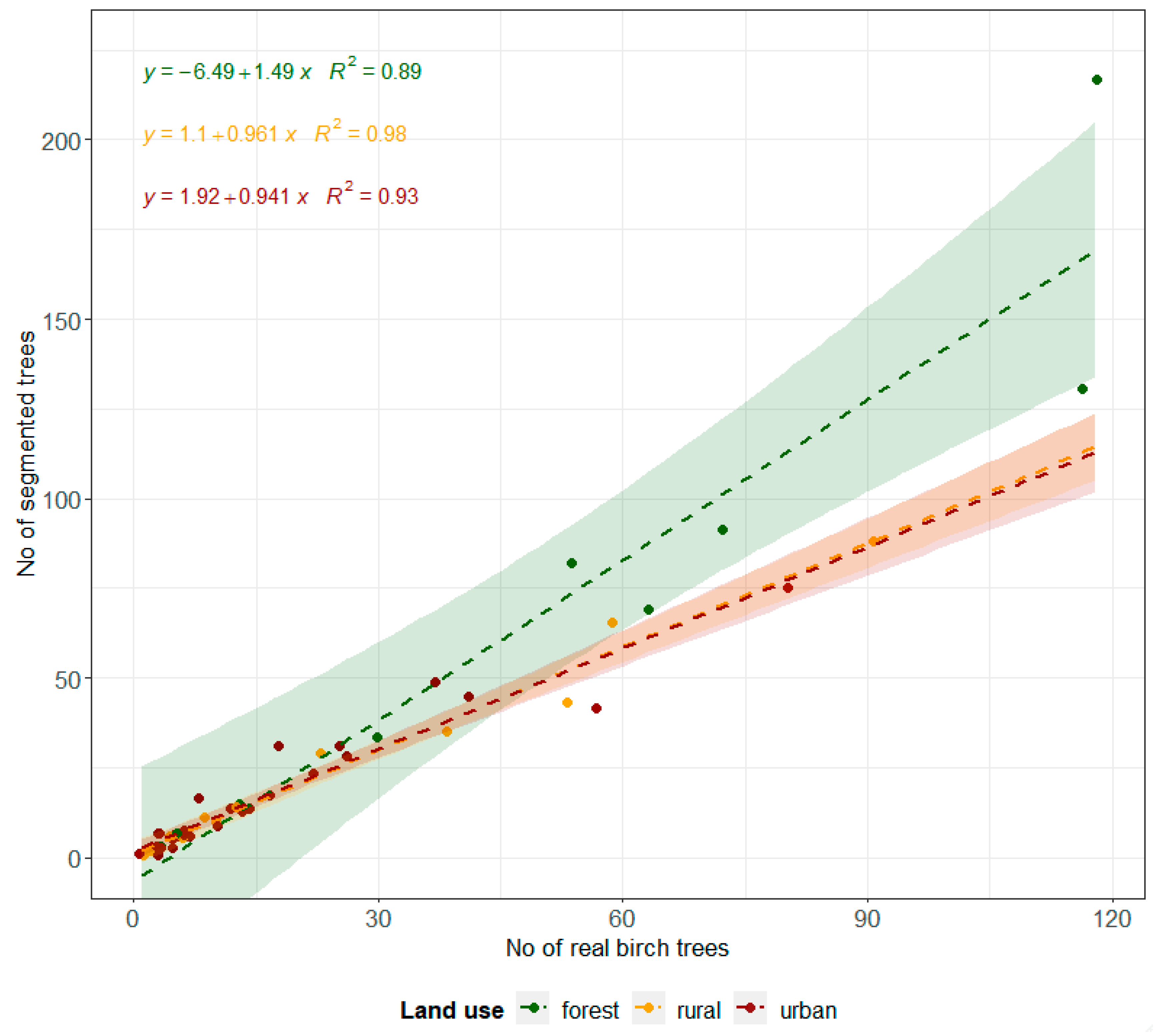
3.2. Birch Inventory
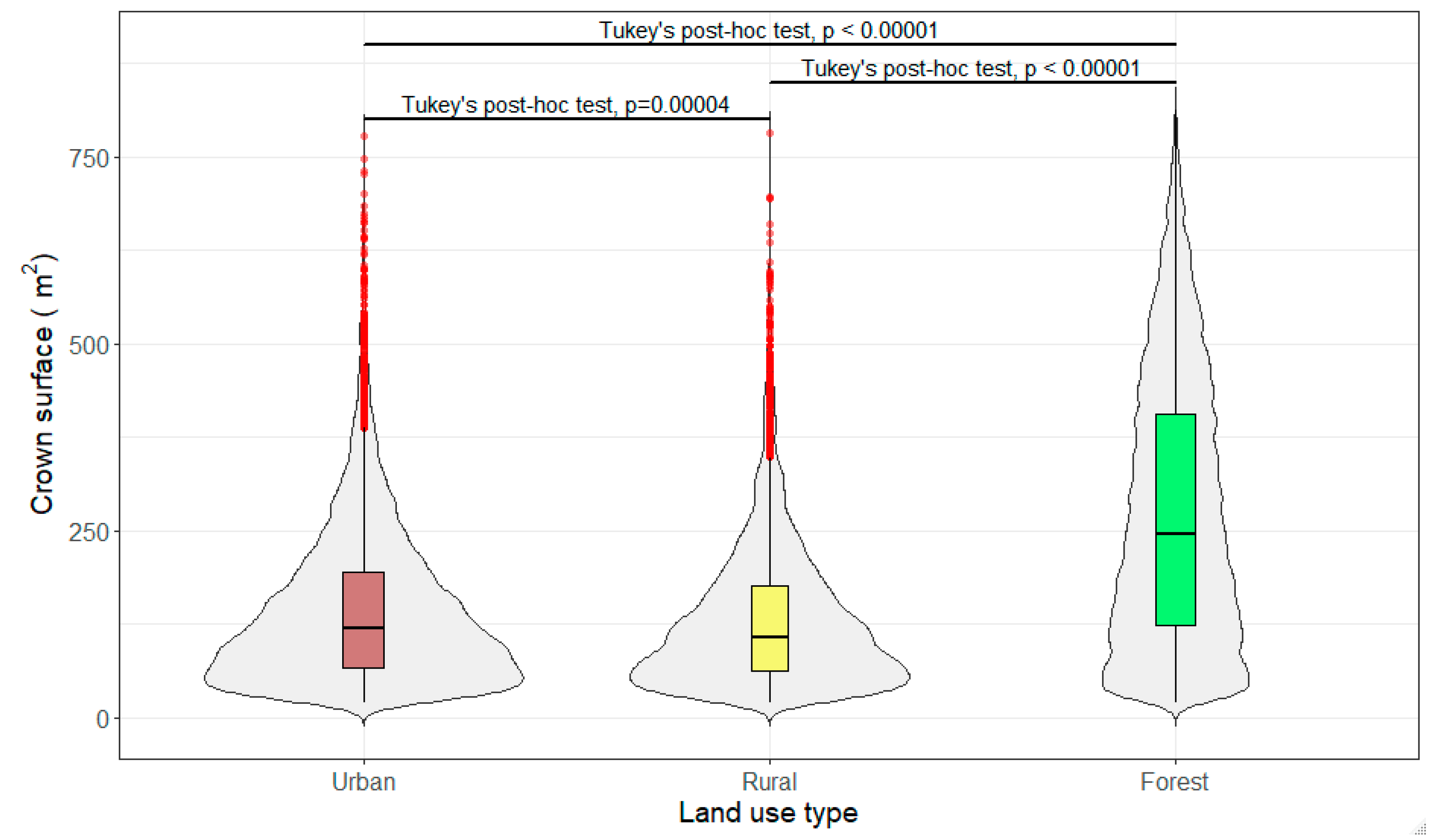
3.3. Crown Volume and Surface
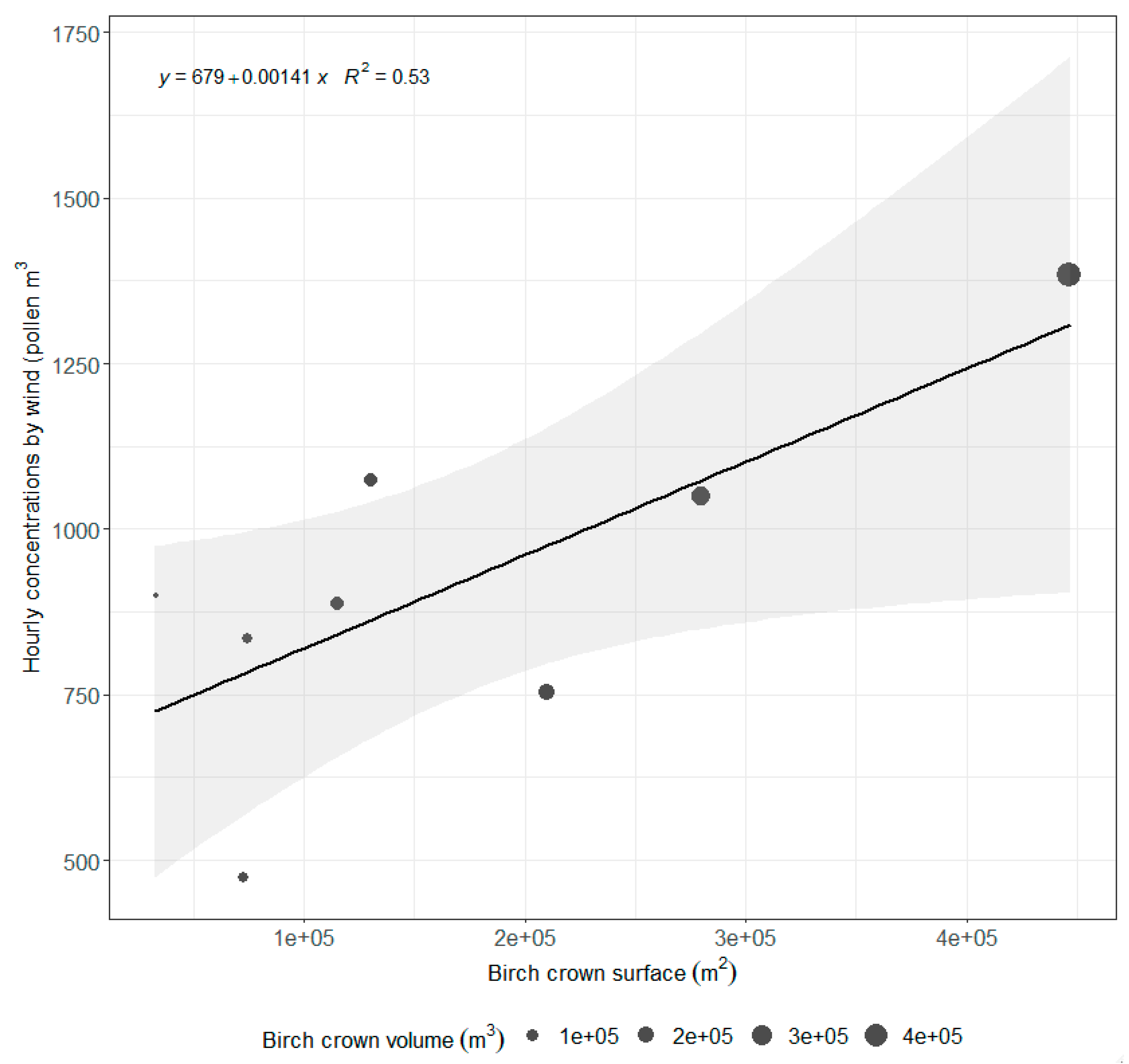
3.4. Relationship between Pollen Concentration and Birch Crown Parameters
4. Discussion
4.1. Impacts of Pollen Sources Vary in Space
4.2. Birch Trees in Different Land-Use Types
4.3. Vertical Dimension of the Pollen Inventory—the Use of LiDAR Data
4.4. Limitations of the Study and Future Work
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beck, P.; Caudullo, G.; de Rigo, D.; Tinner, W. Betula pendula, Betula pubescens and other birches in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publication Office of the European Union: Luxembourg, 2016; p. e010226+. [Google Scholar]
- Atkinson, M.D. Betula Pendula Roth (B. Verrucosa Ehrh.) and B. Pubescens Ehrh. J. Ecol. 1992, 80, 837–870. [Google Scholar] [CrossRef]
- Burbach, G.J.; Heinzerling, L.M.; Edenharter, G.; Bachert, C.; Bindslev-Jensen, C.; Bonini, S. GA2LEN skin test study II: Clinical relevance of inhalant allergen sensitizations in Europe. Allergy 2009, 64, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Warm, K.; Lindberg, A.; Lundbäck, B.; Rönmark, E. Increase in sensitization to common airborne allergens among adults—Two population-based studies 15 years apart. Allergy Asthma Clin. Immunol. 2013, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.A.; Wayne, P.M.; Macklin, E.A.; Muilenberg, M.L.; Wagner, C.J.; Epstein, P.R.; Bazzaz, F.A. Interaction of the Onset of Spring and Elevated Atmospheric CO2 On Ragweed (Ambrosia artemisiifolia L.) Pollen Production. Environ. Health Perspect. 2006, 114, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Newnham, R.M.; Sparks, T.H.; Skjøth, C.A.; Head, K.; Adams-Groom, B.; Smith, M. Pollen season and climate: Is the timing of birch pollen release in the UK approaching its limit? Int. J. Biometeorol. 2013, 57, 391–400. [Google Scholar] [CrossRef]
- Pfaar, O.; Bastl, K.; Berger, U.; Buters, J.; Calderon, M.A.; Clot, B.; Darsow, U.; Demoly, P.; Durham, S.R.; Galan, C. Defining pollen exposure times for clinical trials of allergen immunotherapy for pollen-induced rhinoconjunctivitis—An EAACI Position Paper. Allergy 2017, 72, 713–722. [Google Scholar] [CrossRef]
- Sofiev, M.; Siljamo, P.; Ranta, H.; Rantio-Lehtimaki, A. Towards numerical forecasting of long-range air transport of birch pollen: Theoretical considerations and a feasibility study. Int. J. Biometeorol. 2006, 50, 392–402. [Google Scholar] [CrossRef]
- Bogawski, P.; Borycka, K.; Grewling, Ł.; Kasprzyk, I. Detecting distant sources of airborne pollen for Poland: Integrating back-trajectory and dispersion modelling with a satellite-based phenology. Sci. Total Environ. 2019, 689, 109–125. [Google Scholar] [CrossRef]
- Ranta, H.; Hokkanen, T.; Linkosalo, T.; Laukkanen, L.; Bondestam, K.; Oksanen, A. Male flowering of birch: Spatial synchronization, year-to-year variation and relation of catkins numbers and airborne pollen counts. For. Ecol. Manag. 2008, 255, 643–650. [Google Scholar] [CrossRef]
- Siljamo, P.; Sofiev, M.; Severova, E.; Ranta, H.; Kukkonen, J.; Polevova, S.; Kubin, E.; Minin, A. Sources, impact and exchange of early-spring birch pollen in the Moscow region and Finland. Aerobiologia 2008, 24, 211–230. [Google Scholar] [CrossRef]
- Skjøth, C.A.; Bilińska, D.; Werner, M.; Malkiewicz, M.; Adams-Groom, B.; Kryza, M.; Drzeniecka-Osiadacz, A. Footprint areas of pollen from alder (Alnus) and birch (Betula) in the UK (Worcester) and Poland (Wroclaw) during 2005–2014. Acta Agrobot. 2015, 68, 315–324. [Google Scholar] [CrossRef]
- Sofiev, M.; Vira, J.; Kouznetsov, R.; Prank, M.; Soares, J.; Genikhovich, E. Construction of the SILAM Eulerian atmospheric dispersion model based on the advection algorithm of Michael Galperin. Geosci. Model Dev. 2015, 8, 3497–3522. [Google Scholar] [CrossRef]
- Siljamo, P.; Sofiev, M.; Filatova, E.; Grewling, Ł.; Jäger, S.; Khoreva, E.; Linkosalo, T.; Ortega Jimenez, S.; Ranta, H.; Rantio-Lehtimäki, A.; et al. A numerical model of birch pollen emission and dispersion in the atmosphere. Model. Eval. Sensit. Anal. Int. J. Biometeorol. 2013, 57, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Skjoth, C.A.; Šikoparija, B.; Jager, S. EAN-Network Chapter 2. Pollen Sources. In Allergenic Pollen: A Review of the Production, Release, Distribution and Health Impacts; Sofiev, M., Bergmann, K.C., Eds.; Springer Science+Business Media: Dordrecht, The Nederlands, 2013. [Google Scholar]
- Ritenberga, O.; Sofiev, M.; Kirillova, V.; Kalnina, L.; Genikhovich, E. Statistical modelling of non-stationary processes of atmospheric pollution from natural sources: Example of birch pollen. Agric. For. Meteorol. 2016, 226, 96–107. [Google Scholar] [CrossRef]
- De Rigo, D.; Caudullo, G.; Houston Durrant, T.; San-Miguel-Ayanz, J. The European Atlas of Forest Tree Species: Modelling, data and information on forest tree species. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publication Office of the European Union: Luxembourg, 2016; p. e01aa69+. [Google Scholar]
- McInnes, R.N.; Hemming, D.; Burgess, P.; Lyndsay, D.; Osborne, N.J.; Skjøth, C.A.; Thomas, S.; Vardoulakis, S. Mapping allergenic pollen vegetation in UK to study environmental exposure and human health. Sci. Total Environ. 2017, 599, 483–499. [Google Scholar] [CrossRef] [PubMed]
- Skjøth, C.S.; Sun, Y.; Karrer, G.; Sikoparija, B.; Smith, M.; Schaffner, U.; Müller-Schärer, H. Predicting abundances of invasive ragweed across Europe using a “top-down” approach. Sci. Total Environ. 2019, 686, 212–222. [Google Scholar] [CrossRef]
- Jackowiak, B. Atlas of Distribution of Vascular Plants in Poznań; No. 2; The Department of Plant Taxonomy of the Adam Mickiewicz University: Poznań, Poland, 1993; p. 409. [Google Scholar]
- Skjøth, C.A.; Sommer, J.; Brandt, J.; Hvidberg, M.; Geels, C.; Hansen, K.M.; Hertel, O.; Frohn, L.M.; Christensen, J.H. Copenhagen—A significant source to birch (Betula) pollen? Int. J. Biometeorol. 2008, 52, 453–462. [Google Scholar] [CrossRef]
- Skjøth, C.A.; Geels, C.; Hvidberg, M.; Hertel, O.; Brandt, J.; Frohn, L.M.; Hansen, K.M.; Hedegaard, G.B.; Christensen, J.; Moseholm, L. An inventory of tree species in Europe—An essential data input for air pollution modelling. Ecol. Model. 2008, 217, 292–304. [Google Scholar] [CrossRef]
- Skjøth, C.A.; Ørby, P.V.; Becker, T.; Geels, C.; Schlunssen, V. Identifying urban sources as cause of elevated grass pollen concentrations using GIS and remote sensing. Biogeosciences 2013, 10, 541–554. [Google Scholar] [CrossRef]
- Sjöman, H.; Östberg, J.; Bühler, O. Diversity and distribution of the urban tree population in ten major Nordic cities. Urban For. Urban Green. 2012, 11, 31–39. [Google Scholar]
- Pecero-Casimiro, R.; Fernández-Rodríguez, S.; Tormo-Molina, R.; Monroy-Colín, A.; Silva-Palacios, I.; Cortés-Pérez, J.P.; Gonzalo-Garijo, A.; Maya-Manzano, J.-M. Urban aerobiological risk mapping of ornamental trees using a new index based on LiDAR and Kriging: A case study of plane trees. Sci. Total Environ. 2019, 693, 133576. [Google Scholar] [CrossRef] [PubMed]
- Maya-Manzano, J.M.; Tormo-Molina, R.; Rodríguez, S.F.; Palacios, I.S.; Garijo, Á.G. Distribution of ornamental urban trees and their influence on airborne pollen in the SW of Iberian Peninsula. Landsc. Urban Plan. 2017, 157, 434–446. [Google Scholar] [CrossRef]
- Matthias, I.; Nielsen, A.B.; Giesecke, T. Evaluating the effect of flowering age and forest structure on pollen productivity estimates. Veget. Hist. Archaeobot. 2012, 21, 471–484. [Google Scholar] [CrossRef]
- Longman, K.A.; Wareing, P.F. Early Induction of Flowering in Birch Seedlings. Nature 1959, 184, 203. [Google Scholar] [CrossRef]
- Carson, W.W.; Andersen, H.-E.; Reutebuch, S.E.; McGaughey, R.J. Lidar Applications In Forestry—An Overview 7-2038. In Proceedings of the ASPRS Annual Conference Proceedings, Denver, CO, USA, 23–28 May 2004. [Google Scholar]
- Vauhkonen, J.; Maltamo, M.; McRoberts, R.E.; Næsset, E. Chapter 1: Introduction to Forestry Applications of Airborne Laser Scanning. In Concepts and Case Studies, Managing Forest Ecosystems; Maltamo, M., Næsset, E., Vauhkonen, J., Eds.; Springer Science+Business Media: Dordrecht, The Nederlands, 2014; Volume 27. [Google Scholar]
- MacFaden, S.W.; O’Neil-Dunne, J.P.M.; Royar, A.R.; Lu, J.W.T.; Rundle, A.G. High-resolution tree canopy mapping for New York City using LIDAR and object-based image analysis. J. Appl. Remote Sens. 2012, 6, 063567. [Google Scholar] [CrossRef]
- Vaughn, N.R.; Moskal, M.; Turnblom, E.C. Tree Species Detection Accuracies Using Discrete Point Lidar and Airborne Waveform Lidar. Remote Sens. 2012, 4, 377–403. [Google Scholar] [CrossRef]
- Donager, J.J.; Sankey, T.T.; Sankey, J.B.; Sanchez Meador, A.J.; Springer, A.E.; Bailey, J.D. Examining Forest Structure With Terrestrial Lidar: Suggestions and Novel Techniques Based on Comparisons Between Scanners and Forest Treatments. Earth Space Sci. 2018, 5, 753–776. [Google Scholar] [CrossRef]
- Shang, X.; Chazette, P. Interest of a full-waveform flown UV lidar to derive forest vertical structures and aboveground carbon. Forests 2014, 5, 1454–1480. [Google Scholar] [CrossRef]
- Hancock, S.; Armston, J.; Hofton, M.; Sun, X.; Tang, H.; Duncanson, L.I.; Kellner, J.R.; Dubayah, R. The GEDI Simulator: A Large-Footprint Waveform Lidar Simulator for Calibration and Validation of Spaceborne Missions. Earth Space Sci. 2019, 6, 294–310. [Google Scholar] [CrossRef]
- Roncat, A.; Morsdorf, F.; Briese, C.; Wagner, W.; Pfeifer, N. Chapter 2. Laser Pulse Interaction with Forest Canopy: Geometric and Radiometric Issues. In Forestry Applications of Airborne Laser Scanning; Maltamo, M., Næsset, E., Vauhkonen, J., Eds.; Concepts and Case Studies, Managing Forest Ecosystems; Springer Science+Business Media: Dordrecht, The Nederlands, 2014. [Google Scholar]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Woś, A. Klimat Polski w drugiej połowie XX wieku; Wyd. Naukowe UAM: Poznań, Poland, 2010. [Google Scholar]
- Kolendowicz, L.; Czernecki, B.; Półrolniczak, M.; Taszarek, M.; Tomczyk, A.M.; Szyga-Pluga, K. Homogenization of air temperature and its long-term trends in Poznań (Poland) for the period 1848–2016. Theor. Appl. Climatol. 2019, 136, 1357–1370. [Google Scholar] [CrossRef]
- Hirst, J.M. An automatic volumetric spore trap. Ann. Appl. Biol. 1952, 39, 257–265. [Google Scholar] [CrossRef]
- Galán, C.; Smith, M.; Thibaudon, M.; Frenguelli, G.; Oteros, J.; Gehrig, R.; Berger, U.; Clot, B.; Brandao, R.; EAS QC Working Group. Pollen monitoring: Minimum requirements and reproducibility of analysis. Aerobiologia 2014, 30, 385–395. [Google Scholar]
- Uria-Tellaetxe, I.; Carslaw, D.C. Conditional bivariate probability function for source identification. Environ. Model. Softw. 2014, 59, 1–9. [Google Scholar] [CrossRef]
- Carslaw, D.C.; Beevers, S.D. Characterising and understanding emission sources using bivariate polar plots and k-means clustering. Environ. Model. Softw. 2013, 40, 325–329. [Google Scholar] [CrossRef]
- Carslaw, D.C.; Ropkins, K. Openair—An R package for air quality data analysis. Environ. Model. Softw. 2012, 27, 52–61. [Google Scholar] [CrossRef]
- Google. Explore Street View. 2018. Available online: https://www.google.com/maps/streetview/explore/ (accessed on 15 February 2019).
- European Environment Agency (EEA); European Union. Copernicus Land Monitoring Service CORINE Land Cover. 2012,dataset. Available online: www.eea.europa.eu/data-and-maps/data/copernicus-land-monitoring-service-corine (accessed on 18 May 2019).
- Wężyk, P. (Ed.) Podręcznik dla uczestników szkoleń z wykorzystania produktów LiDAR; Główny Urząd Geodezji i Kartografii: Warszawa, Poland, 2015. (In Polish) [Google Scholar]
- Roussel, J.-R.; Auty, D. lidR: Airborne LiDAR Data Manipulation and Visualization for Forestry Applications. R Package Version 2.0.3; Repository: CRAN. 2019. Available online: https://cran.r-project.org/web/packages/lidR/index.html (accessed on 11 November 2019).
- Li, W.; Guo, Q.; Jakubowski, M.K.; Kelly, M. A new method for segmenting individual trees from the lidar point cloud. Photogramm. Eng. Remote Sens. 2012, 78, 75–84. [Google Scholar] [CrossRef]
- Barber, C.B.; Dobkin, D.P.; Huhdanpaa, H.T. The Quickhull algorithm for convex hulls. ACM Trans. Math. Softw. 1996, 22, 469–483. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 30 July 2018).
- Silva, C.A.; Crookston, N.L.; Hudak, A.T.; Vierling, L.A.; Klauberg, C.; Cardil, A. rLiDAR: LiDAR Data Processing and Visualization. R Package Version 0.1.1; Repository: CRAN. 2017. Available online: https://cran.r-project.org/web/packages/rLiDAR/index.html (accessed on 11 November 2019).
- Habel, K.; Grasman, R.; Gramacy, R.B.; Stahel, A.; Sterratt, D.C. Geometry: Mesh Generation and Surface Tesselation. R Package Version 0.3-6; Repository: CRAN. 2015. Available online: https://cran.r-project.org/web/packages/geometry/index.html (accessed on 11 November 2019).
- Elzinga, C.L.; Salzer, D.W.; Willoughby, J.W. Measuring & monitoring plant populations; U.S. Bureau of Land Management; Papers 17; University of Nebraska Lincoln: Lincoln, NE, USA, 1998. [Google Scholar]
- Jackson, S.T.; Lyford, M. Pollen Dispersal Models in Quaternary Plant Ecology: Assumptions, Parameters, and Prescriptions. Bot. Rev. 1999, 65, 39–75. [Google Scholar] [CrossRef]
- Skjøth, C.A.; Sommer, J.; Stach, A.; Smith, M.; Brandt, J. The long range transport of birch (Betula) pollen from Poland and Germany causes significant pre-season concentrations in Denmark. Clin. Exp. Allergy 2007, 37, 1204–1212. [Google Scholar] [CrossRef]
- Maya-Manzano, J.M.; Sadyś, M.; Tormo-Molina, R.; Fernández-Rodríguez, S.; Oteros, J.; Silva-Palacios, I.; Gonzalo-Garijo, A. Relationships between airborne pollen grains, wind direction and land cover using GIS and circular statistics. Sci. Total Environ. 2017, 584, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Skjøth, C.A.; Baker, P.; Sadyś, M.; Adams-Groom, B. Pollen from alder (Alnus sp.), birch (Betula sp.) and oak (Quercus sp.) in the UK originate from small woodlands. Urban Clim. 2015, 14, 414–428. [Google Scholar]
- Oteros, J.; García-Mozo, H.; Alcázar, P.; Belmonte, J.; Bermejo, D.; Boi, M.; Cariñanos, P.; de la Guardia, C.D.; Fernández-González, D.; González-Minero, F.; et al. A new method for determining the sources of airborne particles. J. Environ. Manag. 2015, 155, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Oteros, J.; Valencia, R.M.; del Río, S.; Vega, A.M.; García-Mozoc, H.; Galánc, C.; Gutiérrez, P.; Mandrioli, P.; Fernández-González, D. Concentric Ring Method for generating pollen maps. Quercus as case study. Sci. Total Environ. 2017, 576, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Seinfeld, J.; Pandis, S. Atmospheric Chemistry and Physics; Wiley: New York, NY, USA, 1998. [Google Scholar]
- Zhang, R.; Duhl, T.; Salam, M.T.; House, J.M.; Flagan, R.C.; Avol, E.L.; Gilliland, F.D.; Guenther, A.; Chung, S.H.; Lamb, B.K.; et al. Development of a regional-scale pollen emission and transport modeling framework for investigating the impact of climate change on allergic airway disease. Biogeosciences 2014, 11, 1461–1478. [Google Scholar] [CrossRef]
- Adams-Groom, B.; Skjøth, C.; Baker, M.; Welch, T.E. Modelled and Observed Surface Soil Pollen Deposition Distance Curves for Isolated Trees of Carpinus Betulus, Cedrus Atlantica, Juglans nigra and Platanus Acerifolia. Aerobiologia 2017, 33, 407–416. [Google Scholar] [CrossRef]
- Corden, J.; Millington, W.; Bailey, J.; Brookes, M.; Caulton, E.; Emberlin, J.; Mullins, J.; Simpson, C.; Wood, A. UK regional variations in Betula pollen (1993–1997). Aerobiologia 2000, 16, 227–232. [Google Scholar] [CrossRef]
- Zajączkowski, S.; Talarczyk, A.; Myszkowski, M.; Kucab, M. Wyniki aktualizacji stanu powierzchni leśnej i zasobów drzewnych w Lasach Państwowych na dzień 1 stycznia 2018 r; Oficyna wydawnicza Forest: Sękocin Stary, Poland, 2019. [Google Scholar]
- Nowak, D.J.; Hoehn, R.E.; Crane, D.E.; Stevens, J.C.; Walton, J.T. Northern Resource Bulletin NRS-8. In Assessing Urban Forest Effects and Values: San Francisco’s Urban Forest; USDA Forest Service: Newtown Square, PA, USA, 2007; p. 24. [Google Scholar]
- Tyrväinen, L.; Mäkinen, L.; Schipperijn, J. Tools for mapping social values for urban woodlands and of other green spaces. Landsc. Urban. Plan. 2007, 79, 5–19. [Google Scholar] [CrossRef]
- Selmi, W.; Weber, C.; Rivière, E.; Blond, N.; Mehdi, L.; Nowak, D. Air pollution removal by trees in public green spaces in Strasbourg City, France. Urban For. Urban Green. 2016, 17, 192–201. [Google Scholar] [CrossRef]
- Ow, L.F.; Ghosh, S. Urban cities and road traffic noise: Reduction through vegetation. Appl. Acoust. 2017, 120, 15–20. [Google Scholar] [CrossRef]
- Elmqvist, T.; Setälä, H.; Handel, S.N.; van der Ploeg, S.; Aronson, J.; Blignaut, J.N.; Gómez-Baggethun, E.; Nowak, D.J.; Kronenberg, J.; de Groot, R. Benefits of restoring ecosystem services in urban areas. Curr. Opin. Environ. Sustain. 2015, 14, 101–108. [Google Scholar]
- Carinanos, P.; Casares-Porcel, M. Urban green zones and related pollen allergy: A review. Some guidelines for designing spaces with low allergy impact. Landsc. Urban Plan. 2011, 101, 205–214. [Google Scholar] [CrossRef]
- Pauleit, S.; Duhme, F. Assessing the environmental performance of land cover types for urban planning. Landsc. Urban. Plan. 2000, 52, 1–20. [Google Scholar] [CrossRef]
- Sæbø, A.; Benedikz, T.; Randrup, T.B. Selection of trees for urban forestry in the Nordic countries. Urban. For. Urban. Green. 2003, 2, 101–114. [Google Scholar] [CrossRef]
- Jochner-Oette, S.; Stitz, T.; Jetschni, J.; Cariñanos, P. The Influence of Individual-Specific Plant Parameters and Species Composition on the Allergenic Potential of Urban Green Spaces. Forests 2018, 9, 284. [Google Scholar] [CrossRef]
- Kozlov, M.V.; Zvereva, E.L. Reproduction of mountain birch along a strong pollution gradient near Monchegorsk: Northwestern Russia. Environ. Pollut. 2004, 132, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Eränen, J.K. Local adaptation of mountain birch to heavy metals in subarctic industrial barrens. For. Snow Landsc. Res. 2006, 80, 161–167. [Google Scholar]
- Kostina, M.V.; Barabanshikova, N.S.; Bityugova, G.V.; Yasinskaya, O.I.; Dubach, A.M. Structural Modifications of Birch (Betula pendula Roth.) Crown in Relation to Environmental Conditions. Contemp. Probl. Ecol. 2015, 8, 584–597. [Google Scholar] [CrossRef]
- Yasaka, M.; Kobayashi, S.; Takeuchi, S.; Tokuda, S.; Takiya, M.; Ohno, Y. Prediction of birch airborne pollen counts by examining male catkin numbers in Hokkaido, northern Japan. Aerobiologia 2009, 25, 111–117. [Google Scholar] [CrossRef]
- Ilomäki, S.; Nikinmaa, E.; Mäkelä, A. Crown rise due to competition drives biomass allocation in silver birch. Can. J. For. Res. 2003, 33, 2395–2404. [Google Scholar] [CrossRef]
- Lintunen, A.; Sievänen, R.; Kaitaniemi, P.; Perttunen, J. Models of 3D crown structure for Scots pine (Pinus sylvestris) and silver birch (Betula pendula) grown in mixed forest. Can. J. Res. 2011, 41, 1779–1794. [Google Scholar] [CrossRef]
- Michel, D. Observations and Modelling of Birch Pollen Emission and Dispersion from an Isolated Source. Ph.D. Thesis, University of Basel, Basel, Switzerland, 2014. [Google Scholar]
- Dalponte, M.; Coomes, D.A. Tree-centric mapping of forest carbon density from airborne laser scanning and hyperspectral data. Methods Ecol. Evol. 2016, 7, 1236–1245. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.A.; Hudak, A.T.; Vierling, L.A.; Loudermilk, E.L.; O’Brien, J.J.; Hiers, J.K.; Khosravipour, A. Imputation of Individual Longleaf Pine (Pinus palustris Mill.) Tree Attributes from Field and LiDAR Data. Can. J. Remote Sens. 2016, 42, 554–573. [Google Scholar]
- Bogawski, P.; Grewling, Ł.; Jackowiak, B. Predicting the onset of Betula pendula flowering in Poznań (Poland) using remote sensing thermal data. Sci. Total Environ. 2019, 658, 1485–1499. [Google Scholar] [CrossRef] [PubMed]
- Bohlmann, S.; Shang, X.; Giannakaki, E.; Filioglou, M.; Saarto, A.; Romakkaniem, S.; Komppula, M. Detection and characterization of birch pollen in the atmosphere using multi-wavelength Raman lidar in Finland. Atmos. Chem. Phys. Discuss. 2019. [Google Scholar] [CrossRef]
- Robichaud, A.; Comtois, P. Statistical modeling, forecasting and time series analysis of birch phenology in Montreal, Canada. Aerobiologia 2017, 33, 529–554. [Google Scholar] [CrossRef]
- Grewling, L.; Jackowiak, B.; Nowak, M.; Uruska, A.; Smith, M. Variations and trends of birch pollen seasons during 15 years (1996–2010) in relation to weather conditions in Poznań (western Poland). Grana 2012, 51, 280–292. [Google Scholar] [CrossRef]
| Descriptive Statistic | Wind Directions/Sectors | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| E | N | NE | NW | S | SE | SW | W | ||
| Crown volume [m3] | sum | 416,903.6 | 253,275.2 | 233,730.9 | 148,841.3 | 419,030.2 | 1,922,507 | 168,407.4 | 225,820.8 |
| mean | 158.4 | 161.1 | 157.5 | 98.2 | 243.6 | 292.2 | 136.3 | 112.9 | |
| standard deviation | 194.0 | 182.2 | 191.6 | 109.7 | 282.4 | 311.8 | 149.6 | 129.3 | |
| skewness | 2.6 | 2.2 | 2.3 | 2.9 | 1.9 | 1.5 | 2.0 | 2.3 | |
| min | 0.8 | 3.9 | 2.7 | 3.8 | 3.0 | 1.6 | 3.7 | 1.7 | |
| 1st quartile | 33.5 | 37.9 | 33.5 | 26.5 | 41.9 | 58.5 | 31.4 | 27.7 | |
| median | 89.1 | 99.0 | 85.8 | 64.4 | 131.8 | 168.3 | 80.9 | 63.7 | |
| 3rd quartile | 205.2 | 215.4 | 198.7 | 131.4 | 346.8 | 433.0 | 187.3 | 150.4 | |
| max | 1561.8 | 1384.5 | 1267.5 | 1067.7 | 1645.1 | 1644.1 | 1038.2 | 984.6 | |
| Crown surface [m2] | sum | 419,437.7 | 257,290.4 | 236,891.4 | 174,051.4 | 374,786.6 | 1,655,425 | 176,389.0 | 250,973.5 |
| mean | 159.4 | 163.7 | 159.6 | 114.8 | 217.9 | 251.6 | 142.7 | 125.4 | |
| standard deviation | 122.4 | 114.8 | 124.2 | 77.4 | 161.8 | 176.2 | 97.5 | 89.7 | |
| skewness | 1.5 | 1.3 | 1.4 | 1.6 | 1.0 | 0.7 | 1.2 | 1.4 | |
| min | 20.0 | 20.8 | 20.1 | 20.1 | 20.1 | 20.0 | 20.1 | 20.2 | |
| 1st quartile | 68.3 | 75.1 | 68.0 | 56.6 | 85.5 | 104.9 | 66.8 | 58.7 | |
| median | 124.6 | 134.8 | 122.1 | 99.2 | 172.9 | 205.1 | 118.9 | 100.7 | |
| 3rd quartile | 211.2 | 219.2 | 207.4 | 151.9 | 315.2 | 375.9 | 199.5 | 166.1 | |
| max | 777.4 | 695.2 | 675.2 | 588.2 | 801.8 | 812.7 | 605.6 | 565.0 | |
| Pollen [count per hour] | total integrated hourly pollen count | 68,253.4 | 38,372 | 21,125.8 | 69,143.8 | 88,372.2 | 171,624.6 | 59,550.8 | 112,964.2 |
| no of hours with wind from the sector | 65 | 51 | 45 | 77 | 100 | 124 | 71 | 105 | |
| mean pollen count per hour | 1050.1 | 752.4 | 469.5 | 898.0 | 883.7 | 1384.1 | 838.7 | 1075.8 | |
| Mean crown volume/pollen ratio | 6.63 | 4.67 | 2.98 | 9.14 | 3.63 | 4.74 | 6.15 | 9.53 | |
| Mean crown surface/pollen ratio | 6.59 | 4.60 | 2.94 | 7.82 | 4.06 | 5.50 | 5.88 | 8.58 | |
| Mean crown volume/surface ratio | 0.99 | 0.98 | 0.99 | 0.86 | 1.12 | 1.16 | 0.96 | 0.90 | |
| Distance to Pollen Sampler [m] | Crown Surface in Sectors [m2] | Crown Volume in Sectors [m3] | ||
|---|---|---|---|---|
| Correlation Coefficient | p-Value | Correlation Coefficient | p-Value | |
| From 0 to 500 | *cor = −0.399 | 0.3268 | cor = −0.383 | 0.3487 |
| From 0 to 1000 | cor = 0.592 | 0.1224 | cor = 0.582 | 0.1302 |
| From 500 to 1000 | cor = 0.701 | 0.0526 | **rho = 0.524 | 0.1966 |
| From 0 to 1500 | cor = 0.674 | 0.0668 | cor = 0.670 | 0.0689 |
| From 500 to 1500 | cor = 0.728 | 0.0406 | cor = 0.729 | 0.0399 |
| From 1000 to 1500 | cor = 0.683 | 0.0617 | cor = 0.696 | 0.0554 |
| From 0 to 2000 | rho = 0.476 | 0.2431 | rho = 0.286 | 0.5008 |
| From 500 to 2000 | rho = 0.595 | 0.1323 | rho = 0.429 | 0.2992 |
| From 1000 to 2000 | rho = 0.571 | 0.1511 | rho = 0.476 | 0.2431 |
| From 1500 to 2000 | rho = 0.286 | 0.5008 | rho = 0.286 | 0.5008 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogawski, P.; Grewling, Ł.; Dziób, K.; Sobieraj, K.; Dalc, M.; Dylawerska, B.; Pupkowski, D.; Nalej, A.; Nowak, M.; Szymańska, A.; et al. Lidar-Derived Tree Crown Parameters: Are They New Variables Explaining Local Birch (Betula sp.) Pollen Concentrations? Forests 2019, 10, 1154. https://doi.org/10.3390/f10121154
Bogawski P, Grewling Ł, Dziób K, Sobieraj K, Dalc M, Dylawerska B, Pupkowski D, Nalej A, Nowak M, Szymańska A, et al. Lidar-Derived Tree Crown Parameters: Are They New Variables Explaining Local Birch (Betula sp.) Pollen Concentrations? Forests. 2019; 10(12):1154. https://doi.org/10.3390/f10121154
Chicago/Turabian StyleBogawski, Paweł, Łukasz Grewling, Katarzyna Dziób, Kacper Sobieraj, Marta Dalc, Barbara Dylawerska, Dominik Pupkowski, Artur Nalej, Małgorzata Nowak, Agata Szymańska, and et al. 2019. "Lidar-Derived Tree Crown Parameters: Are They New Variables Explaining Local Birch (Betula sp.) Pollen Concentrations?" Forests 10, no. 12: 1154. https://doi.org/10.3390/f10121154
APA StyleBogawski, P., Grewling, Ł., Dziób, K., Sobieraj, K., Dalc, M., Dylawerska, B., Pupkowski, D., Nalej, A., Nowak, M., Szymańska, A., Kostecki, Ł., Nowak, M. M., & Jackowiak, B. (2019). Lidar-Derived Tree Crown Parameters: Are They New Variables Explaining Local Birch (Betula sp.) Pollen Concentrations? Forests, 10(12), 1154. https://doi.org/10.3390/f10121154