Impact of Climate, Stand Growth Parameters, and Management on Isotopic Composition of Tree Rings in Chestnut Coppices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Climate
2.3. Growth Stand Conditions
2.4. Dendrochronological Analysis
2.5. Dendroisotopic Analysis
2.6. Statistical Analysis
3. Results
3.1. Dendrometric Characteristics
3.2. Dendrochronology and Dendroclimatology
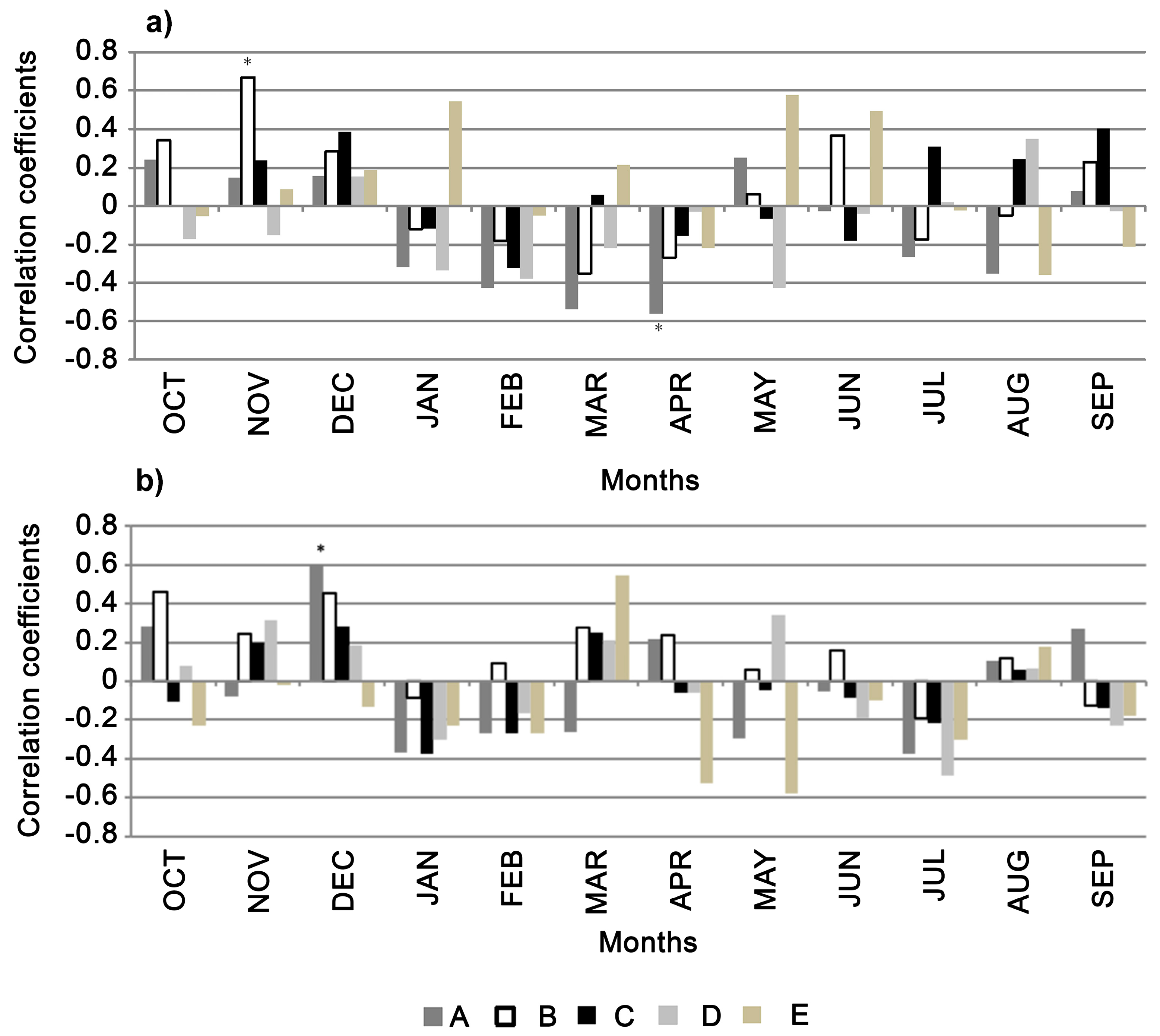
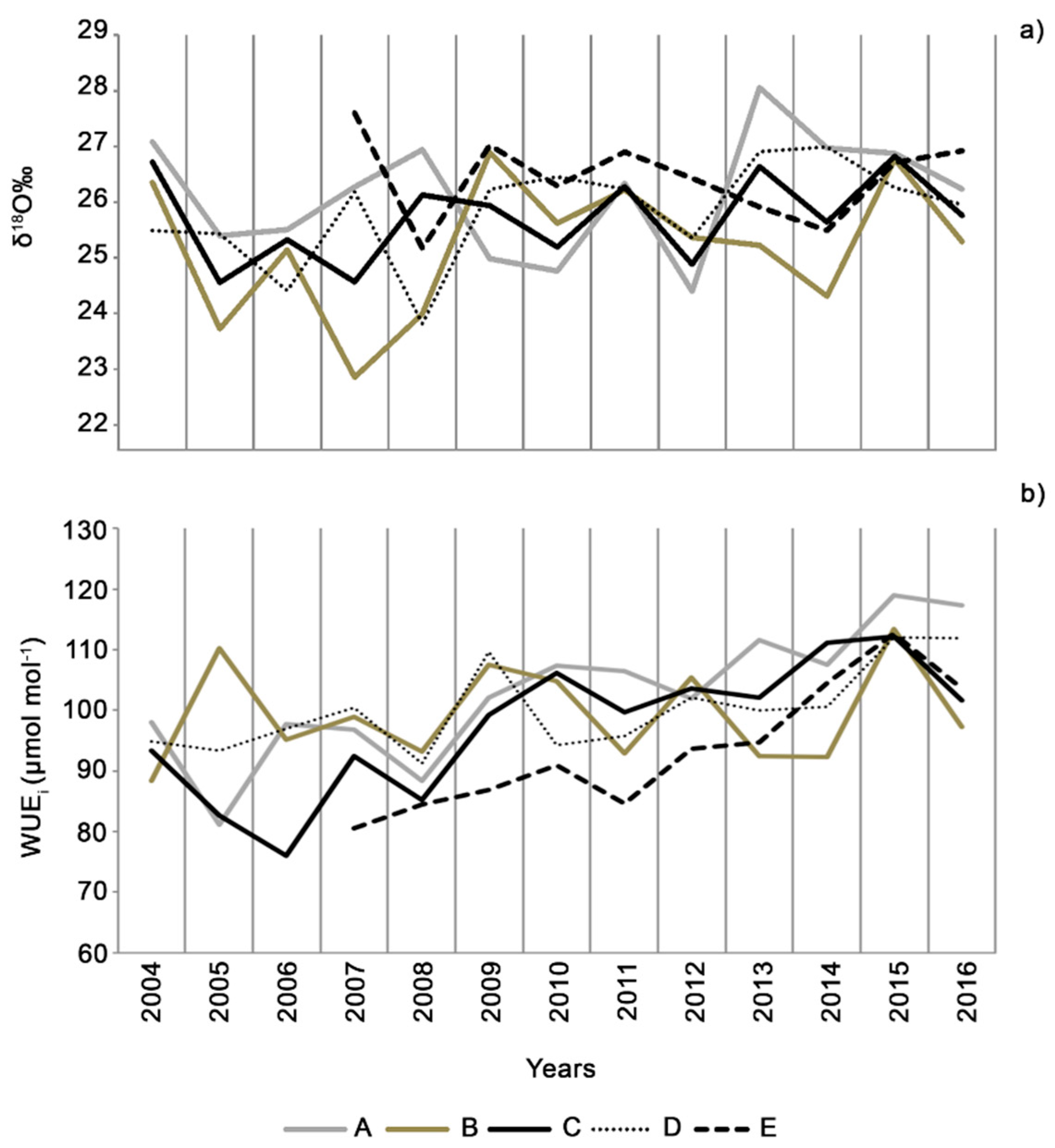
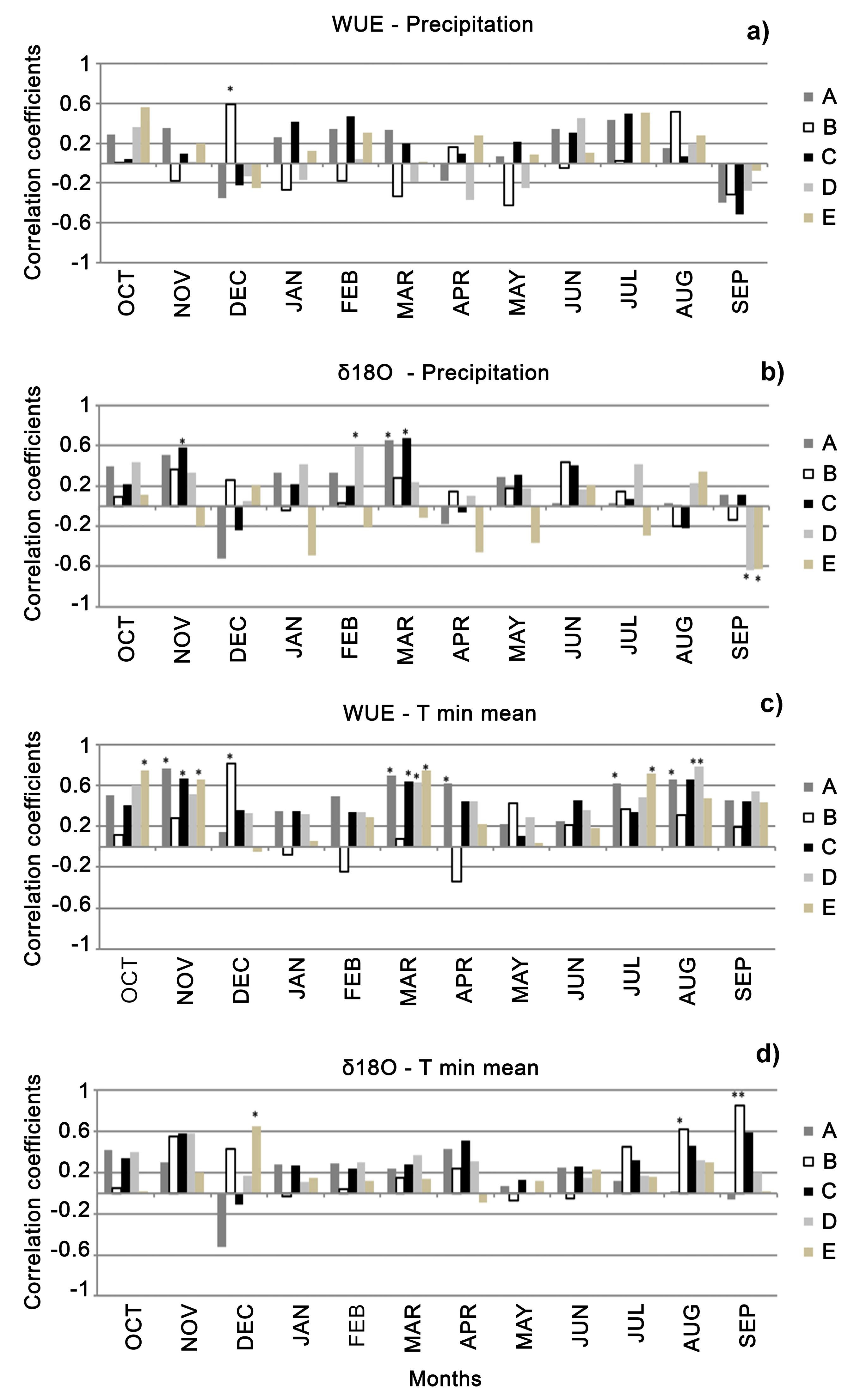
3.3. Stable Isotopes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Conedera, M.; Krebs, P.; Tinner, W.; Pradella, M.; Torriani, D. The cultivation of Castanea sativa (Mill.) in Europe, from its origin to its diffusion on a continental scale. Veg. Hist. Archaeobot. 2004, 13, 161–17029. [Google Scholar] [CrossRef] [Green Version]
- Manetti, M.C.; Becagli, C.; Carbone, F.; Corona, P.; Giannini, T.; Romano, R.; Pelleri, F. Linee Guida Per la Selvicoltura dei Cedui di Castagno; Consiglio Per la Ricerca in Agricoltura e l’analisi dell’economia Agraria (CREA): Roma, Italy, 2017; ISBN 9788899595579. [Google Scholar]
- Manetti, M.C.; Amorini, E.; Becagli, C.; Conedera, M.; Giudici, F. Productive potentiality of chestnut (Castanea sativa Mill.) stands over Europe. For. Snow Landsc. Res. 2001, 76, 471–476. [Google Scholar]
- Spina, S.; Romagnoli, M. Characterization of ring shake defect in chestnut (Castanea sativa Mill.) wood in the Lazio Region (Italy). For. Int. J. For. Res. 2010, 83, 315–327. [Google Scholar] [CrossRef] [Green Version]
- Romagnoli, M.; Spina, S. Physical and mechanical wood properties of ring-shaken chestnut (Castanea sativa) trees. Can. J. For. Res. 2013, 43. [Google Scholar] [CrossRef]
- Romagnoli, M.; Cavalli, D.; Spinaa, S. Wood quality of chestnut: Relationship between ring width, specific gravity, and physical and mechanical properties. BioResources 2014, 9, 1132–1147. [Google Scholar] [CrossRef]
- Romagnoli, M.; Fragiacomo, M.; Brunori, A.; Follesa, M.; Scarascia Mugnozza, G. Solid wood and wood based composites: The challenge of sustainability looking for a short and smart supply chain. Lect. Notes Civ. Eng. 2019, 24, 783–807. [Google Scholar]
- Mipaaf Piano del Settore Castanicolo 2010/2013 1; Documento di Sintesi; Ministero Delle Politiche Agricole e Forestali: Roma, Italy, 2009.
- Romagnoli, M.; Cherubini, M.; Prislan, P.; Gričar, J.; Spina, S.; Čufar, K. Main phases of wood formation in chestnut (Castanea sativa) in Central Italy - comparison of seasons 2008 and 2009. Drv. Ind. 2011, 62, 269–275. [Google Scholar] [CrossRef]
- Becagli, C.; Amorini, E.; Manetti, M.C. Incidenza della cipollatura in popolamenti cedui di castagno da legno del Monte Amiata. Ann. CRA 2006, 33, 245–256. [Google Scholar]
- Lionello, P. The Climate of the Mediterranean Region: From the Past to the Future; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 0124160425. [Google Scholar]
- Manetti, M.C.; Becagli, C.; Sansone, D.; Pelleri, F. Tree-oriented silviculture: A new approach for coppice stands. IForest 2016, 9, 791–800. [Google Scholar] [CrossRef] [Green Version]
- Sohn, J.A.; Saha, S.; Bauhus, J. Potential of forest thinning to mitigate drought stress: A meta-analysis. For. Ecol. Manag. 2016, 380, 261–273. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Leary, M.H.O.; Berry, J.A. Discrimination and the Intercellular carbon dioxide Concentration in Leaves. Aust.J.Plant Physiol 1982, 9, 121–137. [Google Scholar]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon Isotope Discrimination and Photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Battipaglia, G.; De Micco, V.; Brand, W.A.; Saurer, M.; Aronne, G.; Linke, P.; Cherubini, P. Drought impact on water use efficiency and intra-annual density fluctuations in Erica arborea on Elba (Italy). Plant, Cell Environ. 2014, 37, 382–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battipaglia, G.; De Micco, V.; Brand, W.A.; Linke, P.; Aronne, G.; Saurer, M.; Cherubini, P. Variation of vessel diameter and δ13C in false rings of Arbutus unedo L. reflect different environmental conditions. New Phytol. 2010, 188, 1099–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voelker, S.L.; Brooks, J.R.; Meinzer, F.C.; Anderson, R.; Bader, M.K.; Battipaglia, G.; Becklin, K.M.; Beerling, D.; Bert, D.; Betancourt, J.L. A dynamic leaf gas-exchange strategy is conserved in woody plants under changing ambient CO2: Evidence from carbon isotope discrimination in paleo and CO2 enrichment studies. Glob. Chang. Biol. 2016, 22, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, M.; Vinciguerra, V.; Silvestri, A. Heat Treatment Effect on Lignin and Carbohydrates in Corsican Pine Earlywood and Latewood Studied by PY--GC--MS Technique. J. Wood Chem. Technol. 2018, 38, 57–70. [Google Scholar] [CrossRef]
- Fritts, H. Tree Rings and Climate; Academic Press: San Diego, CA, USA, 1976. [Google Scholar]
- Corona, P.; Romagnoli, M.; Torrini, L. Stem annual increments as ecobiological indicators in Turkey oak (Quercus cerris L.). Trees 1995, 10, 13–19. [Google Scholar] [CrossRef]
- Romagnoli, M.; Codipietro, G. Pointer years and growth in Turkey oak (Quercus cerris L) in Latium (central Italy). A dendroclimatic approach. Ann. Des Sci. For. 1996, 53, 671–684. [Google Scholar] [CrossRef] [Green Version]
- Eckstein, D.; Bauch, J. Beitrag zur Rationalisierung eines dendrochronologischen Verfahrens und zur Analyse seiner Aussagesicherheit. Forstwiss. Cent. 1969, 88, 230–250. [Google Scholar] [CrossRef]
- Weigt, R.B.; Bräunlich, S.; Siegwolf, R.T.W.; Saurer, M.; Grams, T.E.E.; Nikolova, P.S.; Weigt, R.B.; Zimmermann, L.; Dietrich, H.-P. Comparison of δ18O and δ13C values between tree-ring whole wood and cellulose in five species growing under two different site conditions. Rapid Commun. Mass Spectrom. 2015, 29, 2233–2244. [Google Scholar] [CrossRef]
- Riechelmann, D.F.C.; Maus, M.; Dindorf, W.; Konter, O.; Schöne, B.R.; Esper, J. Comparison of δ13C and δ18O from cellulose, whole wood, and resin-free whole wood from an old high elevation Pinus uncinata in the Spanish central Pyrenees*. Isotopes Environ. Health Stud. 2016, 52, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Valor, T.; Casals, P.; Altieri, S.; González-Olabarria, J.R.; Piqué, M.; Battipaglia, G. Disentangling the effects of crown scorch and competition release on the physiological and growth response of Pinus halepensis Mill. using δ 13 C and δ 18 O isotopes. For. Ecol. Manag. 2018, 424, 276–287. [Google Scholar] [CrossRef]
- Battipaglia, G.; Pelleri, F.; Lombardi, F.; Altieri, S.; Vitone, A.; Conte, E.; Tognetti, R. Effects of associating Quercus robur L. and Alnus cordata Loisel. on plantation productivity and water use efficiency. For. Ecol. Manag. 2017, 391, 106–114. [Google Scholar] [CrossRef]
- McCarroll, D.; Loader, N.J. Stable isotopes in tree rings. Quat. Sci. Rev. 2004, 23, 771–801. [Google Scholar] [CrossRef]
- Ehleringer, J.; Hall, A.; Farquhar, G.D. Stable Isotopes and Plant Carbon–Water Relations; Academic Press: Cambridge, MA, USA, 1993. [Google Scholar]
- Gessler, A.; Ferrio, J.P.; Hommel, R.; Treydte, K.; Werner, R.A.; Monson, R.K. Stable isotopes in tree rings: Towards a mechanistic understanding of isotope fractionation and mixing processes from the leaves to the wood. Tree Physiol. 2014, 34, 796–818. [Google Scholar] [CrossRef] [Green Version]
- Seibt, U.; Rajabi, A.; Griffiths, H.; Berry, J.A. Carbon isotopes and water use efficiency: Sense and sensitivity. Oecologia 2008, 155, 441–454. [Google Scholar] [CrossRef]
- Altieri, S.; Mereu, S.; Cherubini, P.; Castaldi, S.; Sirignano, C.; Lubritto, C.; Battipaglia, G. Tree-ring carbon and oxygen isotopes indicate different water use strategies in three Mediterranean shrubs at Capo Caccia (Sardinia, Italy). Trees Struct. Funct. 2015, 29, 1593–1603. [Google Scholar] [CrossRef]
- Moreno-Gutiérrez, C.; Dawson, T.E.; Nicolás, E.; Querejeta, J.I. Isotopes reveal contrasting water use strategies among coexisting plant species in a mediterranean ecosystem. New Phytol. 2012, 196, 489–496. [Google Scholar] [CrossRef]
- Moreno-Gutiérrez, C.; Battipaglia, G.; Cherubini, P.; Delgado Huertas, A.; Querejeta, J.I. Pine afforestation decreases the long-term performance of understorey shrubs in a semi-arid Mediterranean ecosystem: A stable isotope approach. Funct. Ecol. 2015, 29, 15–25. [Google Scholar] [CrossRef]
- Romagnoli, M.; Nocetti, M.; Sarlatto, M.; Evangelistella, L. Dendrochronological assessment of chestnut (Castanea sativa Mill.) for dating purposes in Central Italy. Dendrochronologia 2004, 3, 117–130. [Google Scholar] [CrossRef]
- Jarman, R.; Moir, A.K.; Webb, J.; Chambers, F.M.; Russell, K. Dendrochronological assessment of British veteran sweet chestnut (Castanea sativa) trees: Successful cross-matching, and cross-dating with British and French oak (Quercus) chronologies. Dendrochronologia 2018, 51, 10–21. [Google Scholar] [CrossRef]
- Fonti, P.; Solomonoff, N.; García-González, I. Earlywood vessels of Castanea sativa record temperature before their formation. New Phytol. 2007, 173, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Di Matteo, G.; De Angelis, P.; Brugnoli, E.; Cherubini, P.; Scarascia-Mugnozza, G. Tree-ring Δ 13C reveals the impact of past forest management on water-use efficiency in a Mediterranean oak coppice in Tuscany (Italy). Ann. For. Sci. 2010, 67, 510. [Google Scholar] [CrossRef]
- Fonti, P.; Giudici, F. Quantità e qualità della massa legnosa ottenibile da un ceduo invecchiato. Schweiz. Z. Forstwes. 2001, 152, 417–424. [Google Scholar] [CrossRef] [Green Version]
- Schrader, J.; Baba, K.; May, S.T.; Palme, K.; Bennett, M.; Bhalerao, R.P.; Sandberg, G. Polar auxin transport in the wood-forming tissues of hybrid aspen is under simultaneous control of developmental and environmental signals. Proc. Natl. Acad. Sci. USA 2003, 100, 10096–10101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrader, J.; Nilsson, J.; Mellerowicz, E.; Berglund, A.; Nilsson, P.; Hertzberg, M.; Sandberg, G. A high-resolution transcript profile across the wood-forming meristem of poplar identifies potential regulators of cambial stem cell identity. Plant Cell 2004, 16, 2278–2292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deslauriers, A.; Morin, H. Intra-annual tracheid production in balsam fir stems and the effect of meteorological variables. Trees Struct. Funct. 2005, 19, 402–408. [Google Scholar] [CrossRef] [Green Version]
- Gričar, J.; Čufar, K.; Spina, S.; Cherubini, M.; Romagnoli, M.; Prislan, P. Xylem and phloem formation in chestnut (Castanea sativa Mill.) during the 2008 growing season. Dendrochronologia 2011, 29, 127–134. [Google Scholar]
- de Micco, V.; Battipaglia, G.; Brand, W.A.; Linke, P.; Saurer, M.; Aronne, G.; Cherubini, P. Discrete versus continuous analysis of anatomical and δ13C variability in tree rings with intra-annual density fluctuations. Trees Struct. Funct. 2012, 26, 513–524. [Google Scholar] [CrossRef]
- Romagnoli, M.; Moroni, S.; Recanatesi, F.; Salvati, R.; Mugnozza, G.S. Climate factors and oak decline based on tree-ring analysis. A case study of peri-urban forest in the Mediterranean area. Urban For. Urban Green. 2018, 34, 17–28. [Google Scholar] [CrossRef]
- Petrucco, L.; Nardini, A.; Von Arx, G.; Saurer, M.; Cherubini, P. Isotope signals and anatomical features in tree rings suggest a role for hydraulic strategies in diffuse drought-induced die-back of Pinus nigra. Tree Physiol. 2017, 37, 523–535. [Google Scholar] [PubMed]
- Ciampi, C. Evoluzione della cerchia legnosa in Castanea sativa Mill. Plant Biosyst. 1951, 58, 271–292. [Google Scholar]
- Génova, R.; Gracia, C.A. Análisis dendroclimatológico del castaño (Castanea sativa Mill.) en el Macizo del Montseny. Mediterr. Ser. Biol. 1984, 7, 67–82. [Google Scholar] [CrossRef] [Green Version]
- García-González, I.; Fonti, P. Selecting earlywood vessels to maximize their environmental signal. Tree Physiol. 2006, 26, 1289–1296. [Google Scholar] [CrossRef] [Green Version]
- Cochard, H.; Tyree, M.T. Xylem dysfunction in Quercus: Vessel sizes, tyloses, cavitation and seasonal changes in embolism. Tree Physiol. 1990, 6, 393–407. [Google Scholar] [CrossRef] [Green Version]
- Uwe, G.H.; John, S.S. Functional and ecological Xylem anatomy. Funct. Ecol. Xylem Anat. 2001, 1–281. [Google Scholar] [CrossRef] [Green Version]
- von Allmen, E.I.; Sperry, J.S.; Bush, S.E. Contrasting whole-tree water use, hydraulics, and growth in a co-dominant diffuse-porous vs. ring-porous species pair. Trees Struct. Funct. 2015, 29, 717–728. [Google Scholar] [CrossRef]
- Pérez-de-Lis, G.; Rozas, V.; Vázquez-Ruiz, R.; García-González, I. Do ring-porous oaks prioritize earlywood vessel efficiency over safety? Environmental effects on vessel diameter and tyloses formation. Agric. For. Meteorol. 2017, 248, 205–214. [Google Scholar] [CrossRef]
- Castellani, C. Studio sull’incremento diametrico stagionale di alcune delle piuimportanti specie forestali che popolano i boschi italiani. Ann. ISAFA 1979, 7, 3–106. [Google Scholar]
- Reynolds-Henne, C.E.; Saurer, M.; Siegwolf, R.T.W. Temperature versus species-specific influences on the stable oxygen isotope ratio of tree rings. Trees Struct. Funct. 2009, 23, 801–811. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, L.; Zhao, P.; Zhu, L.; Zhang, Z.; Zhao, X.; Ni, G. Difference in response of water use to evaporative demand for codominant diffuse-porous versus ring-porous tree species under N addition in a temperate forest. Ecohydrology 2017, 10, 1–9. [Google Scholar] [CrossRef]
- Gea-Izquierdo, G.; Chaar, H.; Canellas, I.; Fonti, P.; Cherubini, P.; Martin-Benito, D. Xylem hydraulic adjustment and growth response of Quercus canariensis Willd. to climatic variability. Tree Physiol. 2012, 32, 401–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peñuelas, J.; Canadell, J.G.; Ogaya, R. Increased water-use efficiency during the 20th century did not translate into enhanced tree growth. Glob. Ecol. Biogeogr. 2011, 20, 597–608. [Google Scholar] [CrossRef]
- Ferrio, J.P.; Voltas, J. Carbon and oxygen isotope ratios in wood constituents of Pinus halepensis as indicators of precipitation, temperature and vapour pressure deficit. Tellus B Chem. Phys. Meteorol. 2005, 57, 164–173. [Google Scholar] [CrossRef]
- Roden, J.S.; Ehleringer, J.R. Hydrogen and oxygen isotope ratios of tree ring cellulose for field-grown riparian trees. Oecologia 2000, 123, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Treydte, K.; Battipaglia, G.; Fonti, P.; Frank, D.; Graf Pannatier, E.; Saurer, M.; Siegwolf, R.; Ullrich, B.; Gessler, A.; Werner, W.; et al. Seasonal transfer of oxygen isotopes from precipitation and soil to the tree ring: Source water versus needle water enrichment. New Phytol. 2014, 202, 772–783. [Google Scholar] [CrossRef]
- Battipaglia, G.; Saurer, M.; Cherubini, P.; Siegwolf, R.T.W.; Cotrufo, M.F. Tree rings indicate different drought resistance of a native (Abies alba Mill.) and a nonnative (Picea abies (L.) Karst.) species co-occurring at a dry site in Southern Italy. For. Ecol. Manag. 2009, 257, 820–828. [Google Scholar] [CrossRef]
- Castagneri, D.; Battipaglia, G.; Von Arx, G.; Pacheco, A.; Carrer, M. Tree-ring anatomy and carbon isotope ratio show both direct and legacy effects of climate on bimodal xylem formation in Pinus pinea. Tree Physiol. 2018, 38, 1098–1109. [Google Scholar] [CrossRef]
- Umebayashi, T.; Fukuda, K. Seasonal changes in the occurrence of embolisms among broad-leaved trees in a temperate region. Am. J. Bot. 2018, 96, 873–881. [Google Scholar] [CrossRef]
- Fotelli, M.N.; Rennenberg, H.; Holst, T.; Mayer, H.; Geßler, A. Effects of climate and silviculture on the carbon isotope composition of understorey species in a beech (Fagus sylvatica L.) forest. New Phytol. 2003, 159, 229–244. [Google Scholar] [CrossRef]
- Martín-Benito, D.; Del Río, M.; Heinrich, I.; Helle, G.; Cañellas, I. Response of climate-growth relationships and water use efficiency to thinning in a Pinus nigra afforestation. For. Ecol. Manag. 2010, 259, 967–975. [Google Scholar] [CrossRef]
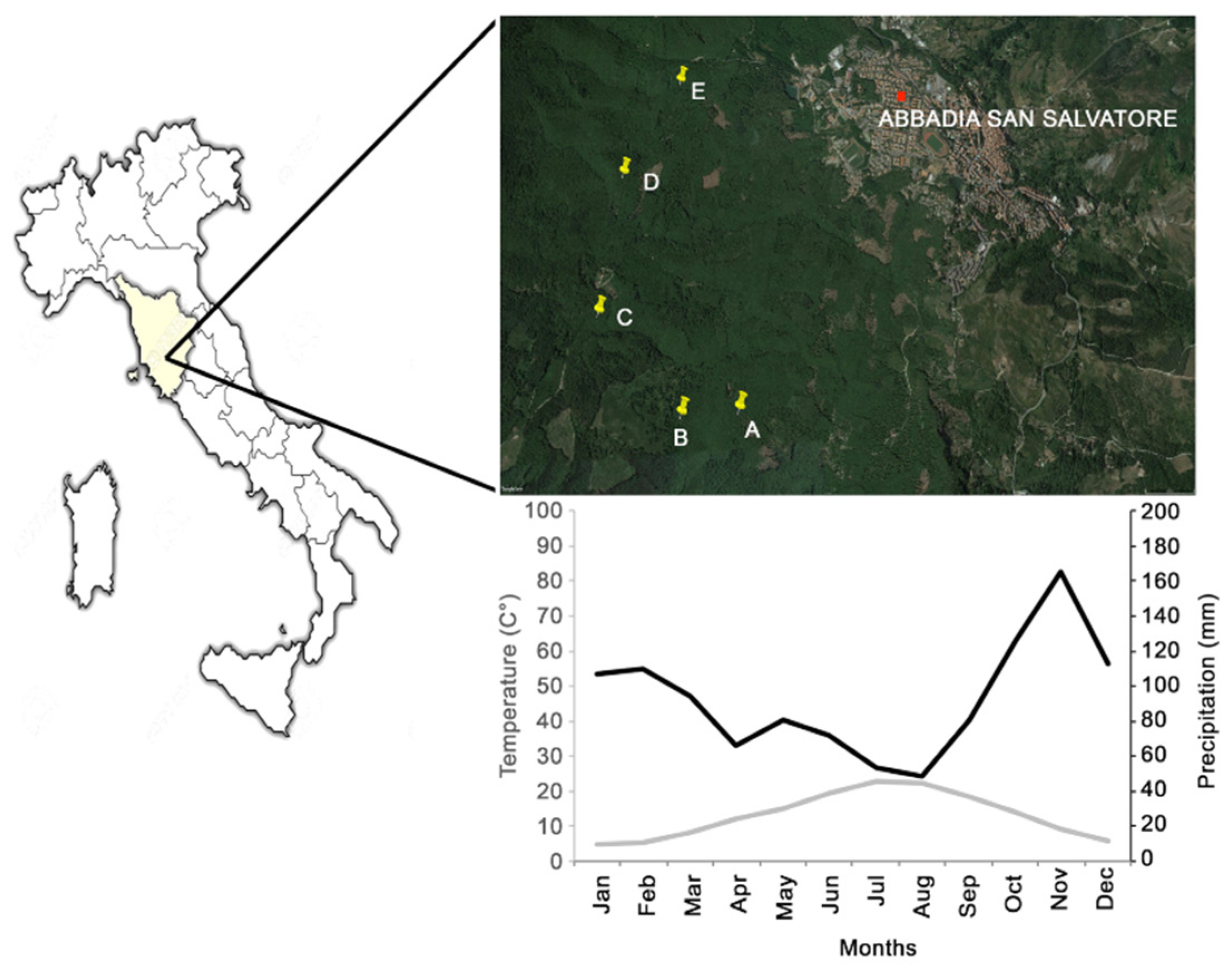
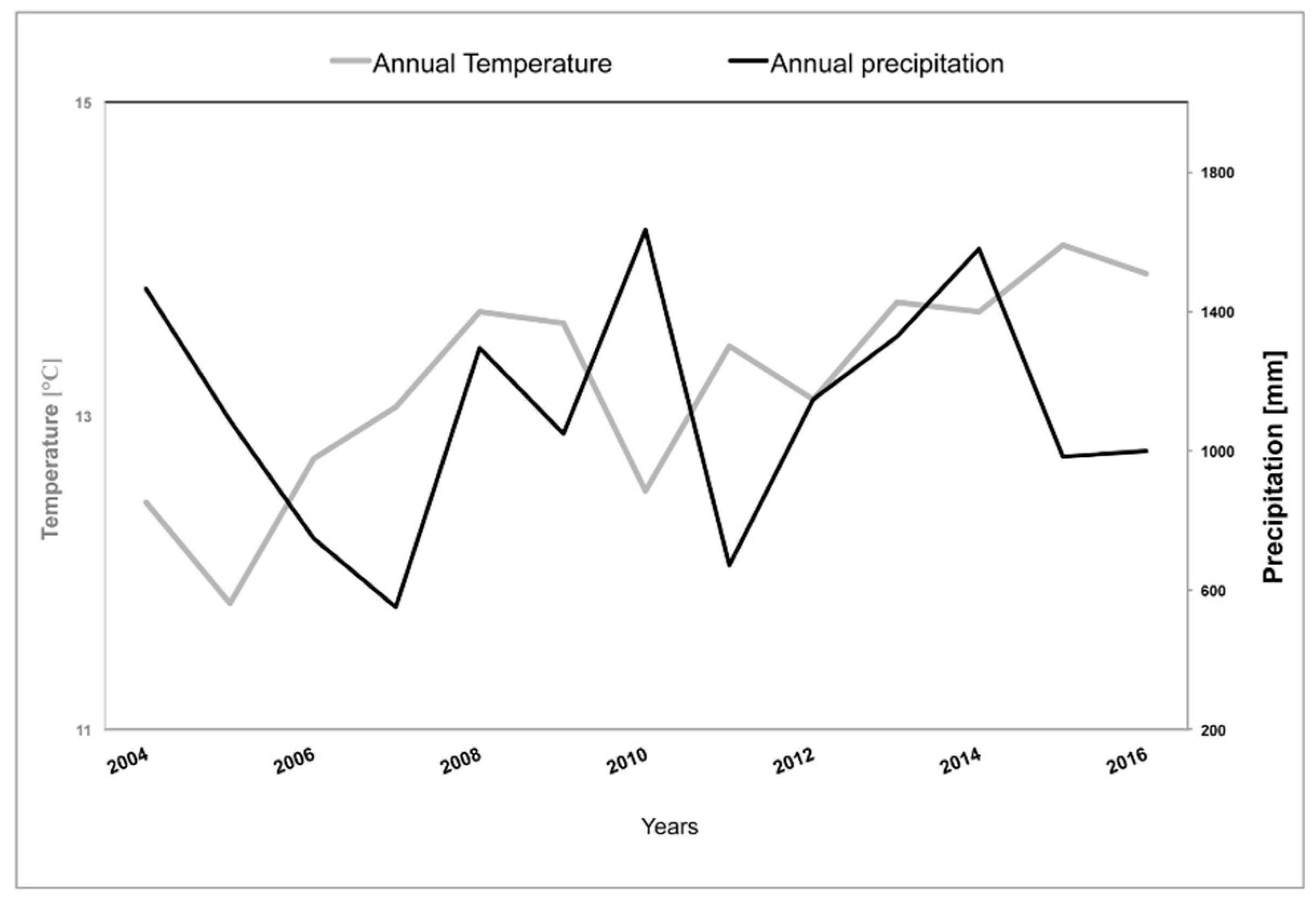
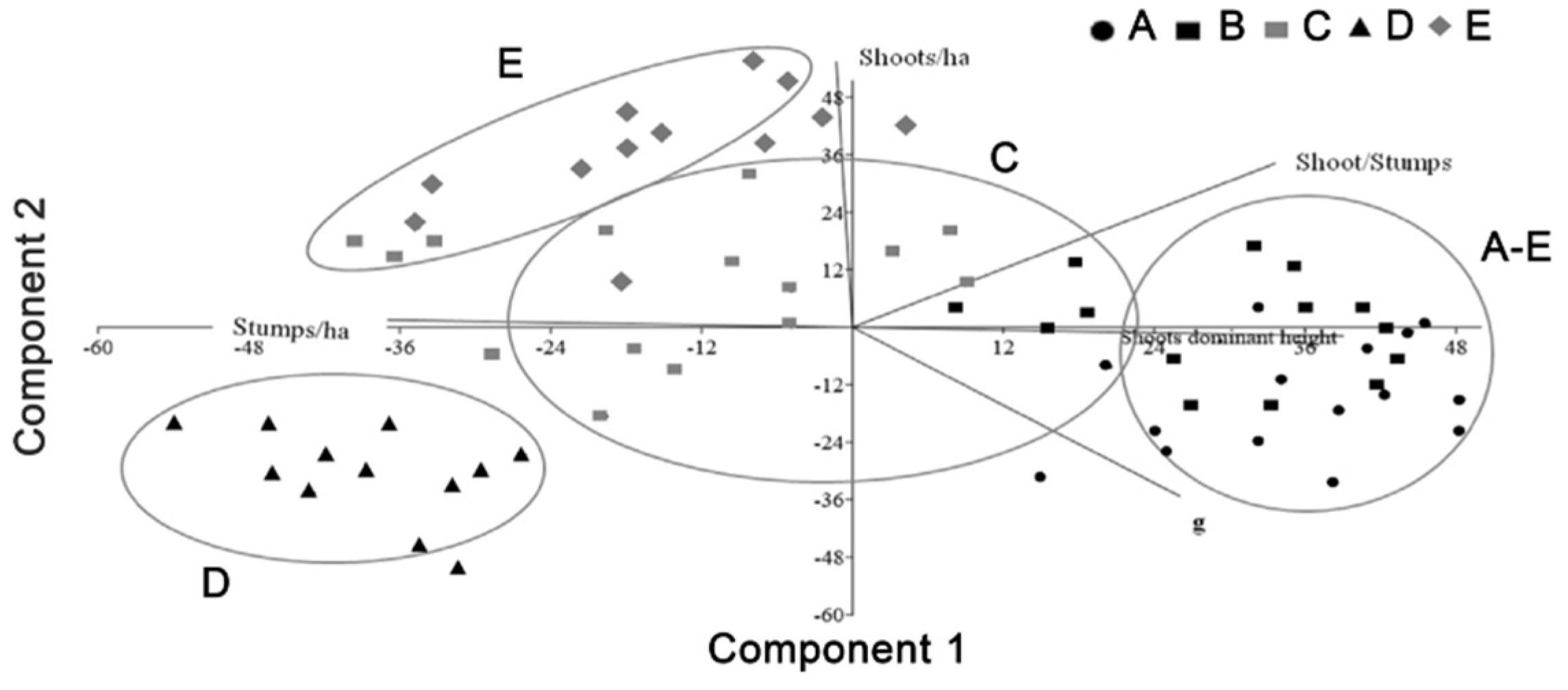
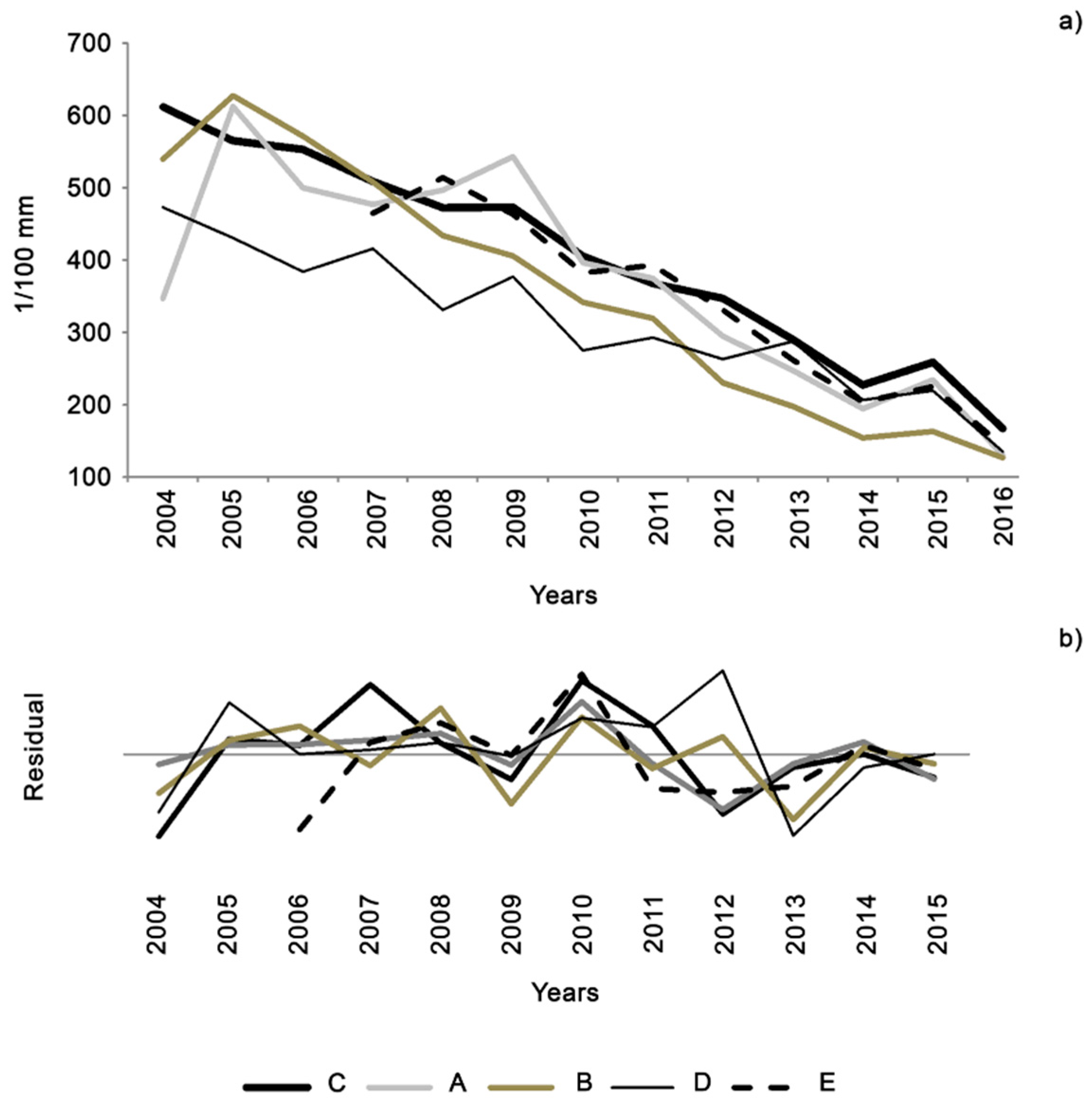
| Stand | A | B | C | D | E |
|---|---|---|---|---|---|
| Altitude (m) | 990 | 1030 | 1145 | 1100 | 1030.0 |
| Exposure | SE | SE | SE | SE | SO |
| Slope (%) | 0 | 30 | 0 | 30 | 40.0 |
| Age (years) | 15 | 11 | 12 | 15 | 11.0 |
| Shoots’ Dominant height (m) | 14.9 | 15.3 | 13.6 | 13.8 | 14.0 |
| Stumps ha−¹ | 580 | 623 | 780 | 1047 | 962.0 |
| Shoots ha−¹ | 3480 | 3860 | 4810 | 2845 | 5210.0 |
| Shoots/stumps | 9.8 | 8.25 | 8 | 4.2 | 9.1 |
| sd | 3.6 | 2.4 | 4.5 | 1.5 | 4.0 |
| Shoots dbh (m) | 9.6 | 10.6 | 8.9 | 10 | 7.0 |
| sd | 2.9 | 2.91 | 2.87 | 2.02 | 2.0 |
| Stumps/ha | 70 | 113 | 56 | 70 | 110.0 |
| Volume (m3 ha−1) | 193 | 269 | 220 | 156 | 136.0 |
| G (m² ha−1) | 25.41 | 34.20 | 29.95 | 22.42 | 20.31 |
| Tree Ring Width (1/100 mm) | 372.95 | 355.34 | 403.78 | 314.75 | 338.2 |
| sd | 147.75 | 172.74 | 140.75 | 98.34 | 125.8 |
| Mean Sensitivity | 0.36 | 0.3 | 0.27 | 0.31 | 0.4 |
| A | B | C | D | E | |
|---|---|---|---|---|---|
| A | Di * g * SS *** | Di *** g *** | |||
| B | SS *** | Di *** g *** | |||
| C | SS | Di *** g ** | |||
| D | Di *** g ** SS *** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marini, F.; Battipaglia, G.; Manetti, M.C.; Corona, P.; Romagnoli, M. Impact of Climate, Stand Growth Parameters, and Management on Isotopic Composition of Tree Rings in Chestnut Coppices. Forests 2019, 10, 1148. https://doi.org/10.3390/f10121148
Marini F, Battipaglia G, Manetti MC, Corona P, Romagnoli M. Impact of Climate, Stand Growth Parameters, and Management on Isotopic Composition of Tree Rings in Chestnut Coppices. Forests. 2019; 10(12):1148. https://doi.org/10.3390/f10121148
Chicago/Turabian StyleMarini, Francesco, Giovanna Battipaglia, Maria Chiara Manetti, Piermaria Corona, and Manuela Romagnoli. 2019. "Impact of Climate, Stand Growth Parameters, and Management on Isotopic Composition of Tree Rings in Chestnut Coppices" Forests 10, no. 12: 1148. https://doi.org/10.3390/f10121148
APA StyleMarini, F., Battipaglia, G., Manetti, M. C., Corona, P., & Romagnoli, M. (2019). Impact of Climate, Stand Growth Parameters, and Management on Isotopic Composition of Tree Rings in Chestnut Coppices. Forests, 10(12), 1148. https://doi.org/10.3390/f10121148







