Functional Composition Changes of a Subtropical Monsoon Evergreen Broad-Leaved Forest Under Environmental Change
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Community Surveys
2.3. Soil Water Content
2.4. Functional Traits
2.5. Analytical Approach
2.5.1. Trends in Functional Composition
2.5.2. The Influence of Disturbance on Functional Composition
2.5.3. The Influence of Environmental Change on Functional Composition
2.5.4. Partitioning Effects of Disturbance and Environment Change on Functional Composition
3. Results
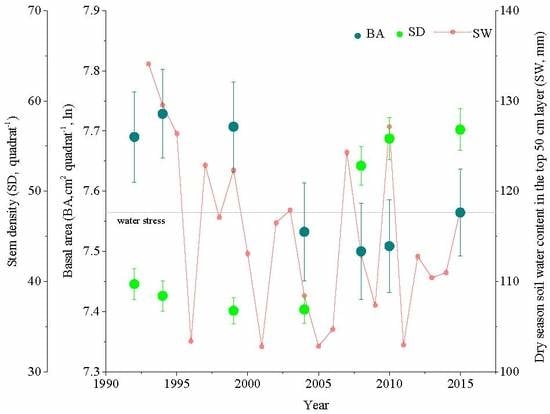
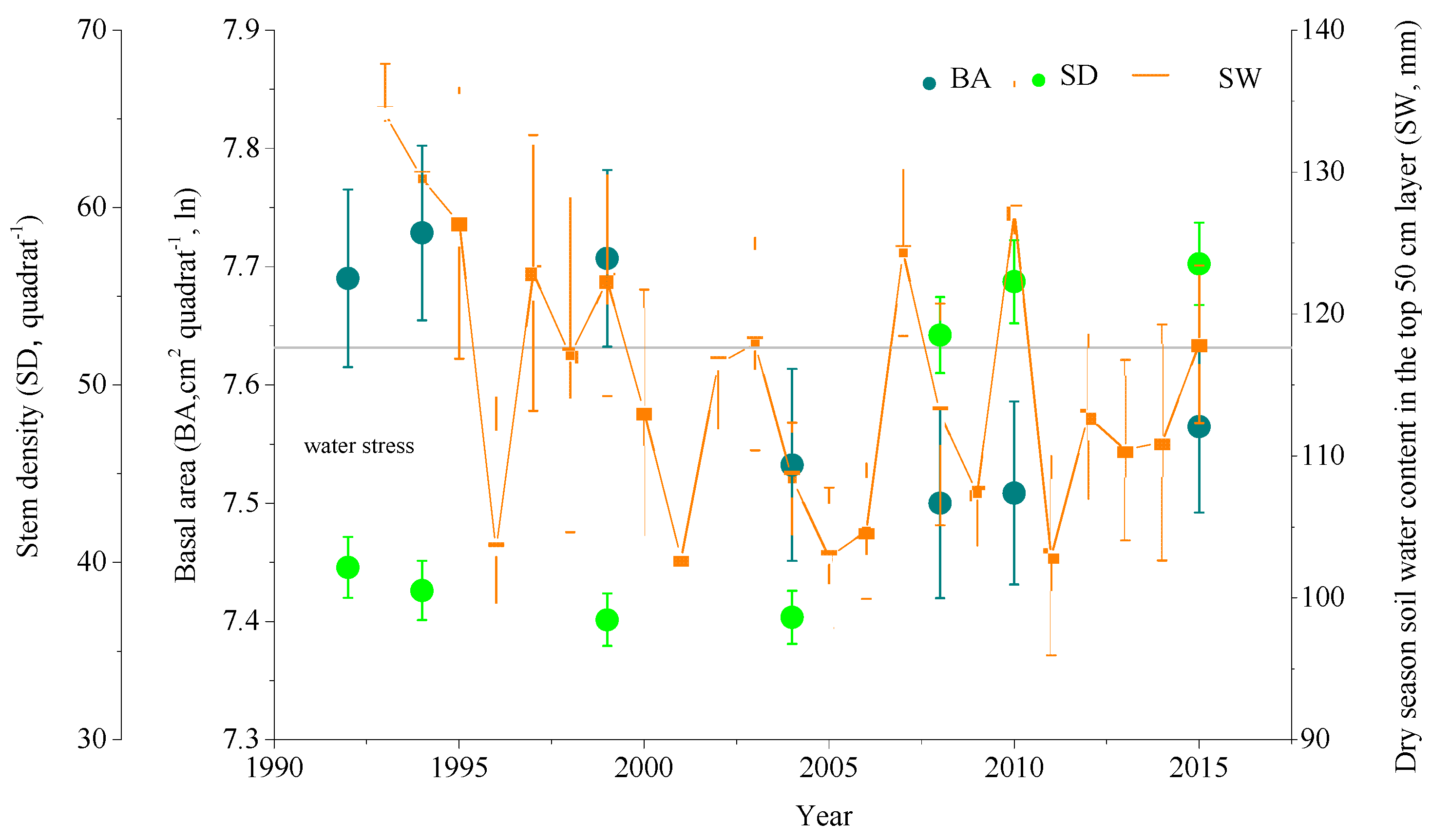
3.1. Community and Soil Water
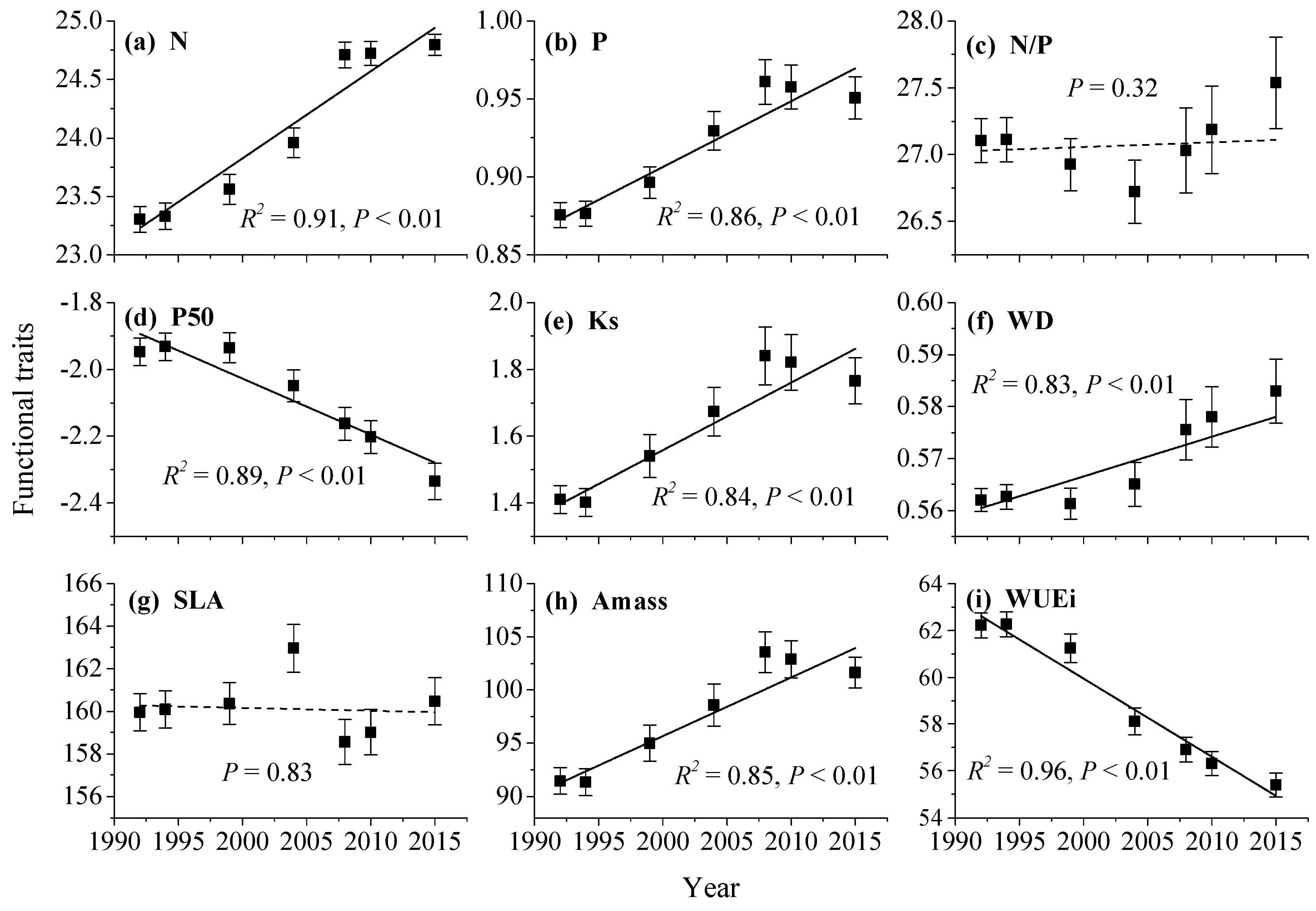
3.2. Trends in Functional Composition
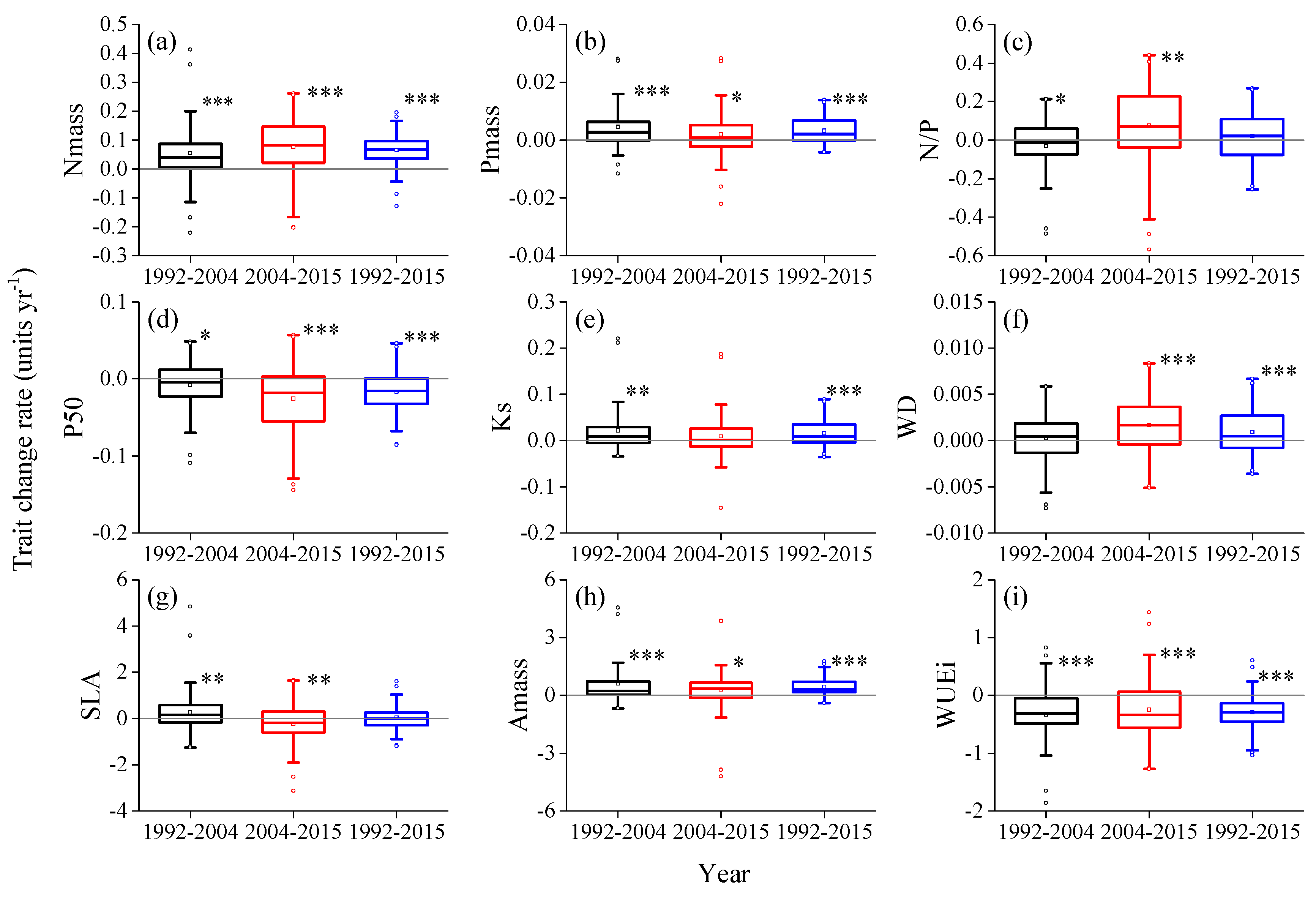
3.3. The Influence of Initial Functional Composition
3.4. Basal Area and Functional Composition
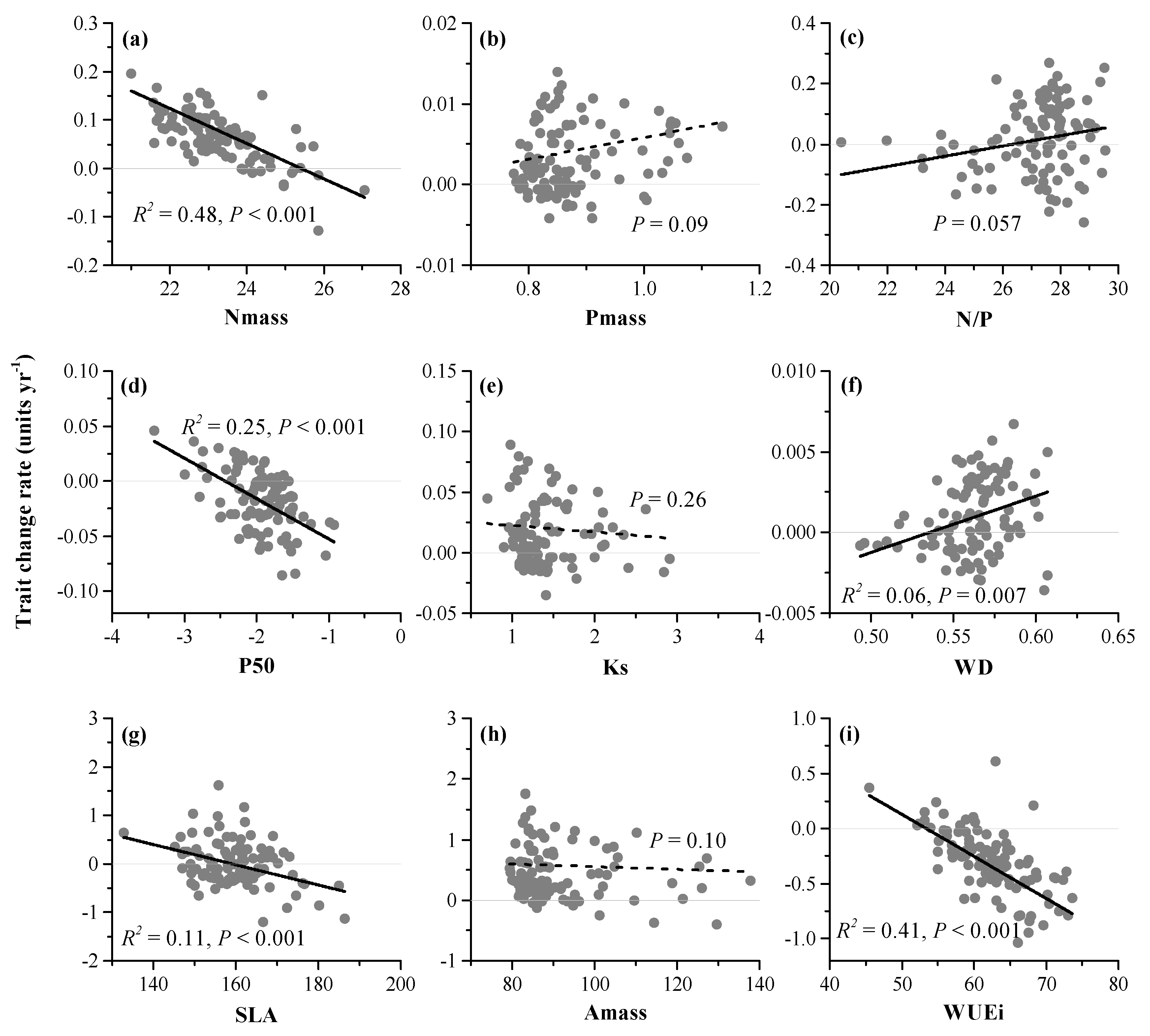
3.5. Soil Dryness and Functional Composition
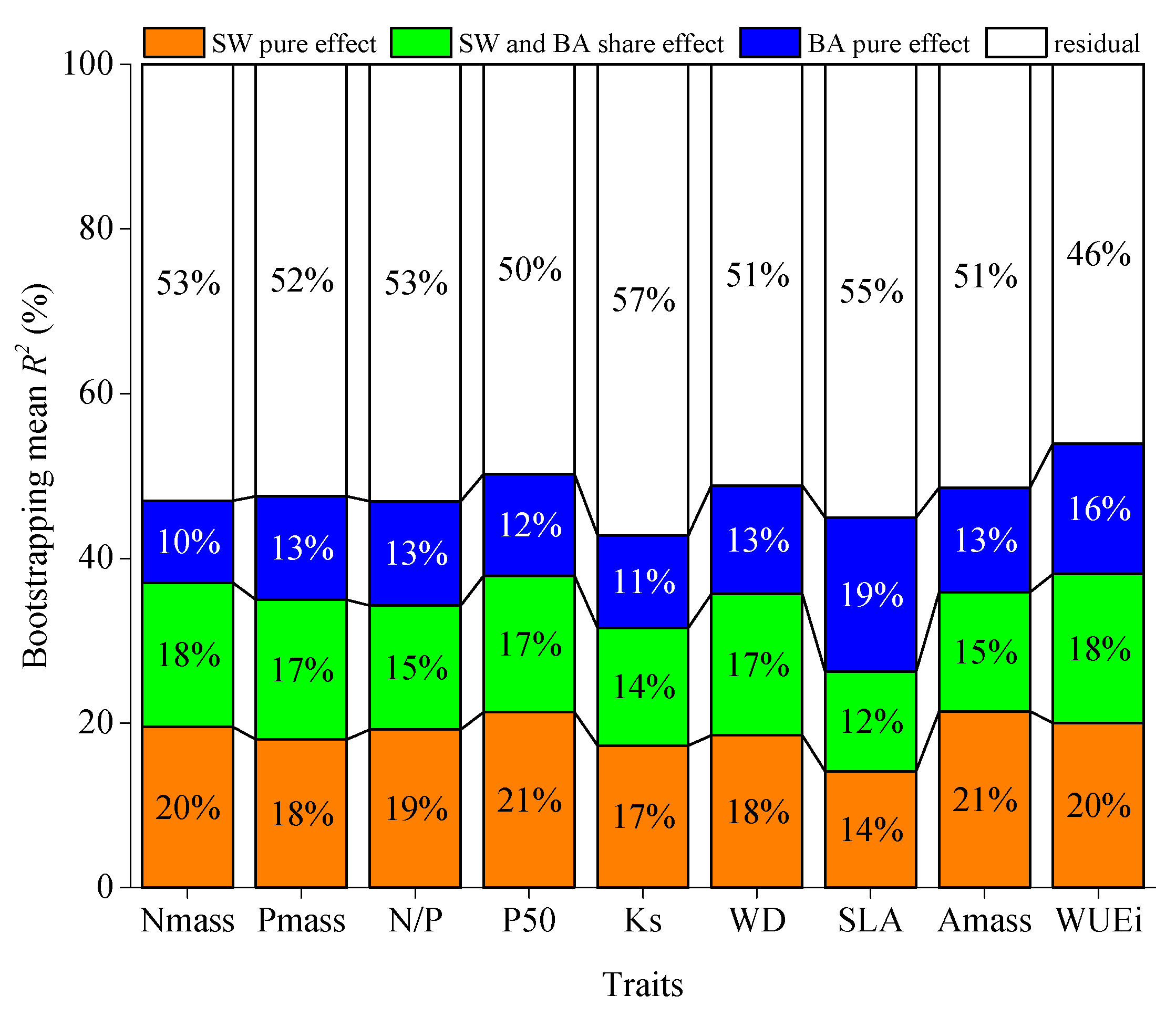
3.6. Partitioning Effects of Soil dryness and BA on Functional Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Traits | Year | Mean Rates (Units yr−1) | Lower 95% CI | Upper 95% CI | Bootstrapping p Values | Ni | Binomial p Values |
|---|---|---|---|---|---|---|---|
| Nmass | 1992–2004 | 0.0544 | 0.0379 | 0.0726 | <0.001 | 79 | <0.001 |
| 2004–2015 | 0.0763 | 0.0574 | 0.0962 | <0.001 | 81 | <0.001 | |
| 1992–2015 | 0.0649 | 0.0550 | 0.0749 | <0.001 | 88 | <0.001 | |
| Pmass | 1992–2004 | 0.0045 | 0.0032 | 0.0058 | <0.001 | 74 | <0.001 |
| 2004–2015 | 0.0019 | 0.0005 | 0.0034 | 0.01 | 56 | 0.27 | |
| 1992–2015 | 0.0033 | 0.0025 | 0.0041 | <0.001 | 73 | <0.001 | |
| N/P | 1992–2004 | −0.0319 | −0.0582 | −0.0068 | 0.02 | 45 | 0.37 |
| 2004–2015 | 0.0741 | 0.0369 | 0.1102 | <0.001 | 68 | <0.001 | |
| 1992–2015 | 0.0188 | −0.0048 | 0.0419 | 0.12 | 56 | 0.27 | |
| P50 | 1992–2004 | −0.0084 | −0.0150 | −0.0022 | 0.01 | 46 | 0.48 |
| 2004–2015 | −0.0260 | −0.0350 | −0.0176 | <0.001 | 30 | <0.001 | |
| 1992–2015 | −0.0168 | −0.0223 | −0.0116 | <0.001 | 28 | <0.001 | |
| Ks | 1992–2004 | 0.0219 | 0.0136 | 0.0311 | <0.001 | 64 | <0.01 |
| 2004–2015 | 0.0084 | −0.0009 | 0.0185 | 0.09 | 54 | 0.48 | |
| 1992–2015 | 0.0154 | 0.0105 | 0.0208 | <0.001 | 65 | <0.01 | |
| WD | 1992–2004 | 0.0002 | −0.0003 | 0.0007 | 0.34 | 57 | 0.19 |
| 2004–2015 | 0.0016 | 0.0010 | 0.0022 | <0.001 | 70 | <0.001 | |
| 1992–2015 | 0.0009 | 0.0005 | 0.0014 | <0.001 | 61 | 0.04 | |
| SLA | 1992–2004 | 0.2514 | 0.1068 | 0.4129 | <0.01 | 65 | <0.01 |
| 2004–2015 | −0.2259 | −0.3779 | −0.0779 | <0.01 | 35 | <0.01 | |
| 1992–2015 | 0.0231 | −0.0650 | 0.1111 | 0.61 | 48 | 0.76 | |
| Amass | 1992–2004 | 0.5953 | 0.4170 | 0.7898 | <0.001 | 78 | <0.001 |
| 2004–2015 | 0.2783 | 0.0611 | 0.4910 | 0.01 | 70 | <0.001 | |
| 1992–2015 | 0.4437 | 0.3657 | 0.5306 | <0.001 | 89 | <0.001 | |
| WUEi | 1992–2004 | −0.3419 | −0.4343 | −0.2549 | <0.001 | 19 | <0.001 |
| 2004–2015 | −0.2479 | −0.3369 | −0.1545 | <0.001 | 31 | <0.001 | |
| 1992–2015 | −0.2969 | −0.3523 | −0.2412 | <0.001 | 14 | <0.001 |
| Traits | Year | Spearman r | n | p Values |
|---|---|---|---|---|
| Nmass | 1992–2004 | −0.21 | 100 | 0.03 |
| 2004–2015 | 0.10 | 100 | 0.31 | |
| 1992–2015 | −0.10 | 100 | 0.34 | |
| Pmass | 1992–2004 | −0.17 | 100 | 0.10 |
| 2004–2015 | −0.01 | 100 | 0.96 | |
| 1992–2015 | −0.15 | 100 | 0.15 | |
| N/P | 1992–2004 | 0.05 | 100 | 0.61 |
| 2004–2015 | 0.09 | 100 | 0.38 | |
| 1992–2015 | 0.10 | 100 | 0.30 | |
| P50 | 1992–2004 | −0.17 | 100 | 0.09 |
| 2004–2015 | −0.05 | 100 | 0.64 | |
| 1992–2015 | −0.06 | 100 | 0.54 | |
| Ks | 1992–2004 | −0.14 | 100 | 0.16 |
| 2004–2015 | −0.07 | 100 | 0.50 | |
| 1992–2015 | −0.20 | 100 | 0.05 | |
| WD | 1992–2004 | 0.07 | 100 | 0.46 |
| 2004–2015 | 0.06 | 100 | 0.54 | |
| 1992–2015 | 0.12 | 100 | 0.23 | |
| SLA | 1992–2004 | −0.02 | 100 | 0.85 |
| 2004–2015 | −0.03 | 100 | 0.75 | |
| 1992–2015 | −0.05 | 100 | 0.63 | |
| Amass | 1992–2004 | −0.19 | 100 | 0.06 |
| 2004–2015 | −0.11 | 100 | 0.29 | |
| 1992–2015 | −0.23 | 100 | 0.02 | |
| WUEi | 1992–2004 | 0.05 | 100 | 0.61 |
| 2004–2015 | −0.05 | 100 | 0.59 | |
| 1992–2015 | −0.03 | 100 | 0.78 |
| Traits | Effects | Mean R2 (%) | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|
| Nmass | a | 21.31 | 15.82 | 27.14 |
| b | 19.10 | 14.65 | 23.96 | |
| c | 10.86 | 6.69 | 15.54 | |
| r | 57.83 | 50.79 | 64.99 | |
| Pmass | a | 19.91 | 14.9 | 25.14 |
| b | 18.77 | 14.50 | 23.27 | |
| c | 13.93 | 9.58 | 18.92 | |
| r | 58.08 | 51.71 | 64.37 | |
| N/P | a | 21.06 | 15.69 | 26.61 |
| b | 16.57 | 12.51 | 20.73 | |
| c | 13.87 | 9.70 | 18.49 | |
| r | 58.23 | 51.36 | 64.88 | |
| P50 | a | 23.04 | 17.23 | 29.14 |
| b | 17.91 | 13.41 | 22.68 | |
| c | 13.34 | 9.14 | 17.74 | |
| r | 53.83 | 47.05 | 60.89 | |
| Ks | a | 18.85 | 14.05 | 23.9 |
| b | 15.58 | 11.97 | 19.39 | |
| c | 12.24 | 8.43 | 16.68 | |
| r | 62.38 | 56.24 | 68.36 | |
| WD | a | 20.20 | 15.34 | 25.27 |
| b | 18.73 | 14.34 | 23.4 | |
| c | 14.40 | 10.25 | 18.88 | |
| r | 55.86 | 49.38 | 62.53 | |
| SLA | a | 15.41 | 10.99 | 20.21 |
| b | 13.15 | 9.31 | 17.25 | |
| c | 20.39 | 14.67 | 26.46 | |
| r | 59.95 | 52.86 | 66.89 | |
| Amass | a | 23.47 | 17.87 | 29.22 |
| b | 15.91 | 12.05 | 20.27 | |
| c | 13.92 | 9.78 | 18.56 | |
| r | 56.38 | 49.40 | 63.03 | |
| WUEi | a | 21.38 | 16.04 | 27.04 |
| b | 19.35 | 14.71 | 24.05 | |
| c | 16.98 | 12.47 | 21.80 | |
| r | 49.23 | 42.41 | 55.98 |
References
- Enquist, B.J.; Enquist, C.A.F. Long-term change within a Neotropical forest: assessing differential functional and floristic responses to disturbance and drought. Glob. Chang. Biol. 2011, 17, 1408–1424. [Google Scholar] [CrossRef]
- Esquivel-Muelbert, A.; Baker, T.R.; Dexter, K.G.; Lewis, S.L.; Brienen, R.J.W.; Feldpausch, T.R.; Lloyd, J.; Monteagudo-Mendoza, A.; Arroyo, L.; Alvarez-Davila, E.; et al. Compositional response of Amazon forests to climate change. Glob. Chang. Biol. 2019, 25, 39–56. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.-Y.M.; Scholl, J.P.; Basinger, U.; Huxman, T.E.; Venable, D.L. Functional trait trade-off and species abundance: insights from a multi-decadal study. Ecol. Lett. 2019, 22, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Van Mantgem, P.J.; Stephenson, N.L.; Byrne, J.C.; Daniels, L.D.; Franklin, J.F.; Fule, P.Z.; Harmon, M.E.; Larson, A.J.; Smith, J.M.; Taylor, A.H.; et al. Widespread Increase of Tree Mortality Rates in the Western United States. Science 2009, 323, 521–524. [Google Scholar] [CrossRef]
- Zeppel, M.J.B.; Harrison, S.P.; Adams, H.D.; Kelley, D.I.; Li, G.; Tissue, D.T.; Dawson, T.E.; Fensham, R.; Medlyn, B.E.; Palmer, A.; et al. Drought and resprouting plants. New Phytol. 2015, 206, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Feeley, K.J.; Davies, S.J.; Perez, R.; Hubbell, S.P.; Foster, R.B. Directional changes in the species composition of a tropical forest. Ecology 2011, 92, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Fauset, S.; Baker, T.R.; Lewis, S.L.; Feldpausch, T.R.; Affum-Baffoe, K.; Foli, E.G.; Hamer, K.C.; Swaine, M.D. Drought-induced shifts in the floristic and functional composition of tropical forests in Ghana. Ecol. Lett. 2012, 15, 1120–1129. [Google Scholar] [CrossRef]
- Butt, N.; Malhi, Y.; New, M.; Macia, M.J.; Lewis, S.L.; Lopez-Gonzalez, G.; Laurance, W.F.; Laurance, S.; Luizao, R.; Andrade, A.; et al. Shifting dynamics of climate-functional groups in old-growth Amazonian forests. Plant. Ecol. Divers. 2014, 7, 267–279. [Google Scholar] [CrossRef]
- McIntyre, P.J.; Thorne, J.H.; Dolanc, C.R.; Flint, A.L.; Flint, L.E.; Kelly, M.; Ackerly, D.D. Twentieth-century shifts in forest structure in California: Denser forests, smaller trees, and increased dominance of oaks. Proc. Natl. Acad. Sci. USA 2015, 112, 1458–1463. [Google Scholar] [CrossRef]
- Li, R.H.; Zhu, S.D.; Chen, H.Y.H.; John, R.; Zhou, G.Y.; Zhang, D.Q.; Zhang, Q.M.; Ye, Q. Are functional traits a good predictor of global change impacts on tree species abundance dynamics in a subtropical forest? Ecol. Lett. 2015, 18, 1181–1189. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, S.; He, F. Half-century evidence from western Canada shows forest dynamics are primarily driven by competition followed by climate. Proc. Natl. Acad. Sci. USA 2015, 112, 4009–4014. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.Y.; Houlton, B.Z.; Wang, W.T.; Huang, W.J.; Xiao, Y.; Zhang, Q.M.; Liu, S.Z.; Cao, M.; Wang, X.H.; Wang, S.L.; et al. Substantial reorganization of China’s tropical and subtropical forests: based on the permanent plots. Glob. Chang. Biol. 2014, 20, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.Y.; Peng, C.H.; Li, Y.L.; Liu, S.Z.; Zhang, Q.M.; Tang, X.L.; Liu, J.X.; Yan, J.H.; Zhang, D.Q.; Chu, G.W. A climate change-induced threat to the ecological resilience of a subtropical monsoon evergreen broad-leaved forest in Southern China. Glob. Chang. Biol. 2013, 19, 1197–1210. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.Y.; Wei, X.H.; Wu, Y.P.; Liu, S.G.; Huang, Y.H.; Yan, J.H.; Zhang, D.Q.; Zhang, Q.M.; Liu, J.X.; Meng, Z.; et al. Quantifying the hydrological responses to climate change in an intact forested small watershed in Southern China. Glob. Chang. Biol. 2011, 17, 3736–3746. [Google Scholar] [CrossRef]
- Shen, C.D.; Liu, D.S.; Peng, S.L.; Sun, Y.M.; Jiang, M.T.; Yi, W.X.; Xing, C.P.; Gao, Q.Z.; Li, Z.; Zhou, G.Y. C-14 measurement of forest soils in Dinghushan Biosphere Reserve. Chin. Sci. Bull. 1999, 44, 251–256. [Google Scholar] [CrossRef]
- Zhou, G.Y.; Liu, S.G.; Li, Z.; Zhang, D.Q.; Tang, X.L.; Zhou, C.Y.; Yan, J.H.; Mo, J.M. Old-growth forests can accumulate carbon in soils. Science 2006, 314, 1417. [Google Scholar] [CrossRef]
- Zhu, S.D.; Song, J.J.; Li, R.H.; Ye, Q. Plant hydraulics and photosynthesis of 34 woody species from different successional stages of subtropical forests. Plant. Cell Environ. 2013, 36, 879–891. [Google Scholar] [CrossRef]
- Chave, J.; Coomes, D.; Jansen, S.; Lewis, S.L.; Swenson, N.G.; Zanne, A.E. Towards a worldwide wood economics spectrum. Ecol. Lett. 2009, 12, 351–366. [Google Scholar] [CrossRef]
- Diaz, S.; Kattge, J.; Cornelissen, J.H.C.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Prentice, I.C.; et al. The global spectrum of plant form and function. Nature 2016, 529, 167–171. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Diaz, S.; Hodgson, J.G.; Thompson, K.; Cabido, M.; Cornelissen, J.H.C.; Jalili, A.; Montserrat-Marti, G.; Grime, J.P.; Zarrinkamar, F.; Asri, Y.; et al. The plant traits that drive ecosystems: Evidence from three continents. J. Veg. Sci. 2004, 15, 295–304. [Google Scholar] [CrossRef]
- Reich, P.B.; Ellsworth, D.S.; Walters, M.B.; Vose, J.M.; Gresham, C.; Volin, J.C.; Bowman, W.D. Generality of leaf trait relationships: A test across six biomes. Ecology 1999, 80, 1955–1969. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Klein, T.; Bartlett, M.; Sack, L.; Pellegrini, A.F.A.; Choat, B.; Jansen, S. Meta-analysis reveals that hydraulic traits explain cross-species patterns of drought-induced tree mortality across the globe. Proc. Natl. Acad. Sci. USA 2016, 113, 5024–5029. [Google Scholar] [CrossRef] [PubMed]
- Kunstler, G.; Falster, D.; Coomes, D.A.; Hui, F.; Kooyman, R.M.; Laughlin, D.C.; Poorter, L.; Vanderwel, M.; Vieilledent, G.; Wright, S.J.; et al. Plant functional traits have globally consistent effects on competition. Nature 2016, 529, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Poorter, L.; McDonald, I.; Alarcon, A.; Fichtler, E.; Licona, J.-C.; Pena-Claros, M.; Sterck, F.; Villegas, Z.; Sass-Klaassen, U. The importance of wood traits and hydraulic conductance for the performance and life history strategies of 42 rainforest tree species. New Phytol. 2010, 185, 481–492. [Google Scholar] [CrossRef]
- Dalu, T.; Wasserman, R.J.; Magoro, M.L.; Mwedzi, T.; Froneman, P.W.; Weyl, O.L.F. Variation partitioning of benthic diatom community matrices: Effects of multiple variables on benthic diatom communities in an Austral temperate river system. Sci. Total Environ. 2017, 601, 73–82. [Google Scholar] [CrossRef]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Feild, T.S.; Gleason, S.M.; Hacke, U.G.; et al. Global convergence in the vulnerability of forests to drought. Nature 2012, 491, 752. [Google Scholar] [CrossRef]
- Chen, Y.J.; Cao, K.F.; Schnitzer, S.A.; Fan, Z.X.; Zhang, J.L.; Bongers, F. Water-use advantage for lianas over trees in tropical seasonal forests. New Phytol. 2015, 205, 128–136. [Google Scholar] [CrossRef]
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 2015, 6, 129. [Google Scholar] [CrossRef]
- McDowell, N.G.; Allen, C.D. Darcy’s law predicts widespread forest mortality under climate warming. Nat. Clim. Chang. 2015, 5, 669–672. [Google Scholar] [CrossRef]
- AghaKouchak, A.; Cheng, L.Y.; Mazdiyasni, O.; Farahmand, A. Global warming and changes in risk of concurrent climate extremes: Insights from the 2014 California drought. Geophys. Res. Lett. 2014, 41, 8847–8852. [Google Scholar] [CrossRef]
- Piao, S.L.; Ciais, P.; Huang, Y.; Shen, Z.H.; Peng, S.S.; Li, J.S.; Zhou, L.P.; Liu, H.Y.; Ma, Y.C.; Ding, Y.H.; et al. The impacts of climate change on water resources and agriculture in China. Nature 2010, 467, 43–51. [Google Scholar] [CrossRef]
- Zscheischler, J.; Westra, S.; van den Hurk, B.; Seneviratne, S.I.; Ward, P.J.; Pitman, A.; AghaKouchak, A.; Bresch, D.N.; Leonard, M.; Wahl, T.; et al. Future climate risk from compound events. Nat. Clim. Chang. 2018, 8, 469–477. [Google Scholar] [CrossRef]
- Lewis, S.L.; Brando, P.M.; Phillips, O.L.; van der Heijden, G.M.F.; Nepstad, D. The 2010 Amazon Drought. Science 2011, 331, 554. [Google Scholar] [CrossRef] [PubMed]
| Traits | Mean Rates (unit yr−1 cm−2) | Lower 95% CI | Upper 95% CI | Bootstrapping p Values | Ni | Binomial p Values |
|---|---|---|---|---|---|---|
| Nmass | −2.48 × 10−4 | −4.62 × 10−4 | −2.25 × 10−5 | 0.03 | 27 | <0.001 |
| Pmass | −1.11 × 10−5 | −1.62 × 10−5 | −5.98 × 10−6 | <0.001 | 28 | <0.001 |
| N/P | −1.10 × 10−4 | −4.17 × 10−4 | 2.09 × 10−4 | 0.50 | 37 | 0.01 |
| P50 | −4.48 × 10−5 | −6.94 × 10−5 | −2.15 × 10−5 | 0.001 | 33 | 0.001 |
| Ks | −4.41 × 10−5 | −6.92 × 10−5 | −2.03 × 10−5 | <0.001 | 28 | <0.001 |
| WD | −5.46 × 10−6 | −1.14 × 10−5 | 7.00 × 10−7 | 0.08 | 30 | <0.001 |
| SLA | 2.80 × 10−7 | −1.02 × 10−3 | 9.88 × 10−4 | 0.99 | 62 | 0.02 |
| Amass | −1.35 × 10−3 | −2.18 × 10−3 | −4.66 × 10−4 | 0.005 | 26 | <0.001 |
| WUEi | 1.45 × 10−3 | 1.21 × 10−4 | 2.69 × 10−3 | 0.04 | 76 | <0.001 |
| Traits | Mean Rates (unit yr−1 mm−1) | Lower 95% CI | Upper 95% CI | Bootstrapping p Values | Ni | Binomial p Values |
|---|---|---|---|---|---|---|
| Nmass | −3.99 × 10−3 | −5.19 × 10−3 | −2.69 × 10−3 | <0.001 | 25 | <0.001 |
| Pmass | −2.46 × 10−4 | −3.27 × 10−4 | −1.72 × 10−4 | <0.001 | 27 | <0.001 |
| N/P | −2.40 × 10−4 | −2.07 × 10−3 | 1.49 × 10−3 | 0.80 | 46 | 0.48 |
| P50 | 1.48 × 10−3 | 0.93 × 10−3 | 2.02 × 10−3 | <0.001 | 72 | <0.001 |
| Ks | −1.56 × 10−3 | −2.07 × 10−3 | −1.06 × 10−3 | <0.001 | 33 | 0.001 |
| WD | −4.00 × 10−5 | −7.37 × 10−5 | −7.63 × 10−6 | 0.02 | 41 | 0.09 |
| SLA | 4.24 × 10−3 | −7.84 × 10−3 | 1.56 × 10−2 | 0.48 | 46 | 0.48 |
| Amass | −3.97 × 10−2 | −5.11 × 10−2 | −2.96 × 10−2 | <0.001 | 16 | <0.001 |
| WUEi | 2.24 × 10−2 | 1.53 × 10−2 | 2.93 × 10−2 | <0.001 | 77 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, S.; Zhang, Q.; Zhou, G.; Liu, S.; Chu, G.; Li, R.; Ye, Q.; Zhang, D.; Tang, X.; Liu, J.; et al. Functional Composition Changes of a Subtropical Monsoon Evergreen Broad-Leaved Forest Under Environmental Change. Forests 2020, 11, 191. https://doi.org/10.3390/f11020191
Zou S, Zhang Q, Zhou G, Liu S, Chu G, Li R, Ye Q, Zhang D, Tang X, Liu J, et al. Functional Composition Changes of a Subtropical Monsoon Evergreen Broad-Leaved Forest Under Environmental Change. Forests. 2020; 11(2):191. https://doi.org/10.3390/f11020191
Chicago/Turabian StyleZou, Shun, Qianmei Zhang, Guoyi Zhou, Shizhong Liu, Guowei Chu, Ronghua Li, Qing Ye, Deqiang Zhang, Xuli Tang, Juxiu Liu, and et al. 2020. "Functional Composition Changes of a Subtropical Monsoon Evergreen Broad-Leaved Forest Under Environmental Change" Forests 11, no. 2: 191. https://doi.org/10.3390/f11020191
APA StyleZou, S., Zhang, Q., Zhou, G., Liu, S., Chu, G., Li, R., Ye, Q., Zhang, D., Tang, X., Liu, J., Huang, C., Li, Y., & Meng, Z. (2020). Functional Composition Changes of a Subtropical Monsoon Evergreen Broad-Leaved Forest Under Environmental Change. Forests, 11(2), 191. https://doi.org/10.3390/f11020191