1. Introduction
Agroforestry systems (AFS), agricultural land with >20% tree cover, account for 0.6 billion hectares and support approximately 1.2 billion people often with limited landholdings that depend on some form of these practices for their livelihoods [
1,
2]. AFS are commonly employed in Brazilian Amazonia by smallholding farmers (<100 ha) and could comprise up to 35% of land under cultivation in that region [
3]. Slash-and-burn land preparation is a common way used by smallholding farmers to prepare land for cultivation, in which the standing vegetation is cut, left to dry, and then burned. Burning vegetation creates an initial pulse of available nutrients for crop production via ash, but leaching, erosion, and crop export lead to declining fertility resulting in crop field abandonment [
4].
Slash-and-burn agricultural production is dependent on the length of time fallow vegetation can accumulate nutrients before being cleared and burned again for cropping, since nutrient accumulation in the fallow vegetation is the primary source of crop nutrients [
5]. For example, N (~500 kg ha
−1) and P (~30 kg ha
−1) accumulation were five- and three-fold greater in 8-year-old compared to 3-year-old regenerating secondary forest [
6]. Improved fallow (i.e., the planting of desired species) is one method used to enhance vegetative growth in secondary succession. Although much of the research into use of improved fallows in AFS has looked at cover crops and herbaceous plants [
7], the use of tree species is also possible and in various tropical locations has increased nutrient accumulation [
8,
9,
10].
Pioneer tree species (e.g.,
Cecropia spp.) that first establish on sites in natural forest succession are typically of low commercial timber value [
11]. Species planted for improved fallow could generate income via timber sales, potentially resulting in increased fallow lengths or even the purchase of fertilizers. Improved fallow plantings with commercial timber (e.g.,
Schizolobium amazonicum) or biomass species (e.g.,
Inga edulis) may be harvested as early as six years after slashing [
12]. In the absence of fire, slower growing species (e.g.,
Parkia multijuga) of higher value that are also planted might be sustained through successive crop–fallow cycles prior to harvest.
In addition to fast-growing nutrient accumulators or timber species, improved fallow can improve soil fertility by using nitrogen (N)-fixing species (i.e.,
Inga sp.). Use of N-fixing cover crops (i.e.,
Centrosema macrocarpum) and hedgerows as green manures are common in continuous cultivation systems, where incorporation of cover crop or hedgerow biomass adds N to the agroecosystem [
7]. Alternatively, as it is done in improved fallow, N fixers are added to the fallow mix to stimulate N acquisition for subsequent release to the cropping phase after the fallow is cleared [
7]. Enrichment plantings with various N-fixing tree species, including
I. edulis, were shown to increase fallow biomass by 6–117%, compared to unimproved fallow [
13]. In Panama, young forests (<12 years) were estimated to have the highest rates of N fixation along a 300-year forest age gradient, and N fixers were estimated to supply >50% of the N necessary for biomass growth [
14]. Other research in mixed-species plantings of non-fixing
Eucalyptus sp. with N-fixing
Acacia mearnsii found 3x the foliar N concentration in eucalypt when grown in a 50–50 mix [
15], and
Theobroma cacao roots contained as much as 60% higher N concentrations when grown in the presence of
I. edulis [
16]. The tropical N-fixing tree species
Gliricidia sepium was reported to supply up to 63% of the N content of neighboring grass species via root exudation [
17]. These results indicate that N-fixing trees can increase neighbor N content.
In contrast, some recent studies in moist tropical forests did not support the notion of N-fixing trees facilitating the growth or nutrient acquisition of neighboring plants during secondary succession [
18] or any effect on the total biomass accumulation of the secondary forest [
19]. Rather than depleting agroecosystem N capital, as happens in slash-and-burn systems due to gaseous losses of N during burning [
5], the inclusion of N-fixing tree species to enhance N stocks in AFS requires continued validation.
The slash-and-mulch technology was developed to reduce losses of soil fertility due to biomass burning and can fell, chop, and evenly distribute secondary forest vegetation using a mulching tractor [
20]. Mulching the forest adds all biomass nutrients to the soil surface, which become available for future plant uptake, and, unlike burning, the mulching tractor can maneuver around planted trees.
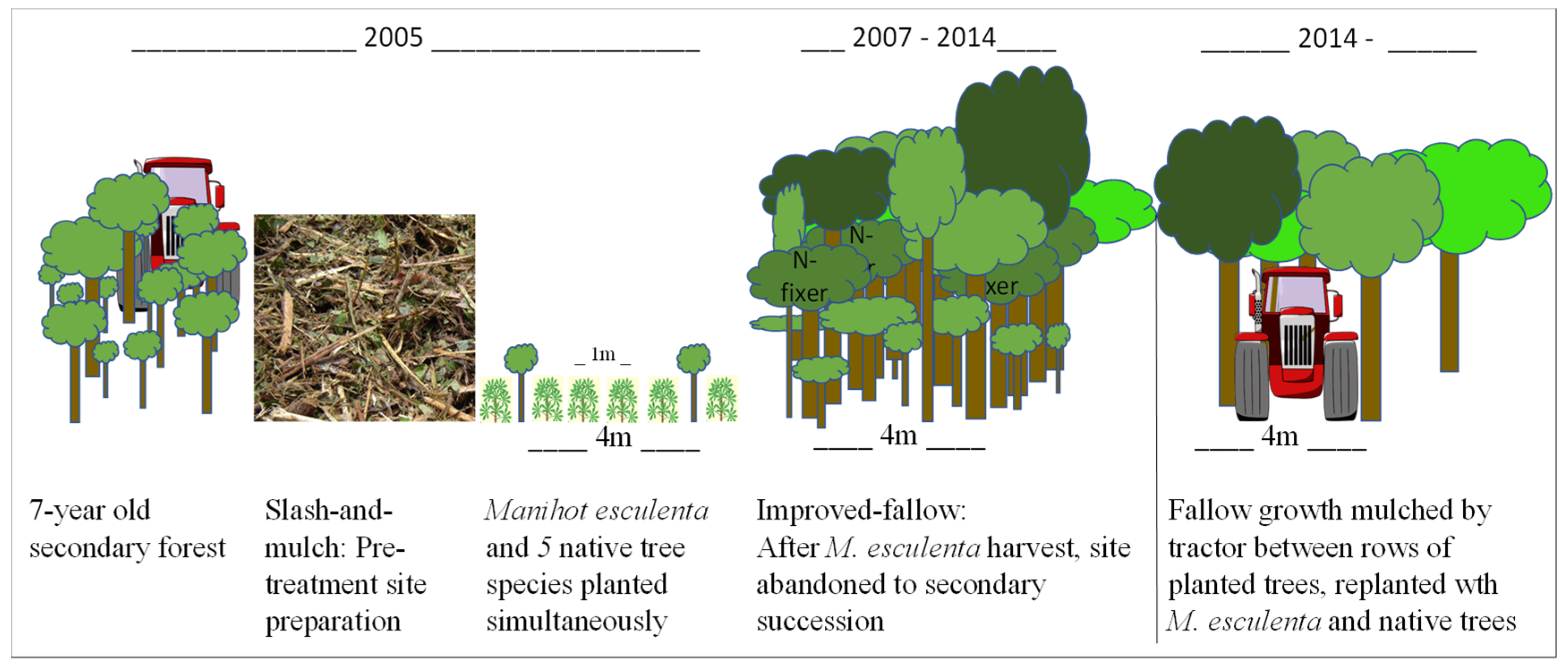
In 2005, a field trial was initiated to develop an improved-fallow AFS that could take advantage of the slash-and-mulch technology and the potential for multiple rotations of tree growth that have economic value [
21]. After mulching of a 7-year-old unimproved secondary forest, tree rows were planted on a 4 m spacing that would allow future passage of the mulching tractor. Trees included four timber species, i.e.,
S. amazonicum, a fast-growing pioneer that could be harvested after one rotation, and
Ceiba pentandra,
Cedrela odorata, and
P. multijuga, all likely to require >2 rotations. Additionally,
I. edulis, an N fixer, was co-planted in some improved-fallow treatments. The expectation was that
I. edulis growth and mulching as a green manure would improve the N stocks of the soil. A P+K fertilization (Fert) treatment was also included, since these are also primary limiting elements along with N. The regional staple food crop
Manihot esculenta was planted simultaneously with the trees.
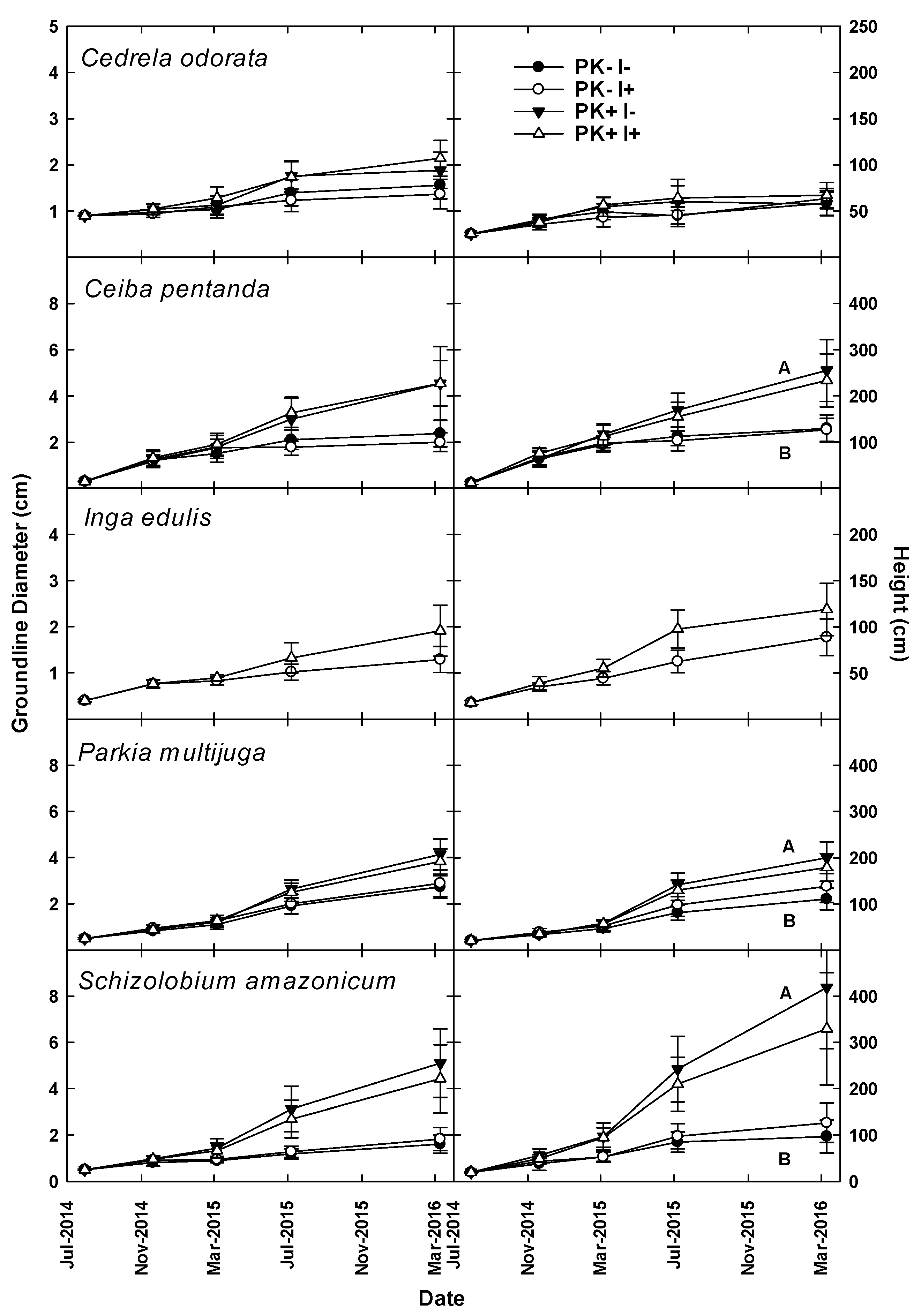
During the first two years of Rotation 1, manioc growth increased significantly with P+K fertilization, but there was no response to the N fixer [
21]. P+K fertilization also enhanced tree growth through Year 6, and estimates of planted tree biomass and nutrient accumulation indicated that inclusion of
I. edulis increased the N stock [
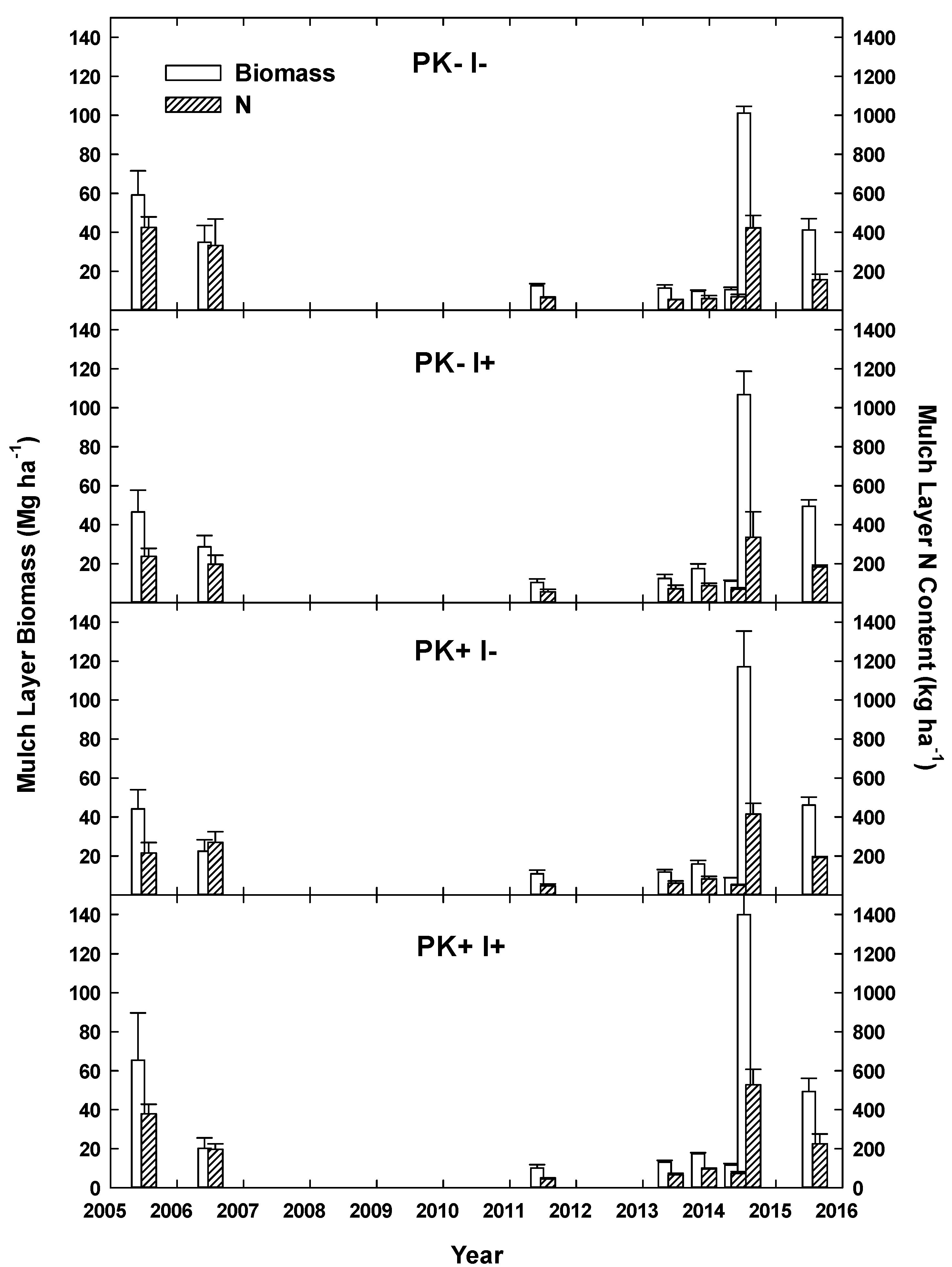
22]. The mulch layer at site initiation had a mean mass of 54 Mg ha
−1 with ~300 kg N ha
−1 [
21] and decreased by Year 6 to 10–15 Mg ha
−1 with N contents of ~130 kg ha
−1 [
22]. In year 6, mulch mass and N content showed no treatment effects. Mineral soil N at that time was not clearly elevated by the presence of
I. edulis, but soil P concentration had clearly increased by 37% and 45% to ~6 and 4 µg/dm
−3 in the 0–10 and 10–20 cm soil layers.
In this paper, we report on the end of the first rotation (Year 9) of this experiment and the initiation of the second rotation through years 10 and 11 that included driving the mulching tractor between planted tree rows, converting
I. edulis trees to green manure, harvesting all
S. amazonicum trees, and planting a second crop of manioc and trees as initially designed. Many AFS studies, including several from this region, have investigated 3–5 years after forest clearing [
4,
5,
20,
23], but only one decade-long study was identified, on shade-grown cacao [
24].
Given the multiple-rotation nature of this experiment, we posed hypotheses relative to the end of the first rotation and others related to the second rotation. In May 2014 at the end of Rotation 1, we hypothesized that the improved-fallow plantings would generate higher biomass and nutrient stocks than the unimproved fallow prior to site conversion. Furthermore, tree growth during Rotation 1 would increase with P+K fertilization and the presence of
I. edulis (Nfix). Since we previously observed increased aboveground stocks of N [
25] under improved fallow, we hypothesized that agroecosystem N stocks would be higher with P+K fertilization and with the presence of
I. edulis at the end of the first Rotation. In July 2014 after mulching to initiate the second rotation, we hypothesized that the growth of newly planted trees would be greater due to P+K fertilization at initiation, as residual effects of fertilization on tree growth have been previously observed up to 20 years after application [
26]. We also expected greater tree growth with
I. edulis due to green manure effects, and that manioc growth would be greater with Fert and N fix treatments in Rotation 2.
2. Materials and Methods
2.1. Site Description
The research was conducted at the Fazenda Experimental de Igarapé Açu (FEIGA) of the Universidade Federal Rural da Amazônia (UFRA) in the Municipality of Igarapé Açu (1°07′41″ S 47°47′15″ W), approximately 110 km East of Belém, Pará, Brazil (
Figure 1a). This region, the Bragantina, is one of the oldest continually inhabited agricultural areas in Amazonia [
4], and the landscape is now dominated by human activities, such as urban, row-crop farms, plantation forests, cattle ranches, and secondary forests.
Soils in the municipality of Igarapé Açu are predominantly Kandiudults [
27]. Particle size analyses for 2 m soil profiles were recently performed in 2016 on soils from one plot from within each block and one of each treatment (n = 4). Each plot location was sampled at increments of 0–10, 10–20, 20–50, 50–100, 100–150, and 150–200 cm. Particle size distribution was determined using the hydrometer method [
28] (
Table S1). Prior bulk density (BD) measures found 1.2 g cm
−3 from 0 to 5 cm and 1.4 g cm
−3 from 5 to 10 cm [
21]. Igarapé Açu has an average annual temperature of 26 °C and annual rainfall of 2500 mm [
29]; the driest months are August–November, and the wettest months are January–May.
2.2. Species Descriptions
The five planted tree species are native to forests of the Bragantina region.
I. edulis (Fabaceae) is the only known planted N fixer, although other N-fixing species may be present due to natural recruitment in secondary succession.
S. amazonicum (Fabaceae) and
C. pentandra (Malvaceae) are rapid-growing pioneers with soft wood.
P. multijuga (Fabaceae) and
C. odorata (Meliaceae) are slower growing tropical hardwoods (see [
21] for a more complete species description).
All five tree species were planted with the food crop manioc (
M. esculenta), which was sampled after 12 months and then harvested after 20 months during the first crop–fallow rotation. Manioc growth response has been reported [
21]. The same tree species were planted with manioc within 10 days of site conversion via mulching tractor in July 2014 to begin the second rotation of the crop–fallow cycle. Manioc sampling of the second rotation was performed 12 months after site preparation, but the majority of the manioc plants were stolen prior to the 20-month sampling event, so that data are not present in this study.
2.3. Plot Establishment
Plot establishment of this site was previously described [
21]. Briefly, in March of 2005, a 1 ha study site within FEIGA (
Figure 1b) was selected and mulched. Experimental treatments were applied in June 2005, and the aforementioned species were planted in four experimental blocks (N = 4). Each block was divided into four plots (n = 16) that measured 24 × 24 m. Trees and manioc were planted simultaneously, with trees planted at 4 × 1.8 m spacing, for a total of 78 trees per plot, or 1354 trees ha
−1. The crop species
M. esculenta was planted at 1 × 1 m spacing, or 10,000 stems ha
−1 (
Figure 2).
A factorial combination of fertilization treatment and N-fixing species was assigned in a split-plot randomized complete block design. The two main plot fertilizer treatments consisted of no fertilization (PK−) and fertilization (PK+) by an application around the base of planted trees of 46 kg P ha−1 as P2O5 (100 kg Simple-Super Phosphate with 46% P) and of 30 kg K ha−1 as KCl (50 kg KCl with 60% K). Sub-plot treatments consisted of planting 26 trees each of the native species C. odorata, C. pentandra, and S. amazonicum together (I−) or in combination with the N-fixing species I. edulis and P. multijuga (I+). In the I+ treatment, half of the trees planted were I. edulis, while the remainder was divided evenly among the remaining species.
In July 2014, the site was prepared for the second rotation of the crop-–fallow system via mulching tractor, as was done during the first rotation. However, the tractor passed between the 4 m-wide spacing between rows of trees, mulching the secondary vegetation but leaving the planted trees to continue growing. Unfortunately, many small trees did not survive the mulching event, either because they were mulched or because other vegetation fell on them. The same five species of trees were planted in both rotations.
2.4. Soil/Litter Sampling and Analysis
During both the end of Rotation 1 and the beginning of Rotation 2, mulch layer samples were collected for plot-level analysis from four random sample locations between rows 2 and 5 and between the 4th and the 10th trees of those rows to avoid edge effects of neighboring plots. Samples were collected within 1 m × 1 m polyvinyl chloride (PVC) quadrats, dried in a forced-air oven at 65 °C, weighed, and averaged per plot. Soil samples were taken from a 0–10 cm depth from the center of each framed location beneath where litter samples had been collected. These samples were air-dried and composited by plot.
All samples were ground to a powder using a vial roller or ball mill grinder (SPEX, Metuchen, NJ). Soil and litter samples were analyzed for carbon (C) and nitrogen (N) concentrations using a CN analyzer (CE Elantech, Lakewood, NJ, USA). Litter samples were analyzed for additional elements using the EPA-3050B (U.S. EPA, 1996) method [
30]. Soil samples were analyzed for cations and P via Mehlich−1 double-acid extraction [
31]. Extracts of 1 M KCl were analyzed via flow-injection spectrometry for NH
4 and NO
3 concentrations (OI Analytical, College Station, TX, USA).
2.5. Plant Biomass
Trees’ diameters at breast height (DBH) and ground line (GLD) were measured in 2013, 2014, 2015, and 2016. Additionally, height was measured in 2014, 2015, and 2016 using a hypsometer (Hägloff, Långsele, Sweden) or a range pole for shorter trees. The diameter was measured using digital calipers for smaller girth trees and seedlings or slide calipers for larger diameters.
For seedlings and saplings that had not achieved DBH, the tree volume was estimated using the frustum formula:
where
h = length of the section measured,
R = radius of the lower end of the section,
r = radius of the upper end of the section
Planted-tree aboveground biomass was estimated by multiplying published wood density values [
32] by the frustum volume estimate:
where
B is biomass,
V is volume, and
ρ is wood density.
Exceptions to this estimate are noted below.
S. amazonicum trees were harvested after mulching at the beginning of the second rotation. Nine trees, out of 42 survivors (21.5%), were selected for measurement of DBH and for diameter at each 5 m increment along the stem. Total length was measured, and branches were measured for diameter at the junction with the main stem. All leaves were collected, dried, and weighed. Discs of the stem were collected, dried, and weighed. The lengths of individual branches were not measured but estimated with a species-specific allometric equation for
S. amazonicum (R
2 = 0.8916) derived from saplings sampled in this research:
where
L = branch length (cm),
D = branch diameter (cm).
Volume estimates of
S. amazonicum at the end of the first rotation (Year 9) used [
33]:
where
V = volume (m
3), DBH = diameter at breast height (cm), H = height (m).
For trees under 2.0 m of height, the frustum volume Equation (1) was used and multiplied by the wood density [
32] to generate the biomass of the stem. Stem biomass was then multiplied by the ratio of the stem biomass to total biomass measured from the nine sample trees mentioned above to generate a total biomass estimate. For trees with height above 2.0 m, biomass (B) was estimated using diameter at breast height (DBH) by the allometric equation [
34]:
I. edulis trees were cut at ground level in May 2014 prior to site conversion via mulching tractor, so that the biomass could be mulched and distributed as green manure with the rest of the secondary vegetation. Twelve trees, 7% of trees in the 2014 sample event, with at least one tree per I+ plot was selected for allometric analysis. These 12 trees were sampled for GLD and DBH, and the mass of each section was weighed wet. The wet weight was converted to wood biomass using data from Reference [
35] in which 39% of wet weight was wood. Additionally, all leaves were collected per tree and dried in a forced-air oven at 65 °C until a constant weight. Height, GLD (cm), and DBH (cm) were used in regression equations to predict total biomass, with the best fit (R² = 0.9127) for trees of GLD < 13.5 cm:
where B is biomass (kg), and GLD is ground line diameter (cm); for trees with
GLD > 13.5 cm (R² = 0.9086):
where
B is in kg, and DBH = diameter at breast height (cm).
Since
I. edulis trees were not measured for height in 2013 due to understory vegetation blocking sight of the tops of most fertilized trees, height, GLD, and DBH measurements from 2011 were used to find the best allometric equation to predict height for the measurements in 2013 and 2014. The best fit was (R
2 = 0.8047):
where H = height (cm).
I. edulis leaf biomass estimates were generated from the leaf-to-stem biomass ratio from the sampled trees, and the best fit was a linear regression (R² = 0.8168):
where LM = leaf mass (g), SB = stem biomass (g).
For
C. odorata saplings without DBH, biomass estimates were generated using the frustum volume (Equation 1) multiplied by the specific gravity of
C. odorata (0.39 g cm
−3; [
32]). For all trees above 2.0 m height, the allometric equation [
36] was used to estimate the biomass:
To separate stem biomass from leaf biomass for trees under 200 cm of height, the leaf biomass was assumed to be 28% of the total biomass [
37], and for trees above 200 cm, the allometric equation [
36] was used to predict leaf biomass:
Foliar and wood N content were estimated using published data: 3.07% N for leaf [
38] and 0.109% N for wood [
39].
P. multijuga biomass estimates for trees <2.0 m of height were generated using the frustum volume (Equation 1) multiplied by the specific gravity of
P. multijuga (0.38 g cm
−3; [
32]). Foliar N content was determined from the mean of published data, i.e., 2.362% [
40,
41,
42], multiplied by leaf biomass estimates. Wood N content was estimated from published data, i.e., 0.109% [
40], multiplied by wood biomass estimates. Wood mass was separated from leaf mass using a 7.3:1 wood/leaf ratio for
P. multijuga from Central Amazonia [
43]. The content of other macronutrients was determined using reported concentrations for wood [
43] and leaf [
39]. For trees with height >2.0 m, the following biomass estimate [
43] was used:
The following power function (R
2 = 0.9218) was used to estimate the height (H) of
P. multijuga that could not be measured due to secondary vegetation interference:
C. pentandra volume was estimated using the frustum method (Equation 1). The volume was then multiplied by the basic density, 0.28 g cm
−3 [
32]. We used reported biomass allometry for
C. pentandra in plantation at 4 years of age [
44] and used the height of 300 cm which was the average height of surviving trees at approximately that age (299 cm ± 182). Leaf and wood biomass distribution was based on the reported aboveground biomass [
43]. Wood N concentration was reported [
45], as well as leaf N concentration [
38].
Manioc stems were planted at the beginning of the second rotation in July 2014, and biomass was measured in July 2015 by placing a 1 × 1 m PVC frame at four randomly selected points in each plot. All biomass from within the vertical plane of the frame was collected, aboveground and belowground. Manioc biomass was divided into three compartments: leaf, stem, and root. Biomass compartments were dried in a forced-air oven at 65 °C and weighed.
2.6. Statistical Analyses
All statistical analyses were performed using SAS University Edition (SAS, Cary, NC, USA). Data distributions were evaluated prior to analysis and transformed as needed. Analyses were performed using Proc MIXED. In the factorial model, the main-plot treatment was with or without P+K fertilization, and the sub-plot treatment was with or without the N-fixing tree species I. edulis. Fert and Nfix were treated as fixed effects, and block as a random effect. Repeated measures analysis was used to account for covariance among dates, with plot as the subject. Contrast statements were used to test differences in growth rates between specific years.
Multivariate regressions (Proc REG) were used to evaluate the role of soil variables (mineral soil and O horizon) on tree and manioc growth. Sampling dates used in the multivariate regressions were: July 2013, May 2014 during Rotation 1, and July 2015 during Rotation 2. Macronutrients N, P, K, Ca, and Mg were used as regressors for both mineral soil and O horizon, as well the C/N ratio. Additionally, O horizon (mulch layer) mass and macronutrient content were used as regressors.
5. Conclusions
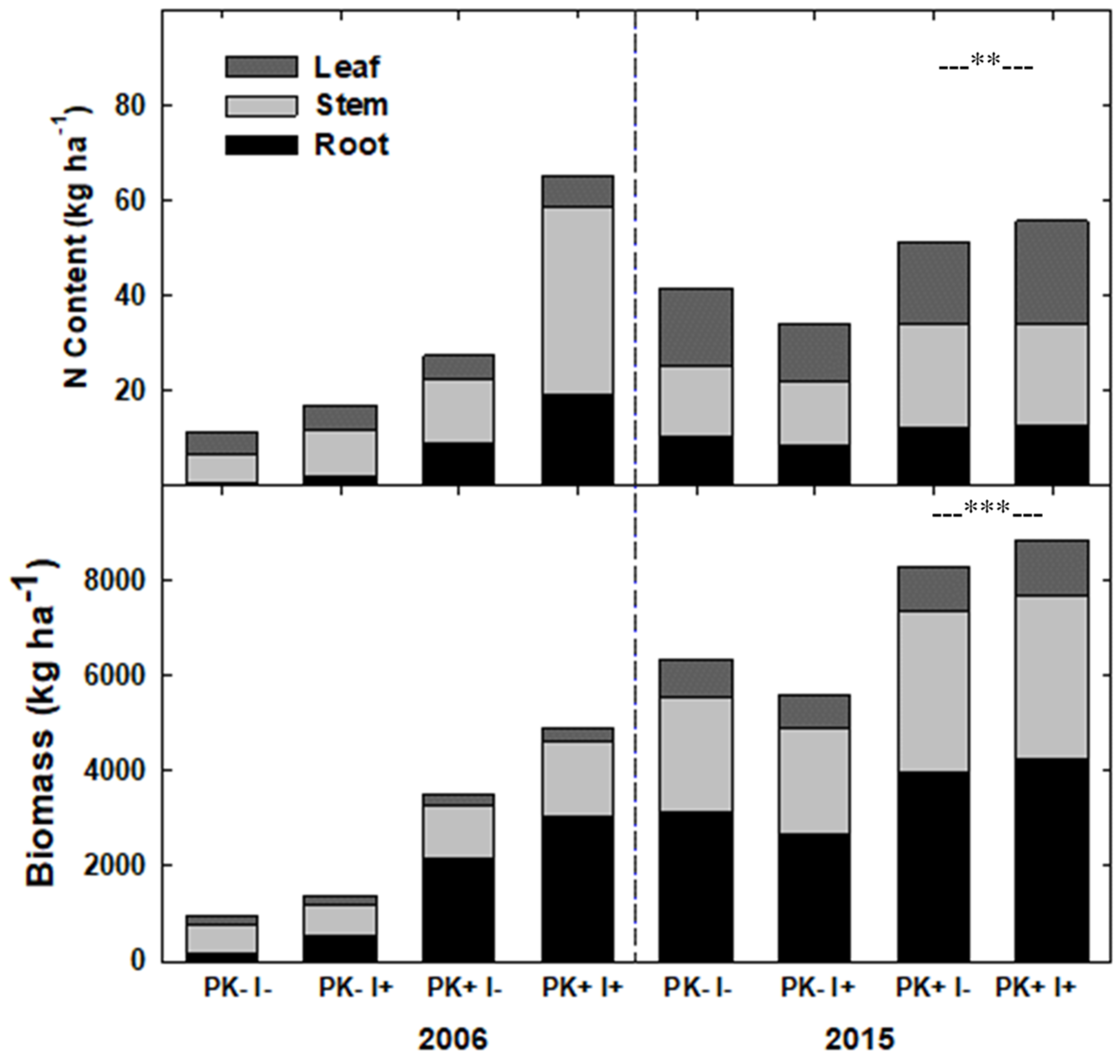
Results from this research indicate that the simultaneous planting of the staple crop manioc with native tree species, in conjunction with slash-and-mulch site preparation, can yield two crop harvests and a merchantable timber harvest through the course of one crop–fallow rotation with P + K fertilization, while leaving other tree species for harvest in future crop–fallow cycles. This combination of site preparation techniques may give producers who utilize agroforestry systems multiple points of income (e.g., during the first two crop harvests at Years 1 and 2 and at the conclusion of the fallow phase at Year 9, in this case), as opposed to only generating income with crop harvests, which is typical without simultaneous improved-fallow plantings.
The improved-fallow design increased the annualized fallow biomass accumulation during Rotation 1, and the increased biomass provided enough nutrients to replace those exported via crop and timber export. The slash-and-mulch site preparation, when combined with improved-fallow planting, can sustain, and even increase, nutrient stocks as compared to nutrient losses prevalent in slash-and-burn site preparation, even with crop export at 12 and 20 months and timber export at Year 9. The inclusion of rapid-growing pioneer species such as S. amazonicum and I. edulis in an improved-fallow design with P + K fertilization will allow producers to harvest manioc and merchantable timber after one crop–fallow rotation, while returning enough nutrients to the agroecosystem via green manure to sustain crop harvest.
After the end of a full rotation and the initiation of a second, this research justifies the use of the leguminous species S. amazonicum and Parkia multijuga in a mixed-species planting design, with the inclusion of I. edulis for use as green manure. Harvest of P+K fertilized S. amazonicum at the end of Rotation 1, mulching, and then re-planting I. edulis and leaving P. multijuga for harvest at the end of Rotation 2 are supported by this study. Due to widespread pest attacks which caused deformation of the stems, and therefore loss of timber value, the results of this study cannot justify the inclusion of the slower growing, although higher value, timber species C. odorata or C. pentandra.