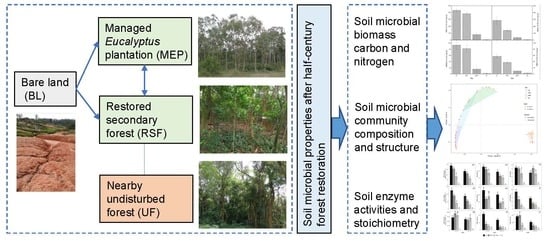
Changes in Soil Microbial Biomass, Community Composition, and Enzyme Activities After Half-Century Forest Restoration in Degraded Tropical Lands
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Soil Sampling
2.3. Soil Physicochemical Analysis
2.4. Soil Microbial Biomass
2.5. Phospholipid Fatty Acids Analyses
2.6. Soil Enzyme Activities Analysis
2.7. Statistical Analysis
3. Results
3.1. Soil Physicochemical Characteristics and Microbial Biomass
3.2. Soil Microbial Community Abundance and Structure
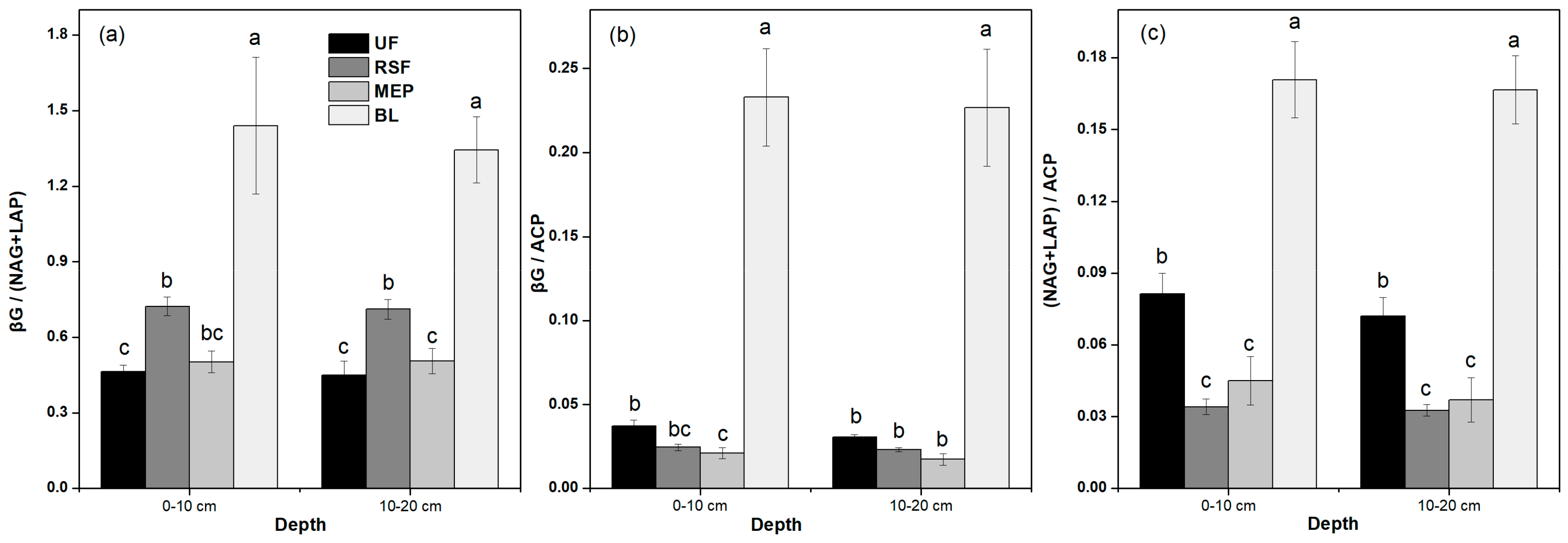
3.3. Soil Enzyme Activities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, J.; Chen, J.Z.; Tan, M.Z.; Gong, Z.T. Soil degradation: A global problem endangering sustainable development. J. Geogr. Sci. 2002, 12, 243–252. [Google Scholar]
- Seymour, F.; Harris, N.L. Reducing tropical deforestation. Science 2019, 365, 756–757. [Google Scholar] [CrossRef]
- Nave, L.E.; Domke, G.M.; Hofmeister, K.L.; Mishra, U.; Perry, C.H.; Walters, B.F.; Swanston, C.W. Reforestation can sequester two petagrams of carbon in US topsoils in a century. Proc. Natl. Acad. Sci. USA 2018. [Google Scholar] [CrossRef]
- Bastin, J.-F.; Finegold, Y.; Garcia, C.; Mollicone, D.; Rezende, M.; Routh, D.; Zohner, C.M.; Crowther, T.W. The global tree restoration potential. Science 2019, 365, 76–79. [Google Scholar] [CrossRef]
- Chen, C.; Park, T.; Wang, X.H.; Piao, S.L.; Xu, B.D.; Chaturvedi, R.K.; Fuchs, R.; Brovkin, V.; Ciais, P.; Fensholt, R.; et al. China and India lead in greening of the world through land-use management. Nat. Sustain. 2019, 2, 122–129. [Google Scholar] [CrossRef]
- Hou, G.L.; Delang, C.O.; Lu, X.X.; Olschewski, R. Valuing Carbon Sequestration to Finance Afforestation Projects in China. Forests 2019, 10, 754. [Google Scholar] [CrossRef]
- Romijn, E.; Coppus, R.; De Sy, V.; Herold, M.; Roman-Cuesta, R.M.; Verchot, L. Land Restoration in Latin America and the Caribbean: An Overview of Recent, Ongoing and Planned Restoration Initiatives and Their Potential for Climate Change Mitigation. Forests 2019, 10, 510. [Google Scholar] [CrossRef]
- Don, A.; Schumacher, J.; Freibauer, A. Impact of tropical land-use change on soil organic carbon stocks—A meta-analysis. Glob. Chang. Biol. 2011, 17, 1658–1670. [Google Scholar] [CrossRef]
- Wang, F.M.; Ding, Y.Z.; Sayer, E.J.; Li, Q.L.; Zou, B.; Mo, Q.F.; Li, Y.W.; Lu, X.L.; Tang, J.W.; Zhu, W.X.; et al. Tropical forest restoration: Fast resilience of plant biomass contrasts with slow recovery of stable soil C stocks. Funct. Ecol. 2017, 31, 1–12. [Google Scholar] [CrossRef]
- Martin, P.A.; Newton, A.C.; Bullock, J.M. Carbon pools recover more quickly than plant biodiversity in tropical secondary forests. Proc. R. Soc. B 2013, 280, 20132236. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, D.F.; Oliet, J.A.; Aronson, J.; Bolte, A.; Bullock, J.M.; Donoso, P.J.; Landhäusser, S.M.; Madsen, P.; Peng, S.; Rey-Benayas, J.M. Restoring forests: What constitutes success in the twenty-first century? New For. 2015, 46, 601–614. [Google Scholar] [CrossRef]
- Asmelash, F.; Bekele, T.; Birhane, E. The Potential Role of Arbuscular Mycorrhizal Fungi in the Restoration of Degraded Lands. Front. Microbiol. 2016, 7, 1095. [Google Scholar] [CrossRef]
- Li, D.J.; Wen, L.; Jiang, S.; Song, T.Q.; Wang, K.L. Responses of soil nutrients and microbial communities to three restoration strategies in a karst area, southwest China. J. Environ. Manag. 2018, 207, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Forrester, D.I.; Bauhus, J.; Cowie, A.L.; Vanclay, J.K. Mixed-species plantations of Eucalyptus with nitrogen-fixing trees: A review. For. Ecol. Manag. 2006, 233, 211–230. [Google Scholar] [CrossRef]
- Chen, F.L.; Zheng, H.; Zhang, K.; Ouyang, Z.Y.; Lan, J.; Li, H.L.; Shi, Q. Changes in soil microbial community structure and metabolic activity following conversion from native Pinus massoniana plantations to exotic Eucalyptus plantations. For. Ecol. Manag. 2013, 291, 65–72. [Google Scholar] [CrossRef]
- Brockett, B.F.T.; Prescott, C.E.; Grayston, S.J. Soil moisture is the major factor influencing microbial community structure and enzyme activities across seven biogeoclimatic zones in western Canada. Soil Biol. Biochem. 2012, 44, 9–20. [Google Scholar] [CrossRef]
- Mangalassery, S.; Mooney, S.J.; Sparkes, D.L.; Fraser, W.T.; Sjögersten, S. Impacts of zero tillage on soil enzyme activities, microbial characteristics and organic matter functional chemistry in temperate soils. Eur. J. Soil Biol. 2015, 68, 9–17. [Google Scholar] [CrossRef]
- Ananbeh, H.; Stojanović, M.; Pompeiano, A.; Voběrková, S.; Trasar-Cepeda, C. Use of soil enzyme activities to assess the recovery of soil functions in abandoned coppice forest systems. Sci. Total Environ. 2019, 694, 133692. [Google Scholar] [CrossRef] [PubMed]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.T.; Landi, L.; Pietramellara, G.; Renella, G. Microbial diversity and soil functions. Eur. J. Soil Sci. 2003, 54, 655–670. [Google Scholar] [CrossRef]
- Singh, B.K.; Bardgett, R.D.; Smith, P.; Reay, D.S. Microorganisms and climate change: Terrestrial feedbacks and mitigation options. Nat. Rev. Microbiol. 2010, 8, 779–790. [Google Scholar] [CrossRef]
- Cusack, D.F.; Silver, W.L.; Torn, M.S.; Burton, S.D.; Firestone, M.K. Changes in microbial community characteristics and soil organic matter with nitrogen additions in two tropical forests. Ecology 2011, 92, 621. [Google Scholar] [CrossRef] [PubMed]
- Sparling, G.P. Ratio of microbial biomass carbon to soil organic carbon as a sensitive indicator of changes in soil organic matter. Aust. J. Soil Res. 1992, 30, 195–207. [Google Scholar] [CrossRef]
- Crowther, T.W.; van den Hoogen, J.; Wan, J.; Mayes, M.A.; Keiser, A.D.; Mo, L.; Averill, C.; Maynard, D.S. The global soil community and its influence on biogeochemistry. Science 2019, 365, eaav0550. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, K.B.; West, C.P.; Acosta-Martinez, V. Assessing the role of interseeding alfalfa into grass on improving pasture soil health in semi-arid Texas High Plains. Appl. Soi. Ecol. 2019. [Google Scholar] [CrossRef]
- Bailey, V.L.; Smith, J.L.; Bolton, H., Jr. Fungal-to-bacterial ratios in soils investigated for enhanced C sequestration. Soil Biol. Biochem. 2002, 34, 997–1007. [Google Scholar] [CrossRef]
- Six, J.; Frey, S.D.; Thiet, R.K.; Batten, K.M. Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Sci. Soc. Am. J. 2006, 70, 555–569. [Google Scholar] [CrossRef]
- Malik, A.A.; Chowdhury, S.; Schlager, V.; Oliver, A.; Puissant, J.; Vazquez, P.G.M.; Jehmlich, N.; von Bergen, M.; Griffiths, R.I.; Gleixner, G. Soil Fungal: Bacterial Ratios are Linked to Altered Carbon Cycling. Front. Microbiol. 2016, 7, 1247. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L. Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol. Biochem. 2010, 42, 391–404. [Google Scholar] [CrossRef]
- Burns, R.G.; Deforest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Waring, B.G.; Averill, C.; Hawkes, C.V. Differences in fungal and bacterial physiology alter soil carbon and nitrogen cycling: Insights from meta-analysis and theoretical models. Ecol. Lett. 2013, 16, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Waldrop, M.P.; Balser, T.C.; Firestone, M.K. Linking microbial community composition to function in a tropical soil. Soil Biol. Biochem. 2000, 32, 1837–1846. [Google Scholar] [CrossRef]
- Teixeira, Z.; Teixeira, H.; Marques, J.C. Systematic processes of land use/land cover change to identify relevant driving forces: Implications on water quality. Sci. Total Environ. 2014, 470–471, 1320–1335. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef]
- Smith, A.P.; Marin-Spiotta, E.; Balser, T. Successional and seasonal variations in soil and litter microbial community structure and function during tropical postagricultural forest regeneration: A multiyear study. Glob. Chang. Biol. 2015, 21, 3532–3547. [Google Scholar] [CrossRef]
- Suseela, V.; Conant, R.T.; Wallenstein, M.D.; Dukes, J.S. Effects of soil moisture on the temperature sensitivity of heterotrophic respiration vary seasonally in an old-field climate change experiment. Glob. Chang. Biol. 2012, 18, 336–348. [Google Scholar] [CrossRef]
- Bhandari, K.B.; West, C.P.; Longing, S.D.; Brown, C.P.; Green, P.E. Comparison of Arthropod Communities among Different Forage Types on the Texas High Plains using Pitfall Traps. Crop Forage Turfgrass Manag. 2018, 4. [Google Scholar] [CrossRef]
- Bhandari, K.B.; West, C.P.; Longing, S.D. Communities of Canopy-Dwelling Arthropods in Response to Diverse Forages. Agric. Environ. Lett. 2018, 3. [Google Scholar] [CrossRef]
- Kuťáková, E.; Cesarz, S.; Münzbergová, Z.; Eisenhauer, N. Soil microarthropods alter the outcome of plant-soil feedback experiments. Sci. Rep. 2018, 8, 11898. [Google Scholar] [CrossRef]
- Fichtner, A.; von Oheimb, G.; Härdtle, W.; Wilken, C.; Gutknecht, J.L.M. Effects of anthropogenic disturbances on soil microbial communities in oak forests persist for more than 100 years. Soil Biol. Biochem. 2014, 70, 79–87. [Google Scholar] [CrossRef]
- Deng, Q.; Cheng, X.L.; Hui, D.F.; Zhang, Q.; Li, M.; Zhang, Q.F. Soil microbial community and its interaction with soil carbon and nitrogen dynamics following afforestation in central China. Sci. Total Environ. 2016, 541, 230–237. [Google Scholar] [CrossRef]
- Jangid, K.; Williams, M.A.; Franzluebbers, A.J.; Schmidt, T.M.; Coleman, D.C.; Whitman, W.B. Land-use history has a stronger impact on soil microbial community composition than aboveground vegetation and soil properties. Soil Biol. Biochem. 2011, 43, 2184–2193. [Google Scholar] [CrossRef]
- Laganiere, J.; Angers, D.A.; Pare, D. Carbon accumulation in agricultural soils after afforestation: A meta-analysis. Glob. Chang. Biol. 2010, 16, 439–453. [Google Scholar] [CrossRef]
- Ren, H.; Li, Z.A.; Shen, W.J.; Yu, Z.Y.; Peng, S.L.; Liao, C.H.; Ding, M.M.; Wu, J.G. Changes in biodiversity and ecosystem function during the restoration of a tropical forest in south China. Sci. China C Life Sci. 2007, 50, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.Y.; Pi, Y.F. The path and its effects to rehabilitating vegetation on the tropical littoral eroded land in Guangdong province. Trop. Subtrop. For. Ecosyst. Res. 1985, 3, 97–108. (In Chinese) [Google Scholar]
- Tang, X.Y.; Liu, S.G.; Liu, J.X.; Zhou, G.Y. Effects of vegetation restoration and slope positions on soil aggregation and soil carbon accumulation on heavily eroded tropical land of Southern China. J. Soils Sed. 2010, 10, 505–513. [Google Scholar] [CrossRef]
- Cao, H.L.; Yu, Z.Y. Comparative study on the community structure of four different forests in Xiaoliang Experimental Station, Dianbai County, south Guangdong. Chin. J. Appl. Environ. Biol. 1998, 4, 315–319. (In Chinese) [Google Scholar]
- Ding, M.M.; Yi, W.M.; Liao, L.Y.; Martens, R.; Insam, H. Effect of afforestation on microbial biomass and activity in soils of tropical China. Soil Biol. Biochem. 1992, 24, 865–872. [Google Scholar] [CrossRef]
- Zhang, H.L.; Deng, Q.; Hui, D.F.; Wu, J.P.; Xiong, X.; Zhao, J.Q.; Zhao, M.D.; Chu, G.W.; Zhou, G.Y.; Zhang, D.Q. Recovery in soil carbon stock but reduction in carbon stabilization after 56-year forest restoration in degraded tropical lands. For. Ecol. Manag. 2019, 441, 1–8. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Bossio, D.A.; Scow, K.M. Impacts of carbon and flooding on soil microbial communities: Phospholipid fatty acid profiles and substrate utilization patterns. Microb. Ecol. 1998, 35, 265–278. [Google Scholar] [CrossRef]
- Fang, X.; Zhou, G.Y.; Li, Y.L.; Liu, S.Z.; Chu, G.W.; Xu, Z.H.; Liu, J.X. Warming effects on biomass and composition of microbial communities and enzyme activities within soil aggregates in subtropical forest. Biol. Fertil. Soils 2015, 52, 353–365. [Google Scholar] [CrossRef]
- Zelles, L. Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: A review. Biol. Fertil. Soils 1999, 29, 111–129. [Google Scholar] [CrossRef]
- Frostegard, A.; Baath, E. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol. Fertil. Soils 1996, 22, 59–65. [Google Scholar] [CrossRef]
- Turpeinen, R.; Kairesalo, T.; Haggblom, M.M. Microbial community structure and activity in arsenic-, chromium- and copper-contaminated soils. FEMS Microbiol. Ecol. 2004, 47, 39–50. [Google Scholar] [CrossRef]
- Olsson, P.A. Signature fatty acids provide tools for determination of the distribution and interactions of mycorrhizal fungi in soil. FEMS Microbiol. Ecol. 1999, 29, 303–310. [Google Scholar] [CrossRef]
- Bell, C.W.; Fricks, B.E.; Rocca, J.D.; Steinweg, J.M.; McMahon, S.K.; Wallenstein, M.D. High-throughput Fluorometric Measurement of Potential Soil Extracellular Enzyme Activities. J. Vis. Exp. 2013. [Google Scholar] [CrossRef]
- Wang, C.; Lu, X.K.; Mori, T.; Mao, Q.G.; Zhou, K.J.; Zhou, G.Y.; Nie, Y.X.; Mo, J.M. Responses of soil microbial community to continuous experimental nitrogen additions for 13 years in a nitrogen-rich tropical forest. Soil Biol. Biochem. 2018, 121, 103–112. [Google Scholar] [CrossRef]
- Caldwell, B.A. Enzyme activities as a component of soil biodiversity: A review. Pedobiologia 2005, 49, 637–644. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L. Enzymic analysis of microbial pattern and process. Biol. Fertil. Soils 1994, 17, 69–74. [Google Scholar] [CrossRef]
- Trasar-Cepeda, C.; Leiros, M.C.; Gil-Sotres, F. Hydrolytic enzyme activities in agricultural and forest soils. Some implications for their use as indicators of soil quality. Soil Biol. Biochem. 2008, 40, 2146–2155. [Google Scholar] [CrossRef]
- Wang, B.; Xue, S.; Liu, G.B.; Zhang, G.H.; Li, G.; Ren, Z.P. Changes in soil nutrient and enzyme activities under different vegetations in the Loess Plateau area, Northwest China. Catena 2012, 92, 186–195. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, J.J.; Koide, R.T.; Li, T.; Yang, H.S.; Chu, J.M. A meta-analysis of soil microbial biomass levels from established tree plantations over various land uses, climates and plant communities. Catena 2017, 150, 256–260. [Google Scholar] [CrossRef]
- Fierer, N.; Strickland, M.S.; Liptzin, D.; Bradford, M.A.; Cleveland, C.C. Global patterns in belowground communities. Ecol. Lett. 2009, 12, 1238–1249. [Google Scholar] [CrossRef]
- Allison, S.D.; Vitousek, P.M. Responses of extracellular enzymes to simple and complex nutrient inputs. Soil Biol. Biochem. 2005, 37, 937–944. [Google Scholar] [CrossRef]
- Jeddi, K.; Cortina, J.; Chaieb, M. Acacia salicina, Pinus halepensis and Eucalyptus occidentalis improve soil surface conditions in arid southern Tunisia. J. Arid Environ. 2009, 73, 1005–1013. [Google Scholar] [CrossRef]
- Korkanç, S.Y. Effects of afforestation on soil organic carbon and other soil properties. Catena 2014, 123, 62–69. [Google Scholar] [CrossRef]
- Yüksek, T.; Yüksek, F. The effects of restoration on soil properties in degraded land in the semi-arid region of Turkey. Catena 2011, 84, 47–53. [Google Scholar] [CrossRef]
- Robinson, N.; Harper, R.J.; Smettem, K.R.J. Soil water depletion by Eucalyptus spp. integrated into dryland agricultural systems. Plant Soil 2006, 286, 141–151. [Google Scholar] [CrossRef]
- Behera, N.; Sahani, U. Soil microbial biomass and activity in response to Eucalyptus plantation and natural regeneration on tropical soil. For. Ecol. Manag. 2003, 174, 1–11. [Google Scholar] [CrossRef]
- Ritz, K.; Young, I.M. Interactions between soil structure and fungi. Mycologist 2004, 18, 52–59. [Google Scholar] [CrossRef]
- De Menezes, A.B.; Richardson, A.E.; Thrall, P.H. Linking fungal-bacterial co-occurrences to soil ecosystem function. Curr. Opin. Microbiol. 2017, 37, 135–141. [Google Scholar] [CrossRef]
- Holland, E.A.; Coleman, D.C. Litter Placement Effects on Microbial and Organic Matter Dynamics in an Agroecosystem. Ecology 1987, 68, 425–433. [Google Scholar] [CrossRef]
- Hanson, C.A.; Allison, S.D.; Bradford, M.A.; Wallenstein, M.D.; Treseder, K.K. Fungal Taxa Target Different Carbon Sources in Forest Soil. Ecosystems 2008, 11, 1157–1167. [Google Scholar] [CrossRef]
- Schimel, J.P.; Schaeffer, S.M. Microbial control over carbon cycling in soil. Front. Microbiol. 2012, 3, 11. [Google Scholar] [CrossRef]
- Wan, X.H.; Huang, Z.Q.; He, Z.M.; Yu, Z.P.; Wang, M.H.; Davis, M.R.; Yang, Y.S. Soil C:N ratio is the major determinant of soil microbial community structure in subtropical coniferous and broadleaf forest plantations. Plant Soil 2015, 387, 103–116. [Google Scholar] [CrossRef]
- Ren, C.J.; Kang, D.; Wu, J.P.; Zhao, F.Z.; Yang, G.H.; Han, X.H.; Feng, Y.Z.; Ren, G.X. Temporal variation in soil enzyme activities after afforestation in the Loess Plateau, China. Geoderma 2016, 282, 103–111. [Google Scholar] [CrossRef]
- Moghimian, N.; Hosseini, S.M.; Kooch, Y.; Darki, B.Z. Impacts of changes in land use/cover on soil microbial and enzyme activities. Catena 2017, 157, 407–414. [Google Scholar] [CrossRef]
- Stock, S.C.; Köster, M.; Dippold, M.A.; Nájera, F.; Matus, F.; Merino, C.; Boy, J.; Spielvogel, S.; Gorbushina, A.; Kuzyakov, Y. Environmental drivers and stoichiometric constraints on enzyme activities in soils from rhizosphere to continental scale. Geoderma 2019, 337, 973–982. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen–phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C.; Contosta, A.R.; Cusack, D.; Frey, S.; Gallo, M.E. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Shah, J.J.F. Ecoenzymatic Stoichiometry and Ecological Theory. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 313–343. [Google Scholar] [CrossRef]
- Deng, Q.; Zhang, D.Q.; Han, X.; Chu, G.W.; Zhang, Q.F.; Hui, D.F. Changing rainfall frequency rather than drought rapidly alters annual soil respiration in a tropical forest. Soil Biol. Biochem. 2018, 121, 8–15. [Google Scholar] [CrossRef]
- Zhao, Q.; Shen, W.J.; Chen, Q.; Helmisaari, H.S.; Sun, Q.Q.; Jian, S.G. Spring drying and intensified summer rainfall affected soil microbial community composition but not enzyme activity in a subtropical forest. Appl. Soi. Ecol. 2018, 130, 219–225. [Google Scholar] [CrossRef]
- Schimel, J.; Balser, T.C.; Wallenstein, M. Microbial Stress-Response Physiology and its Implications for Ecosystem Function. Ecology 2007, 88, 1386–1394. [Google Scholar] [CrossRef]
- Landesman, W.J.; Dighton, J. Response of soil microbial communities and the production of plant-available nitrogen to a two-year rainfall manipulation in the New Jersey Pinelands. Soil Biol. Biochem. 2010, 42, 1751–1758. [Google Scholar] [CrossRef]


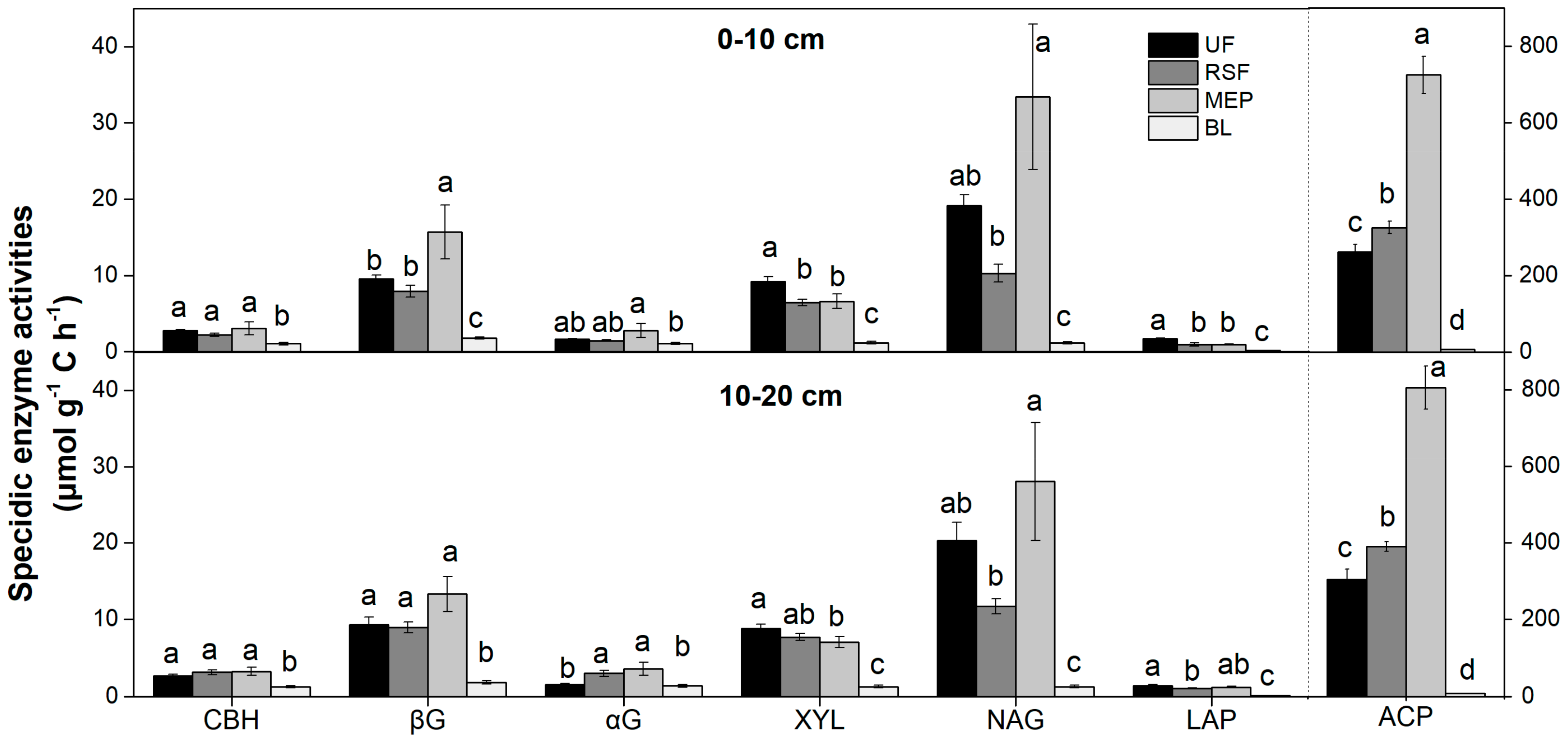
| Element Cycling | Enzyme | Abbr. | Function | Substrate |
|---|---|---|---|---|
| C-cycling enzyme | Cellobiohydrolase | CBH | Cellulose degradation | 4-MUB-β-D-cellobioside |
| β-1,4-glucosidase | βG | Cellulose degradation | 4-MUB-β-D-glucoside | |
| α-1,4-glucosidase | αG | Starch and disaccharides degradation | 4-MUB-α-D-glucoside | |
| β-1,4-xylosidase | XYL | Hemicellulose degradation | 4-MUB-β-D-xyloside | |
| phenol oxidase | PhOx | Polyphenols degradation, such as lignin | L-DOPA | |
| peroxidase | Perox | Polyphenols degradation, such as lignin | L-DOPA | |
| N-cycling enzyme | β-1,4-N-acetyl-glucosaminnidase | NAG | Chitin degradation | 4-MUB-N-acetyl-β-D-glucosaminide |
| L-leucine aminopeptidase | LAP | Cellulose degradation | L-Leucine-T-amino-4-methylcoumarin | |
| P-cycling enzyme | Acid phosphatase | ACP | Organic P mineralization | 4-MUB-phosphate |
| Depth | Research Site | Sol Water Content (%) | TC (g kg−1) | TN (g kg−1) | Soil C/N Ratio | |
|---|---|---|---|---|---|---|
| Dry Season | Wet Season | |||||
| 0–10 cm | UF | 19.42 ± 0.30 a | 21.00 ± 0.28 a | 27.08 ± 0.87 a | 2.12 ± 0.06 a | 12.80 ± 0.19 a |
| RSF | 16.40 ± 0.26 b | 19.75 ± 0.64 a | 29.02 ± 1.98 a | 2.10 ± 0.12 a | 13.77 ± 0.26 a | |
| MEP | 7.97 ± 0.42 c | 8.88 ± 0.56 b | 11.93 ± 1.86 b | 0.87 ± 0.11 b | 13.53 ± 0.42 a | |
| BL | 8.95 ± 0.39 c | 9.74 ± 0.19 b | 2.95 ± 0.28 c | 0.30 ± 0.04 c | 10.04 ± 0.49 b | |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| 10–20 cm | UF | 18.18 ± 0.22 a | 19.93 ± 0.28 a | 20.32 ± 0.72 a | 1.67 ± 0.04 a | 12.18 ± 0.22 a |
| RSF | 16.10 ± 0.20 b | 18.58 ± 0.42 b | 14.85 ± 1.42 b | 1.18 ± 0.09 b | 12.46 ± 0.32 a | |
| MEP | 9.88 ± 0.40 c | 9.66 ± 0.51 c | 8.45 ± 0.92 c | 0.73 ± 0.06 c | 11.45 ± 0.57 a | |
| BL | 8.79 ± 0.27 d | 10.68 ± 0.23 c | 2.73 ± 0.30 d | 0.37 ± 0.03 d | 7.44 ± 0.51 b | |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Season | Site | MBC | MBN | ||
|---|---|---|---|---|---|
| 0–10 cm | 10–20 cm | 0–10 cm | 10–20 cm | ||
| Dry season | UF | 358.47 ± 26.45 a | 255.84 ± 91.67 a | 80.92 ± 5.07 a | 40.27 ± 12.07 a |
| RSF | 273.98 ± 25.20 b | 80.43 ± 21.10 b | 58.86 ± 5.54 a | 23.93 ± 5.38 ab | |
| MEP | 47.52 ± 16.12 c | 11.91 ± 5.47 b | 9.90 ± 3.33 b | 4.63 ± 2.16 b | |
| BL | 0.37 ± 0.29 c | 2.79 ± 0.01 b | 1.00 ± 0.33 b | 1.35 ± 0.75 b | |
| p-value | <0.001 | 0.047 | <0.001 | 0.010 | |
| Wet season | UF | 506.09 ± 36.51 a | 316.71 ± 19.99 a | 106.02 ± 7.63 a | 74.69 ± 4.08 a |
| RSF | 492.87 ± 20.34 a | 204.26 ± 20.61 b | 106.15 ± 5.17 a | 51.73 ± 3.55 b | |
| MEP | 112.77 ± 29.82 b | 60.84 ± 17.15 c | 21.48 ± 5.92 b | 13.81 ± 2.77 c | |
| BL | 6.39 ± 5.29 c | 10.32 ± 1.65 c | 3.44 ± 0.66 b | 4.94 ± 1.50 d | |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | |
| Soil Depth | Site | The Absolute Abundances of PLFAs (nmol g−1 soil) | ||||||
|---|---|---|---|---|---|---|---|---|
| B | G+ | G− | F | Total PLFAs | F/B | G+/G− | ||
| Dry season | ||||||||
| 0–10 cm | UF | 28.16 (1.34) a | 22.53 (1.03) a | 4.25 (0.27) a | 5.91 (0.28) a | 44.51 (2.19) a | 0.21 (0.00) b | 5.33 (0.19) |
| RSF | 17.25 (2.05) b | 13.09 (1.61) b | 3.24 (0.33) b | 4.99 (0.54) a | 29.19 (3.45) b | 0.29 (0.00) b | 4.00 (0.12) | |
| MEP | 7.26 (0.69) c | 5.54 (0.56) c | 1.32 (0.12) c | 1.74 (0.13) b | 11.60 (1.08) c | 0.25 (0.03) b | 4.20 (0.25) | |
| BL | 0.25 (0.04) d | 0.18 (0.03) d | 0.02 (0.01) d | 0.14 (0.01) c | 0.41 (0.05) d | 0.63 (0.12) a | 4.53 (2.16) | |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.578 | |
| 10–20 cm | UF | 18.10 (1.32) a | 15.28 (1.03) a | 1.83 (0.39) a | 2.91 (0.13) a | 26.40 (2.39) a | 0.16 (0.01) b | 9.82 (1.76) a |
| RSF | 8.24 (1.55) b | 6.40 (1.31) b | 1.45 (0.19) a | 1.88 (0.32) b | 13.43 (2.59) b | 0.23 (0.01) b | 4.27 (0.45) b | |
| MEP | 3.15 (0.22) c | 2.55 (0.19) c | 0.49 (0.05) b | 0.74 (0.12) c | 4.88 (0.36) c | 0.23 (0.02) b | 5.38 (0.50) ab | |
| BL | 0.17 (0.02) d | 0.12 (0.01) d | 0.01 (0.01) c | 0.14 (0.03) d | 0.32 (0.05) d | 0.78 (0.09) a | 2.12 (1.32) c | |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.002 | |
| Wet season | ||||||||
| 0–10 cm | UF | 27.66 (1.40) a | 21.78 (1.21) a | 4.52 (0.13) a | 6.59 (0.28) a | 44.99 (2.04) a | 0.24 (0.01) c | 4.80 (0.17) b |
| RSF | 22.83 (0.82) b | 16.81 (0.60) b | 4.81 (0.16) a | 7.22 (0.34) a | 39.62 (1.47) b | 0.32 (0.01) b | 3.49 (0.03) b | |
| MEP | 6.55 (0.93) c | 5.14 (0.73) c | 1.08 (0.15) b | 1.42 (0.18) b | 10.34 (1.50) c | 0.22 (0.01) c | 4.70 (0.12) b | |
| BL | 0.22 (0.01) d | 0.15 (0.01) d | 0.02 (0.00) c | 0.12 (0.00) c | 0.69 (0.02) d | 0.56 (0.04) a | 9.84 (1.87) a | |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| 10–20 cm | UF | 22.52 (1.76) a | 18.53 (1.47) a | 3.11 (0.28) a | 3.92 (0.36) a | 33.83 (2.64) a | 0.17 (0.01) c | 6.02 (0.27) ab |
| RSF | 13.36 (0.76) b | 9.65 (0.63) b | 3.07 (0.13) a | 3.61 (0.20) a | 22.22 (1.27) b | 0.27 (0.01) b | 3.14 (0.15) b | |
| MEP | 4.43 (0.73) c | 3.48 (0.58) c | 0.78 (0.11) b | 0.92 (0.16) b | 7.00 (1.19) c | 0.21 (0.01) c | 4.43 (0.13) ab | |
| BL | 0.19 (0.01) d | 0.14 (0.01) d | 0.01 (0.00) c | 0.09 (0.00) c | 0.16 (0.02) d | 0.47 (0.02) a | 11.52 (4.57) a | |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.081 | |
| Soil Depth | Site | The Relative Abundances PLFAs (mol %) | |||||
|---|---|---|---|---|---|---|---|
| B | G+ | G− | F | ACT | AMF | ||
| Dry season | |||||||
| 0–10 cm | UF | 60.30 (0.31) | 50.66 (0.42) a | 9.53 (0.27) a | 13.29 (0.08) b | 18.74 (0.45) a | 4.67 (0.13) a |
| RSF | 59.09 (0.25) | 44.74 (0.31) b | 11.22 (0.28) a | 17.19 (0.27) b | 19.18 (0.22) a | 4.55 (0.11) a | |
| MEP | 62.50 (0.96) | 47.62 (1.27) ab | 11.42 (0.40) a | 15.40 (1.54) b | 18.78 (0.78) a | 3.31 (0.32) b | |
| BL | 59.21 (3.31) | 45.12 (2.55) b | 4.06 (1.92) b | 36.17 (4.31) a | 4.62 (1.89) b | 0.00 (0.00) c | |
| p-value | 0.236 | 0.038 | 0.003 | < 0.001 | < 0.001 | < 0.001 | |
| 10–20 cm | UF | 69.09 (1.75) a | 58.43 (1.62) a | 6.60 (0.91) b | 11.23 (0.63) c | 16.87 (1.69) a | 2.81 (0.71) ab |
| RSF | 61.59 (0.77) b | 47.10 (0.77) b | 11.55 (1.24) a | 14.43 (0.81) b | 19.95 (0.72) a | 4.03 (0.25) a | |
| MEP | 64.79 (0.99) ab | 52.38 (1.39) ab | 9.97 (0.64) a | 14.83 (1.54) b | 17.65 (1.58) a | 2.72 (0.32) b | |
| BL | 54.83 (3.65) c | 38.10 (3.48) c | 2.41 (1.49) c | 41.51 (2.41) a | 3.65 (2.39) b | 0.00 (0.00) c | |
| p-value | 0.002 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Wet season | |||||||
| 0–10 cm | UF | 61.42 (0.59) a | 48.29 (0.69) a | 10.10 (0.24) b | 14.72 (0.53) b | 18.99 (0.55) b | 4.87 (0.25) a |
| RSF | 57.64 (0.19) b | 42.45 (0.19) b | 12.15 (0.11) a | 18.19 (0.27) a | 19.00 (0.18) b | 5.17 (0.15) a | |
| MEP | 63.48 (0.22) a | 49.76 (0.28) a | 10.61 (0.26) b | 14.02 (0.61) b | 18.90 (0.57) b | 3.60 (0.30) b | |
| BL | 31.37 (1.27) c | 21.70 (1.45) c | 2.46 (0.29) c | 17.44 (0.58) a | 50.93 (1.24) a | 0.27 (0.27) c | |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| 10–20 cm | UF | 66.59 (0.71) a | 54.82 (0.92) a | 9.16 (0.27) c | 11.56 (0.42) c | 17.91 (0.45) b | 3.95 (0.18) b |
| RSF | 60.13 (0.45) c | 43.33 (0.66) c | 13.92 (0.51) a | 16.33 (0.71) a | 18.87 (0.37) b | 4.68 (0.30) a | |
| MEP | 63.67 (0.85) b | 49.97 (0.81) b | 11.32 (0.31) b | 13.13 (0.62) bc | 19.68 (0.67) b | 3.52 (0.06) b | |
| BL | 31.13 (1.53) d | 23.33 (1.58) d | 1.21 (0.57) d | 14.43 (0.42) b | 54.44 (1.85) a | 0.00 (0.00) c | |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Depth | CBH | βG | αG | XYL | NAG | LAP | ACP | PhOx | Perox | βG/ (NAG+LAP) | βG/ACP | (NAG+LAP)/ ACP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–10 cm | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| 10–20 cm | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Xiong, X.; Wu, J.; Zhao, J.; Zhao, M.; Chu, G.; Hui, D.; Zhou, G.; Deng, Q.; Zhang, D. Changes in Soil Microbial Biomass, Community Composition, and Enzyme Activities After Half-Century Forest Restoration in Degraded Tropical Lands. Forests 2019, 10, 1124. https://doi.org/10.3390/f10121124
Zhang H, Xiong X, Wu J, Zhao J, Zhao M, Chu G, Hui D, Zhou G, Deng Q, Zhang D. Changes in Soil Microbial Biomass, Community Composition, and Enzyme Activities After Half-Century Forest Restoration in Degraded Tropical Lands. Forests. 2019; 10(12):1124. https://doi.org/10.3390/f10121124
Chicago/Turabian StyleZhang, Huiling, Xin Xiong, Jianping Wu, Jianqi Zhao, Mengdi Zhao, Guowei Chu, Dafeng Hui, Guoyi Zhou, Qi Deng, and Deqiang Zhang. 2019. "Changes in Soil Microbial Biomass, Community Composition, and Enzyme Activities After Half-Century Forest Restoration in Degraded Tropical Lands" Forests 10, no. 12: 1124. https://doi.org/10.3390/f10121124
APA StyleZhang, H., Xiong, X., Wu, J., Zhao, J., Zhao, M., Chu, G., Hui, D., Zhou, G., Deng, Q., & Zhang, D. (2019). Changes in Soil Microbial Biomass, Community Composition, and Enzyme Activities After Half-Century Forest Restoration in Degraded Tropical Lands. Forests, 10(12), 1124. https://doi.org/10.3390/f10121124