Past Management Spurs Differential Plant Communities within a Giant Single-Clone Aspen Forest
Abstract
1. Introduction
2. Methods
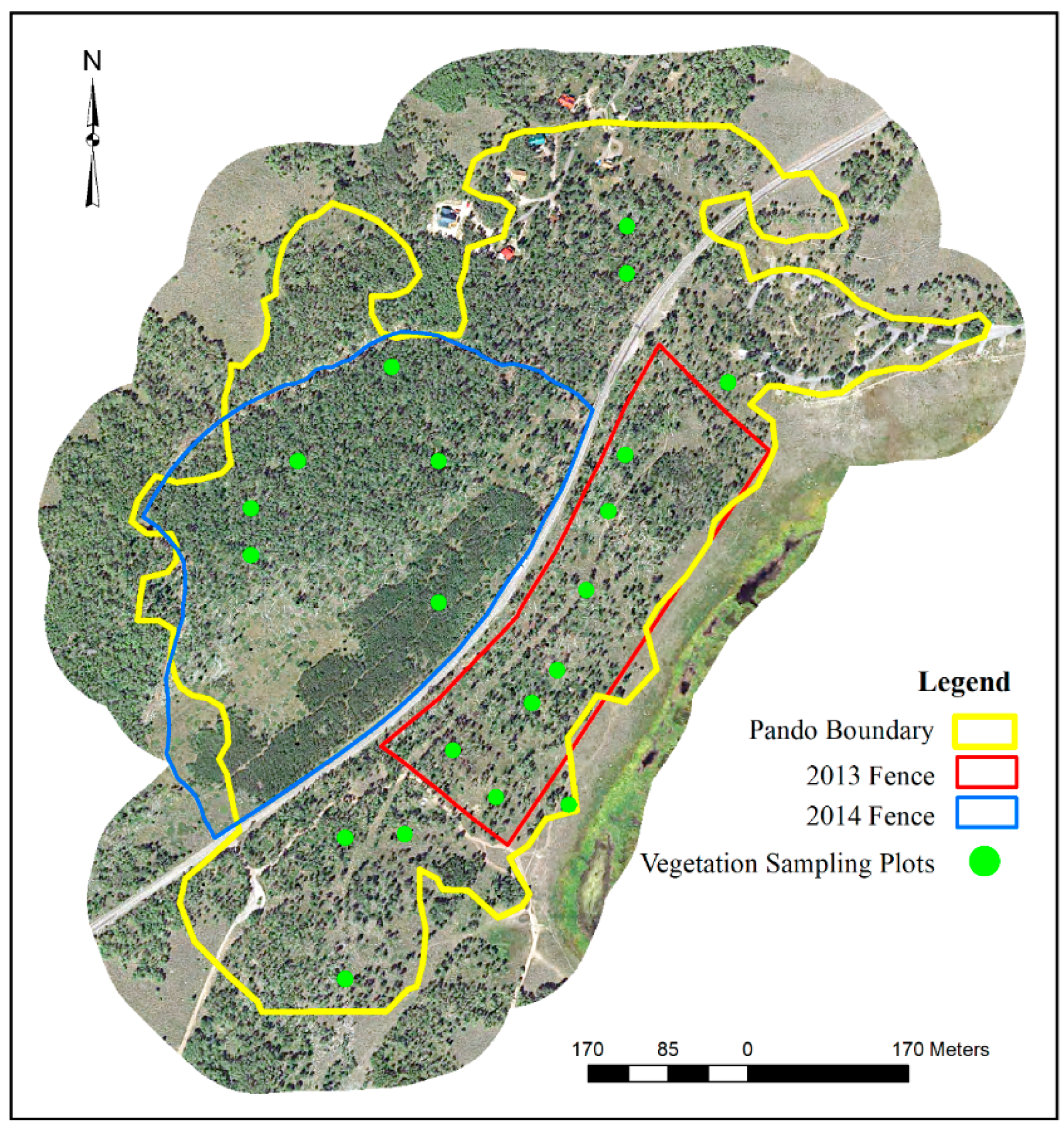
2.1. Study Site
2.2. Field Methods
- No Fence—area is unrestricted from browsing herbivores.
- 2013 Fence—area is restricted from browsing herbivores AND implemented fire, shrub removal, and 50% overstory tree cutting. Approximately half this area was undisturbed as a control.
- 2014 Fence—area is restricted from browsing herbivores and no recent disturbance has taken place. A previous study, however, determined that mule deer were entering and browsing aspen suckers (cattle had no access).
2.3. Analytical Methods
3. Results
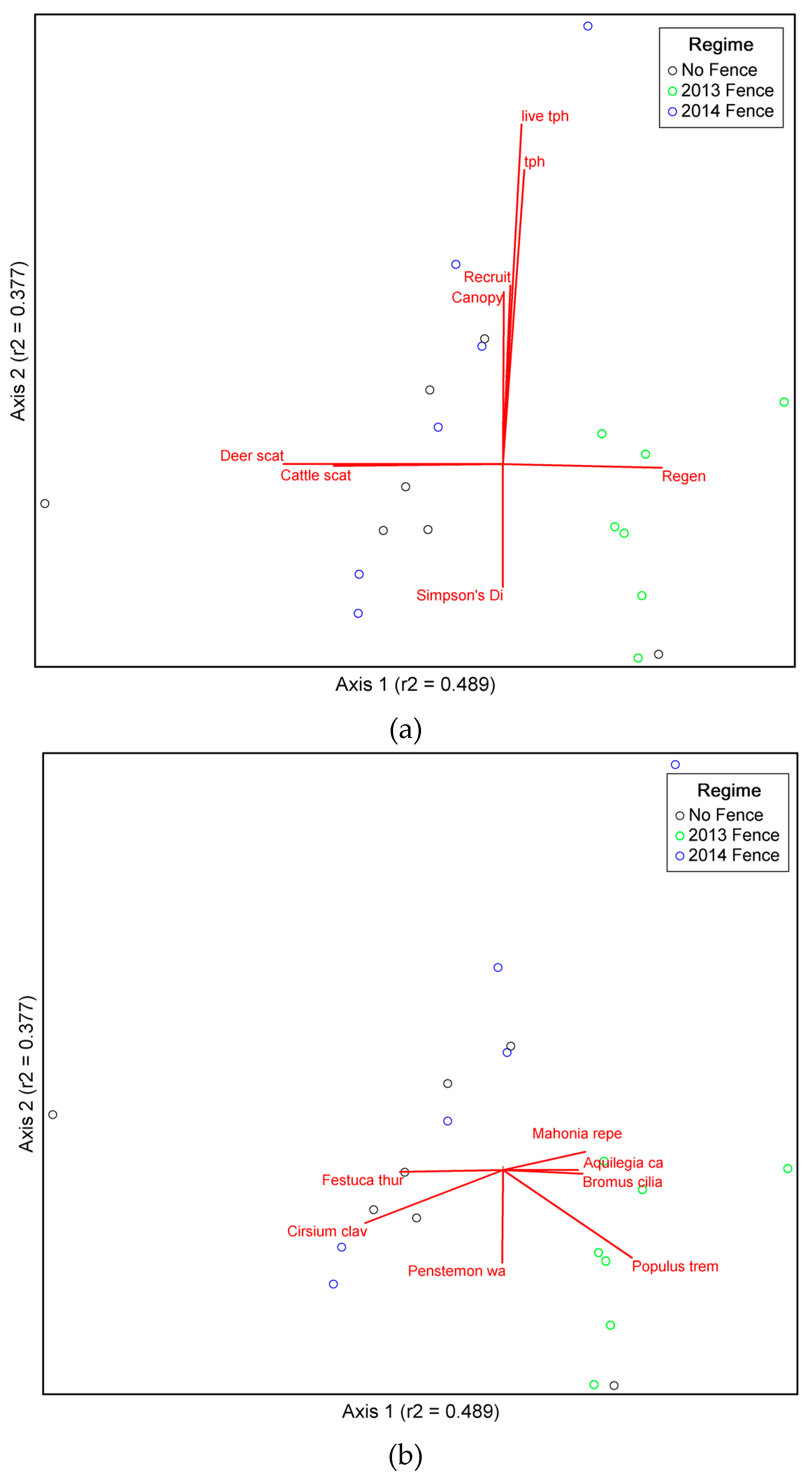
3.1. Is Plant Community Composition Different between Groups?
3.2. Species Preference for Management Groups
3.3. Exploring Factors Affecting Species Make-Up and Habitat Preferences
4. Discussion
4.1. Group and Species Preferences in a Single-Genotype Forest
4.2. Key Factors Influence Pando’s Plant Make-Up
4.3. Broader Implications for Sustained Aspen Diversity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Betters, D.R.; Woods, R.F. Uneven-aged stand structure and growth of Rocky Mountain aspen. J. For. 1981, 79, 673–676. [Google Scholar]
- Harniss, R.O.; Harper, K.T. Tree Dynamics in Seral and Stable Aspen Stands of Central Utah; RP-INT-297; United States Department of Agriculture Forest Service, Intermountain Research Station: Ogden, UT, USA, 1982; 7p.
- Mueggler, W.F. Aspen Community Types of the Intermountain Region; GTR-INT-250; United States Department of Agriculture Forest Service, Intermountain Research Station: Ogden, UT, USA, 1988; 135p.
- Rogers, P.C.; Landhäusser, S.M.; Pinno, B.D.; Ryel, R.J. A Functional Framework for Improved Management of Western North American Aspen (Populus tremuloides Michx.). For. Sci. 2014, 60, 345–359. [Google Scholar] [CrossRef]
- Shinneman, D.J.; Baker, W.L.; Rogers, P.C.; Kulakowski, D. Fire regimes of quaking aspen in the Mountain West. For. Ecol. Manag. 2013, 299, 22–34. [Google Scholar] [CrossRef]
- Kurzel, B.P.; Veblen, T.T.; Kulakowski, D. A typology of stand structure and dynamics of Quaking aspen in northwestern Colorado. For. Ecol. Manag. 2007, 252, 176–190. [Google Scholar] [CrossRef]
- Franklin, J.F.; Spies, T.A.; Van Pelt, R.; Carey, A.B.; Thornburgh, D.A.; Berg, D.R.; Lindenmayer, D.B.; Harmon, M.E.; Keeton, W.S.; Shaw, D.C.; et al. Disturbances and structural development of natural forest ecosystems with silvicultural implications, using Douglas-fir forests as an example. For. Ecol. Manag. 2002, 155, 399–423. [Google Scholar] [CrossRef]
- Holling, C.S.; Meffe, G.K. Command and control and the pathology of natural resource management. Conserv. Biol. 1996, 10, 328–337. [Google Scholar] [CrossRef]
- Chong, G.W.; Simonson, S.E.; Stohlgren, T.J.; Kalkhan, M.A. Biodiversity: Aspen stands have the lead, but will nonnative species take over? In Sustaining Aspen in Western Landscapes, Grand Junction, CO, USA, 13–15 June 2000; Shepperd, W.D., Binkley, D., Bartos, D.L., Stohlgren, T.J., Eskew, L.G., Eds.; RMRS-P-18; United States Department of Agriculture Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2001; pp. 261–271. [Google Scholar]
- Bartos, D.L.; Campbell, R.B.J. Water Depletion and other ecosystem values forfeited when conifer forests displace aspen communities. In Proceedings of the AWRA Specialty Conference, Rangeland Management and Water Resources, American Water Resources Association, Reno, NV, USA, 27–29 May 1998; p. 472. [Google Scholar]
- Bailey, J.K.; Whitham, T.G. Interactions among fire, aspen, and elk affect insect diversity: Reversal of a community response. Ecology 2002, 83, 1701–1712. [Google Scholar] [CrossRef]
- Bartos, D.L.; Campbell, R.B.J. Decline of quaking aspen in the Interior West-examples from Utah. Rangelands 1998, 20, 17–24. [Google Scholar]
- Vehmas, M.; Kouki, J.; Eerikäinen, K. Long-term spatio-temporal dynamics and historical continuity of European aspen (Populus tremula L.) stands in the Koli National Park, eastern Finland. Forestry 2008, 82, 135–148. [Google Scholar] [CrossRef][Green Version]
- Edenius, L.; Ericsson, G. Aspen demographics in relation to spatial context and ungulate browsing: Implications for conservation and forest management. Biol. Conserv. 2007, 135, 293–301. [Google Scholar] [CrossRef]
- Berrill, J.-P.; Dagley, C.M.; Coppeto, S.A.; Gross, S.E. Curtailing succession: Removing conifers enhances understorey light and growth of young aspen in mixed stands around Lake Tahoe, California and Nevada, USA. For. Ecol. Manag. 2017, 400, 511–522. [Google Scholar] [CrossRef]
- Bates, J.D.; Davies, K.W. Quaking aspen woodland after conifer control: Herbaceous dynamics. For. Ecol. Manag. 2018, 409, 307–316. [Google Scholar] [CrossRef]
- Worrall, J.J.; Rehfeldt, G.E.; Hamann, A.; Hogg, E.H.; Marchetti, S.B.; Michaelian, M.; Gray, L.K. Recent declines of Populus tremuloides in North America linked to climate. For. Ecol. Manag. 2013, 299, 35–51. [Google Scholar] [CrossRef]
- Rogers, P.C.; Ryel, R.J. Lichen community change in response to succession in aspen forests of the Rocky Mountains, USA. For. Ecol. Manag. 2008, 256, 1760–1770. [Google Scholar] [CrossRef]
- Rogers, P.C.; Mittanck, C.M. Herbivory strains resilience in drought-prone aspen landscapes of the western United States. J. Veg. Sci. 2014, 25, 457–469. [Google Scholar] [CrossRef]
- Rhodes, A.C.; Wan, H.Y.; St. Clair, S.B. Herbivory impacts of elk, deer and cattle on aspen forest recruitment along gradients of stand composition, topography and climate. For. Ecol. Manag. 2017, 397, 39–47. [Google Scholar] [CrossRef]
- Kuhn, T.J.; Safford, H.D.; Jones, B.E.; Tate, K.W. Aspen (Populus tremuloides) stands and their contribution to plant diversity in a semiarid coniferous landscape. Plant Ecol. 2011, 212, 1451–1463. [Google Scholar]
- Kouki, J.; Arnold, K.; Martikaninen, P. Long-term persistence of aspen-a key host for many threatened species-is endangered in old-growth conservation areas in Finland. J. Nat. Conserv. 2004, 12, 41–52. [Google Scholar] [CrossRef]
- Oaten, D.K.; Larsen, K.W. Aspen stands as small mammal “hotspots” within dry forest ecosystems of British Columbia. Northwest Sci. 2008, 82, 276–285. [Google Scholar] [CrossRef]
- Martin, T.E. Consequences of habitat change and resource selection specialization for population limitation in cavity nesting birds. J. Appl. Ecol. 2014, 52, 475–485. [Google Scholar] [CrossRef]
- Long, J.N.; Mock, K. Changing perspectives on regeneration ecology and genetic diversity in western quaking aspen: Implications for silviculture. Can. J. For. Res. 2012, 42, 2011–2021. [Google Scholar] [CrossRef]
- Lindroth, R.L.; St Clair, S.B. Adaptations of quaking aspen (Populus tremuloides Michx.) for defense against herbivores. For. Ecol. Manag. 2013, 299, 14–21. [Google Scholar] [CrossRef]
- Kemperman, J.A.; Barnes, B.V. Clone size in American aspens. Can. J. Bot. 1976, 54, 2603–2607. [Google Scholar] [CrossRef]
- Mitton, J.B.; Grant, M.C. Genetic variation and the natural history of quaking aspen. BioScience 1996, 46, 25–31. [Google Scholar] [CrossRef]
- DeWoody, J.; Rowe, C.A.; Hipkins, V.D.; Mock, K.E. “Pando” lives: Molecular genetic evidence of a giant aspen clone in central Utah. West. N. Am. Nat. 2008, 68, 493–497. [Google Scholar] [CrossRef]
- Rogers, P.C.; Gale, J.A. Restoration of the iconic Pando aspen clone: Emerging evidence of recovery. Ecosphere 2017, 8, e01661. [Google Scholar] [CrossRef]
- Rogers, P.C.; McAvoy, D.J. Mule deer impede Pando’s recovery: Implications for aspen resilience from a single-genotype forest. PLoS ONE 2018, 13, e0203619. [Google Scholar] [CrossRef]
- Myking, T.; Bohler, F.; Austrheim, G.; Solberg, E.J. Life history strategies of aspen (Populus tremula L.) and browsing effects: A literature review. Forestry 2011, 84, 61–71. [Google Scholar] [CrossRef]
- Schulz, B.K.; Bechtold, W.A.; Zarnoch, S.J. Sampling and Estimation Procedures for the Vegetation Diversity and Structure Indicator; PNW-GTR-781; United States Department of Agriculture Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 2009; 53p.
- McCune, B.; Lesica, P. The trade-off between species capture and quantitative accuracy in ecological inventory of lichens and bryophytes in forests in Montana. Bryologist 1992, 95, 296–304. [Google Scholar] [CrossRef]
- McCune, B.; Grace, J.B.; Urban, D.L. Analysis of Ecological Communities; MjM Software: Gleneden Beach, OR, USA, 2002. [Google Scholar]
- Welsh, S.L.; Atwood, N.D.; Goodrich, S.; Higgins, L.C. A Utah Flora; Brigham Young University Press: Provo, UT, USA, 1987. [Google Scholar]
- McCune, B.; Mefford, M.J. PC-ORD: Multivariate Analysis of Ecological Data; MjM Software: Gleneden Beach, OR, USA, 2016. [Google Scholar]
- Peck, J.E. Multivariate Analysis of Community Ecologists: Step-By-Step Using PC-ORD; MjM Sortware Design: Gleneden Beach, OR, USA, 2010. [Google Scholar]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need of a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Kruskal, J.B. Nonmetric multidimensional scaling: A numerical method. Psychometrika 1964, 29, 115–129. [Google Scholar] [CrossRef]
- Ding, C.; Schreiber, S.G.; Roberts, D.R.; Hamann, A.; Brouard, J.S. Post-glacial biogeography of trembling aspen inferred from habitat models and genetic variance in quantitative traits. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kashian, D.M.; Romme, W.H.; Regan, C.M. Reconciling divergent interpretations of the quaking aspen decline on the northern Colorado Front Range. Ecol. Appl. 2007, 17, 1296–1311. [Google Scholar] [CrossRef] [PubMed]
- Binkley, D.; Moore, M.M.; Romme, W.H.; Brown, P.M. Was Aldo Leopold right about the Kaibab deer herd? Ecosystems 2006, 9, 227–241. [Google Scholar] [CrossRef]
- Beschta, R.L.; Ripple, W.J. Are wolves saving Yellowstone’s aspen? A landscape-level test of a behaviorally mediated trophic cascade: Comment. Ecology 2013, 94, 1420–1425. [Google Scholar]
- Kauffman, M.J.; Brodie, J.F.; Jules, E.S. Are wolves saving Yellowstone’s aspen? A landscape-level test of a behaviorally mediated trophic cascade: Reply. Ecology 2013, 94, 1425–1431. [Google Scholar]
- Painter, L.E.; Beschta, R.L.; Larsen, E.J.; Ripple, E. Recovering aspen follow changing elk dynamics in Yellowstone: Evidence of a trophic cascade? Ecology 2014, 329, 108–117. [Google Scholar] [CrossRef]
- Fedrowitz, K.; Kuusinen, M.; Snäll, T. Metapopulation dynamics and future persistence of epiphytic cyanolichens in a European boreal forest ecosystem. J. Appl. Ecol. 2012, 49, 493–502. [Google Scholar] [CrossRef]
- Edenius, L.; Ericsson, G.; Kempe, G.; Bergström, R.; Danell, K. The effects of changing land use and browsing on aspen abundance and regeneration: A 50-year perspective from Sweden. J. Appl. Ecol. 2011, 4, 301–309. [Google Scholar] [CrossRef]
- Rogers, P.C.; Pinno, B.D.; Šebesta, J.; Albrectsen, A.; Li, G.; Ivanova, N.; Kulakowski, D.; Kusbach, A.; Kuuluvainen, T.; Landhäusser, S.M.; et al. A global view of aspen: Conservation science for widespread keystone systems. Glob. Ecol. Conserv. 2020, 21, e00828. [Google Scholar] [CrossRef]
| Management Group | T | A | p |
|---|---|---|---|
| No Fence vs. 2013 Fence | −1.29 | 0.06 | 0.100 |
| No Fence vs. 2014 Fence | −2.66 | 0.13 | 0.016 |
| 2013 Fence vs. 2014 Fence | −5.58 | 0.29 | <0.001 |
| Overall test result | 4.43 | 0.22 | <0.001 |
| Species | Maximum Score Group | Indicator Value | Indicator Values from Randomization | ||
|---|---|---|---|---|---|
| Mean | Standard Deviation | p | |||
| Artemisia tridentata | 2 | 50.0 | 35.5 | 8.46 | 0.0684 |
| Populus tremuloides | 2 | 60.6 | 40.0 | 4.51 | 0.0002 |
| Bromus ciliatus | 2 | 42.9 | 18.4 | 9.97 | 0.0814 |
| Calamagrostis spp. | 3 | 70.0 | 28.0 | 9.94 | 0.0040 |
| Lupinus argenteus | 3 | 55.4 | 38.3 | 5.52 | 0.0022 |
| Potentilla hippiana | 2 | 56.2 | 33.9 | 8.69 | 0.0198 |
| Silene menziesii | 3 | 38.9 | 20.5 | 9.49 | 0.0764 |
| Variable Name | r-Value | |
|---|---|---|
| Axis 1 | Axis 2 | |
| Fence | 0.138 | 0.309 |
| Juniper cover | −0.048 | 0.319 |
| Aspen cover | 0.056 | 0.683 |
| Regeneration ha−1 | 0.656 | −0.101 |
| Browse level | −0.456 | −0.244 |
| Recruitment ha−1 | 0.142 | 0.694 |
| Trees ha−1 | 0.242 | 0.892 |
| Live trees ha−1 | 0.223 | 0.957 |
| BA live | 0.014 | 0.533 |
| BA dead | −0.111 | 0.319 |
| BA dead as % total BA | −0.090 | 0.066 |
| BA total | −0.025 | 0.506 |
| Cattle scat | −0.677 | −0.068 |
| Deer scat | −0.770 | 0.020 |
| Species richness | 0.150 | −0.385 |
| Species evenness | −0.504 | −0.185 |
| Shannon’s diversity | 0.036 | −0.488 |
| Simpson’s diversity | −0.031 | −0.576 |
| Species Name | r-Value | Species Name | r-Value | ||
|---|---|---|---|---|---|
| Axis 1 | Axis 2 | Axis 1 | Axis 2 | ||
| Abies lasiocarpa | −0.144 | −0.003 | Clematis hirsutissima | 0.250 | −0.120 |
| Amelanchier alnifolia | 0.252 | −0.023 | Comandra umbellata | −0.423 | −0.088 |
| Arctostaphyllos uva-ursi | −0.008 | 0.328 | Cynoglossum officinale | −0.180 | −0.176 |
| Artemisia ludoviciana | −0.082 | 0.141 | Dactylis glomerata | 0.340 | 0.249 |
| Artemisia tridentata | −0.190 | −0.282 | Elymus glaucus | 0.152 | 0.138 |
| Chrysothamnus viscidiflorus | −0.087 | −0.085 | Elymus trachycaulus | −0.058 | 0.035 |
| Juniperus communis | 0.336 | 0.351 | Erigeron sp. | −0.213 | −0.354 |
| Mahonia repens | 0.474 | 0.222 | Eriogonum racemosum | −0.224 | −0.043 |
| Populus tremuloides | 0.592 | −0.488 | Festuca idahoensis | 0.001 | −0.104 |
| Prunus virginiana | −0.144 | −0.003 | Festuca thurberi | −0.530 | −0.075 |
| Ribes montigenum | −0.144 | −0.104 | Fragaria virginiana | 0.167 | −0.396 |
| Ribes inerme | −0.144 | −0.003 | Frasera speciosa | 0.206 | −0.358 |
| Rosa woodsii | −0.076 | −0.171 | Geranium richardsonii | 0.165 | −0.117 |
| Symphoricarpos oreophilus | 0.173 | −0.124 | Hackelia micrantha | 0.337 | 0.182 |
| Agoseris laciniata | −0.237 | −0.124 | Hesperostipa comata | 0.079 | 0.045 |
| Achillea millefolium | −0.040 | −0.205 | Juncus arcticus | −0.190 | −0.064 |
| Achnatheron nelsonii | −0.311 | −0.232 | Koeleria macrantha | −0.379 | 0.135 |
| Allium bisceptrum | −0.250 | −0.184 | Lactuca sp. | −0.286 | −0.136 |
| Antennaria microphylla | 0.074 | −0.242 | Lithospermum multiflorum | 0.344 | −0.036 |
| Aquilegia caerulea | 0.451 | −0.020 | Lotus wrightii | 0.201 | −0.180 |
| Arabis sp. | 0.157 | −0.250 | Lupinus argenteus | −0.155 | 0.142 |
| Arenaria macrophylla | 0.163 | −0.348 | Penstemon watsonii | −0.038 | −0.502 |
| Astragalus tenellus | 0.258 | −0.129 | Poa fendleriana | 0.171 | −0.134 |
| Astragalus laxmannii | 0.140 | −0.134 | Poa pratensis | 0.111 | −0.064 |
| Bromus ciliatus | 0.464 | −0.098 | Potentilla hippiana | −0.081 | −0.318 |
| Bromus inermis | −0.040 | −0.010 | Senecio streptentifolius | 0.416 | −0.179 |
| Calamagrostis sp. | −0.062 | 0.217 | Silene menziesii | 0.100 | 0.204 |
| Carex douglasii | −0.065 | −0.079 | Smilacina stellata | −0.098 | 0.182 |
| Carex obtusata | 0.171 | −0.124 | Taraxacum officinale | −0.205 | −0.169 |
| Carex occidentalis | −0.082 | 0.080 | Thalictrum fendlerii | −0.073 | 0.298 |
| Carex rossii | 0.228 | −0.112 | Thermopsis montana | 0.174 | −0.074 |
| Carex tahoensis | −0.237 | −0.124 | Tragopogon dubius | 0.148 | 0.015 |
| Castilleja sp. | 0.157 | −0.250 | Viola adunca | 0.104 | 0.132 |
| Cirsium clavatum | −0.613 | −0.381 | Wyethia amplexicaule | 0.204 | −0.031 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogers, P.C.; Šebesta, J. Past Management Spurs Differential Plant Communities within a Giant Single-Clone Aspen Forest. Forests 2019, 10, 1118. https://doi.org/10.3390/f10121118
Rogers PC, Šebesta J. Past Management Spurs Differential Plant Communities within a Giant Single-Clone Aspen Forest. Forests. 2019; 10(12):1118. https://doi.org/10.3390/f10121118
Chicago/Turabian StyleRogers, Paul C., and Jan Šebesta. 2019. "Past Management Spurs Differential Plant Communities within a Giant Single-Clone Aspen Forest" Forests 10, no. 12: 1118. https://doi.org/10.3390/f10121118
APA StyleRogers, P. C., & Šebesta, J. (2019). Past Management Spurs Differential Plant Communities within a Giant Single-Clone Aspen Forest. Forests, 10(12), 1118. https://doi.org/10.3390/f10121118




