1. Introduction
Arctic greening is one of the world’s most significant large-scale environmental responses to global climate change [
1]. Numerous studies have reported rising average temperatures over the past century, with the most noticeable and rapid changes occurring at high latitudes [
2]. Here, temperatures have risen by 0.6 °C per decade during the past 30 years, which is twice as fast as in average in the world [
1,
3,
4]. Earth-derived satellite imagery indicates a global increase of the normalized differential vegetation index (NDVI, average value), over the past decades [
5]. This value reflects the degree of ‘greening’ in high-latitudes areas, due to an increase of above ground phytomass production during the growing season [
6]. Satellite imagery data from high latitudes of the Canadian Arctic, northern Alaska, and western Greenland, showed an increase in NDVI by up to 15% between 1982 and 2008 [
6,
7]. A temperature increase in the Arctic may cause shifts of vegetation boundaries by several kilometres northward in lowlands, and by hundreds of meters difference in altitude in the mountains, thus leading to significant changes in species composition and biomass production processes [
2,
8,
9].
Changes in vegetation cover productivity and treelines are also obvious in the Russian Arctic [
10,
11,
12]. The increase of NDVI associated with climate warming was 10–15% during last 15 years [
12,
13]. At the same time, the actual monitoring of the effects of climate change on the Arctic biota and on Arctic ecosystems cannot be limited to remote methods only: they give only an idea of large-scale vegetation processes (in spatial terms). After satellite verification, more detailed analyses of dynamic processes in Arctic ecosystems are required. Changes in vegetation are accounting for phytomass reserves and products. In order to obtain a more detailed picture, measurements of the current soil respiration and soil moisture as well as carbon transpiration data of the vegetation, differentiated by plant groups, are required. They should be complemented by an assessment of projective cover, measurements of the height of phytocenotic horizons (for calculating the specific density of phytomass), and other important data. The latest synthesis of data on the productivity of Arctic ecosystems, based on field measurements in the Russian Arctic, was carried out 20–30 years ago [
14]. In that period, it was already clear that plant productivity of the Arctic vegetation started to increase. This was due to the advancement of the forest to the north, rising of tundra shrubs from the valley bottom to the upland, an increase in the proportion of sedges and cereals in the cover, a decrease in the coverage of lichens, etc. [
15]. At the end of the last century and the beginning of the present, an increasingly deep thawing of Arctic soils was also observed, leading to an increase in the activity of soil and litter microbiota [
7,
16].
The reaction of different groups of organisms of the Arctic Flora and Fauna to the ongoing greening and treeline dynamics caused by climate change have been studied for a long time [
12,
15], but the ecologically highly significant fungi have rarely been included in such studies [
17,
18]. The dynamics of fungal diversity could be very indicative for climate transformation (e.g., indirectly through trophic or symbiontic interactions, etc.) [
19,
20,
21,
22]. The Fungi are a crucial part of the heterotrophic block of ecosystems. Also, in the Arctic, the functioning of fungi (especially of mycelia activity) strongly depends on the length of the vegetation period, the plant activity and the amount of phytomass.
The relationship between warming in the Arctic and fungal dynamics has been addressed for various groups of macromycetes [
22,
23,
24,
25]. However, the relationship between fungal diversity and substrate availability has been included in a few works only [
26,
27,
28,
29,
30], thus not excluding the possibility that fungi might rather react to changes in the structure of the Arctic vegetation, than to the actual increase in temperature [
31,
32,
33].
In the vast expanses of the Russian Arctic, the study of biodiversity and ecology of macromycetes has been conducted for almost a century [
34]. However, the majority of such studies were short-term, lasting only one to two years. It is quite difficult to find long term mycological research areas, where also other parameters such as vegetation dynamics, soil properties, and climate data, were studied. This means that weather stations should be located in the same area, and the dynamics of other elements of the biota should be studied. In Russia, such a territory is the eastern macro slope of the Polar Urals. The Arctic Research Station of the Institute of Plant and Animal Ecology UrB RAS was established in the town Labytnangi (Yamal-Nenets Administrative District) in 1954. On the basis of this research station, ecological studies concerning various components of the high-latitude biota have been conducted for 60 years. Over the past 20 years, it has become extremely important to compare the results obtained in 1950–1960 with the current state of the Arctic biota. In particular, the treeline dynamics was studied in the region of Mount Rai-Iz, showing that since 1900 the timberline has risen by 60 m (relative to sea level). Moreover, the crown density and the height of the stand increased significantly [
35]. Earlier, larch stands prevailed in the forest, but now spruce and other boreal plants are increasing [
36]. Changes in the grass-shrub layer have also been studied in detail [
37], and a connection of these changes with climate warming has been established [
38]. Litter decomposition rate, soil thaw depth, and changes in floristic richness are also being investigated in the region [
39,
40,
41]. Over the past 60 years, the structure of the plant cover has transformed from forest-tundra to north boreal forests in the area of town Labytnangi and Mountains Slantsevaya and Rai-Iz, and this could be associated to climate warming and increased rainfall, especially in winter [
36]. Thus, various elements of the biota and bioclimatic parameters have changed very much in the area, posing the question whether or not the mycobiota reacts to such changes.
Thus, the aim of this study was to evaluate the response of the mycobiota to ongoing changes in climate and vegetation cover in the Polar Urals. We addressed the following questions: (1) How big is the impact of climate change occurring during the last 60 years on treeline position, forest areas, crown density, aboveground phytomass productivity, and permafrost thaw depth? (2) Was the associated mycobiota changing as well? If yes, which species and ecological or biogeographical groups of macromycetes were most affected by warming? Can selected groups of fungal species act as indicators of warming in arctic areas?
2. Materials and Methods
2.1. Study Area and Environmental Conditions
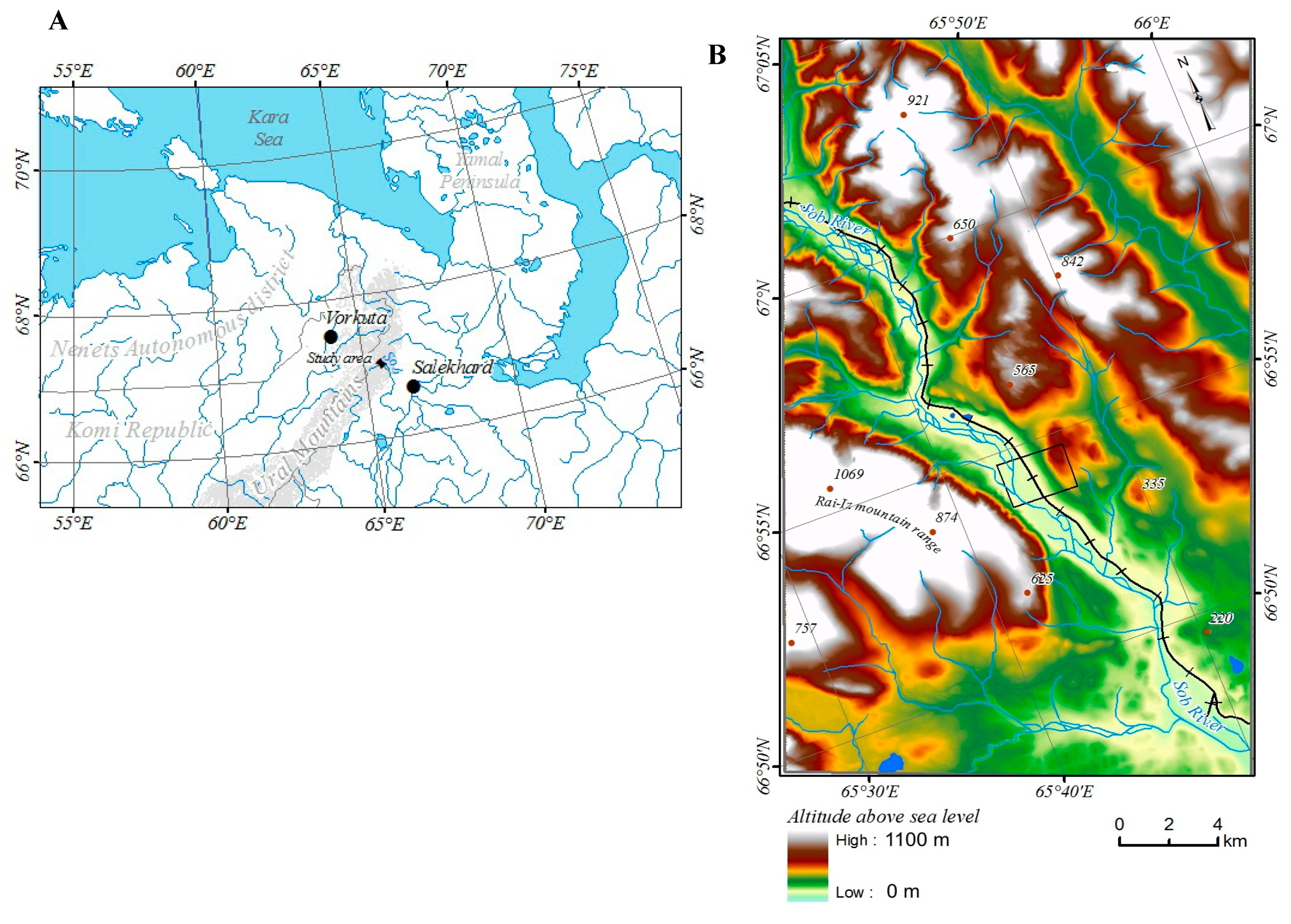
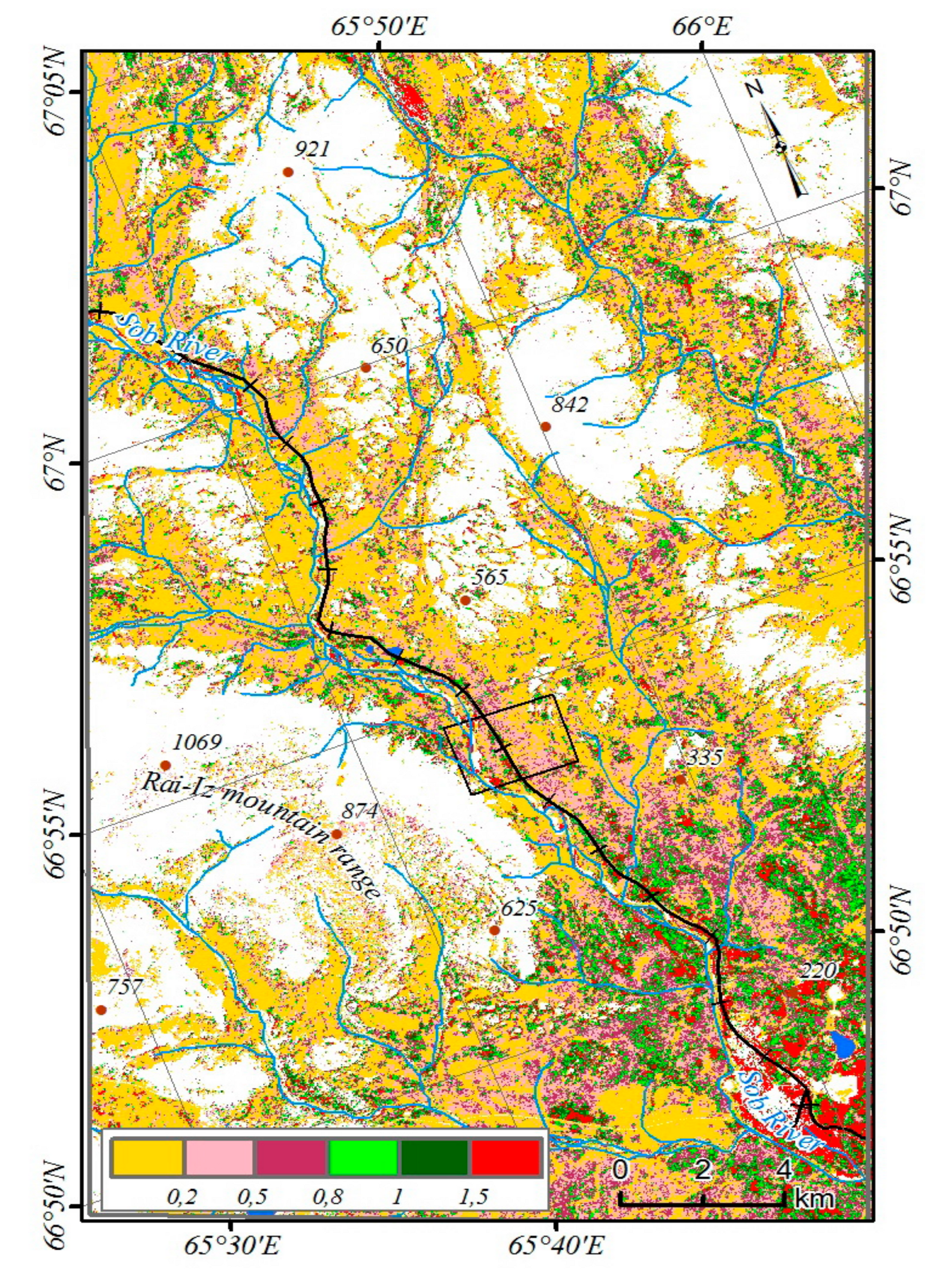
The study area is located where the Sob River cuts through the eastern slope of the Polar Urals at the territory of the Yamal-Nenets Autonomous District. The river flows from northwest to south-east, with the valley framed from west and south-west of Mountain Rai-Iz, and from north and north-east of Mountain Slantsevaya. The area is located 30 km north of the Arctic Circle (N 66°54’; E 65°44’) and the border between Europe and Asia, 60 km east of Vorkuta town (Komi Republic), 50 km west of Salekhard town (capital of the Yamal-Nenets Autonomous District) and 30 km west of Labytnangi town (
Figure 1).
The digital relief model of the study area was taken from ArcticDEM (
http://www.pgc.umn.edu/data/arcticdem/). This model is used for topographic correction of figures of changes in crown density, NDVI, and types of vegetation [
42].
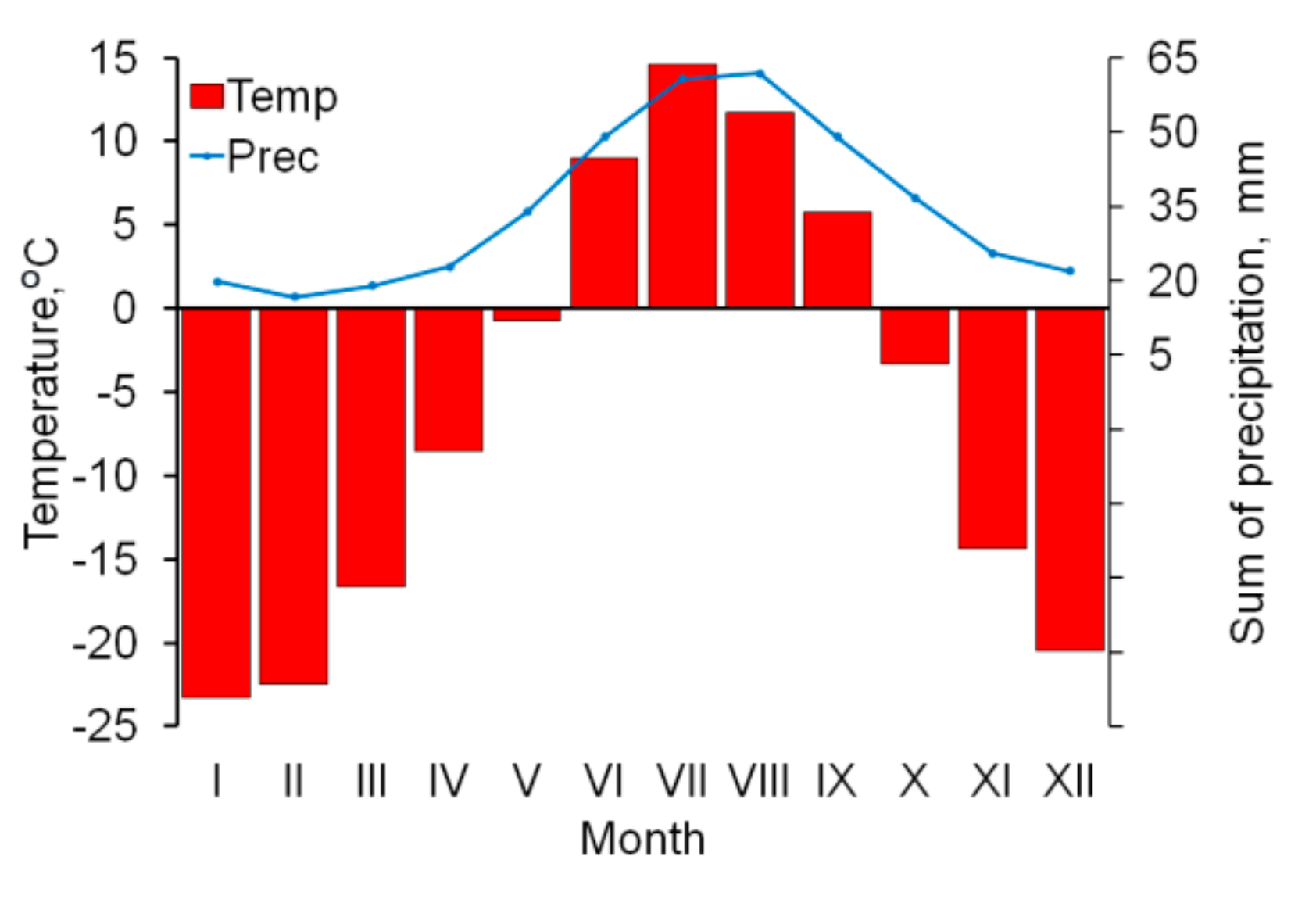
The nearest weather station with a continuous series of meteorological observations is Salekhard, located 50 km south-east from the area under investigation. The average annual temperature is negative (−6.4 °C), as indicative for the harsh thermal regime in the region (
Figure 2). Mean annual precipitation is 415 mm, solid precipitation reaches 45% of the total amount. The region is characterized by a complex wind regime with a predominance of the westerly winds. The average wind speed is 8.5–8.8 m/s in winter and 6.5–7.0 m/s in summer.
Anomalies of the 10-day course of surface air temperature and of the amount of precipitation were calculated according to the generalized data of VNIIGMI-WCD (meteo.ru) for the 65-year observation period (1960–2015) at Salekhard weather station.
Currently, the anthropogenic impact on nature in the study area is minimal. As a first important step for exploitation of the area, the Vorkuta–Labytnangi railway was built in the 1940s. However, there are no permanent settlements in the area of investigation, with the exception of a single house near the railway. Only two people permanently live in this valley of 6 km2 at the moment (population density: 300 ha/individual). The territory is occasionally used during winter in reindeer husbandry. The pasture load on phytocenoses is low (64.1 ha/individual) compared to the average load for the Yamal-Nenets Autonomous District (59.0 ha/individual), or to adjacent farms of the Vorkuta region in the Komi Republic (46.4 ha/individual).
2.2. Litter Decomposition and Soil Defrost Estimation
The litter decomposition rate was estimated by weight loss of standard samples of pure cellulose (laboratory filter paper photo soft extra, Borregaard A/S Sarpsborg, Norway) during a fixed period of time. Despite the simplicity of the method, it is quite informative, since it allows direct measurements of velocity at specific points in space and is currently widely used in soil biology [
43], with different modifications (for example, bait lamina test, litter bag test, cotton strip assay). Cellulose samples were placed in nylon mesh bags of 5×10 cm with a mesh size of 0.5 mm. They were deposited between the litter layer and the humus-accumulating layer (soil). Two series of experiments (1978–1980 and 2013–2015), each lasting three years, were carried. For both series, nylon mesh bags were deposited at the same places in the middle of August. Every year in August, mesh bags were replaced. Mesh bags were placed every 50 cm along a line (at the same height above the sea level) for 25 m, i.e., 50 packets in each line. Each year, three lines were laid along an altitudinal gradient: line one was situated on the treeline of 1978 (230 m a.s.l.); line two was situated in the forest on the slope (180 m a.s.l.); line three was established in the closed forest of the valley bottom (110 m a.s.l.). Thus, during one time series, the data of nine lines were analyzed, amounting for 450 mesh bags. These results in a total of 900 mesh bags for two series.
A study of the soil thaw depth was carried out at the same place (altitudes, transects, and during the same years: 1978–1980 and 2013–2015) as the study of litter decomposition, on the slope of the south-western exposure of Mountain Slantsevaya. Briefly, depth of the soil thaw was studied at 230 m, 180 m, and 110 m a.s.l. The measurements were carried out using a pole of 4 m length. Measurements were carried out on each altitudinal line in one-meter increments for a total of 25 m length. Additional excavations were carried out with a shovel if necessary. As in the case of mesh bags, the depth of soil thaw was studied in mid-August, when the soil melting was most pronounced during the summer period.
2.3. Studies of NDVI and Vegetation Cover
Changes in the vegetation cover of the region were estimated based on the normalized differencevegetation index (NDVI) extrapolated for summer from 1988–2017. To calculate the values, the albedo values spectral reflectance (SRefl) were used. To analyze changes, we selected summer images with similar shooting dates: Landsat TM4 (07.15.1988), Landsat TM5 (07.17.1994), Landsat 8 OLI (07.21.2013 and 07.07.2017).
The total change in crown density of tree species and partial shrub layer (the thickness of the snow cover reaches 0.5–1.0 m) was determined from winter images of Landsat TM5 (04.25.1987) and Landsat 8 OLI (04.23.2018).
The reliability to detect the correct index of crown density increases when using winter images, because manifold forms of the Earth’s surface microrelief are masked by snow cover, foliage is lacking and lichen, moss, grass, shrub layers are covered, leading to a greater contrast of the studied components [
44]. This effect was previously shown based on an analysis of changes in the density of stands of slope forests of Subpolar Urals [
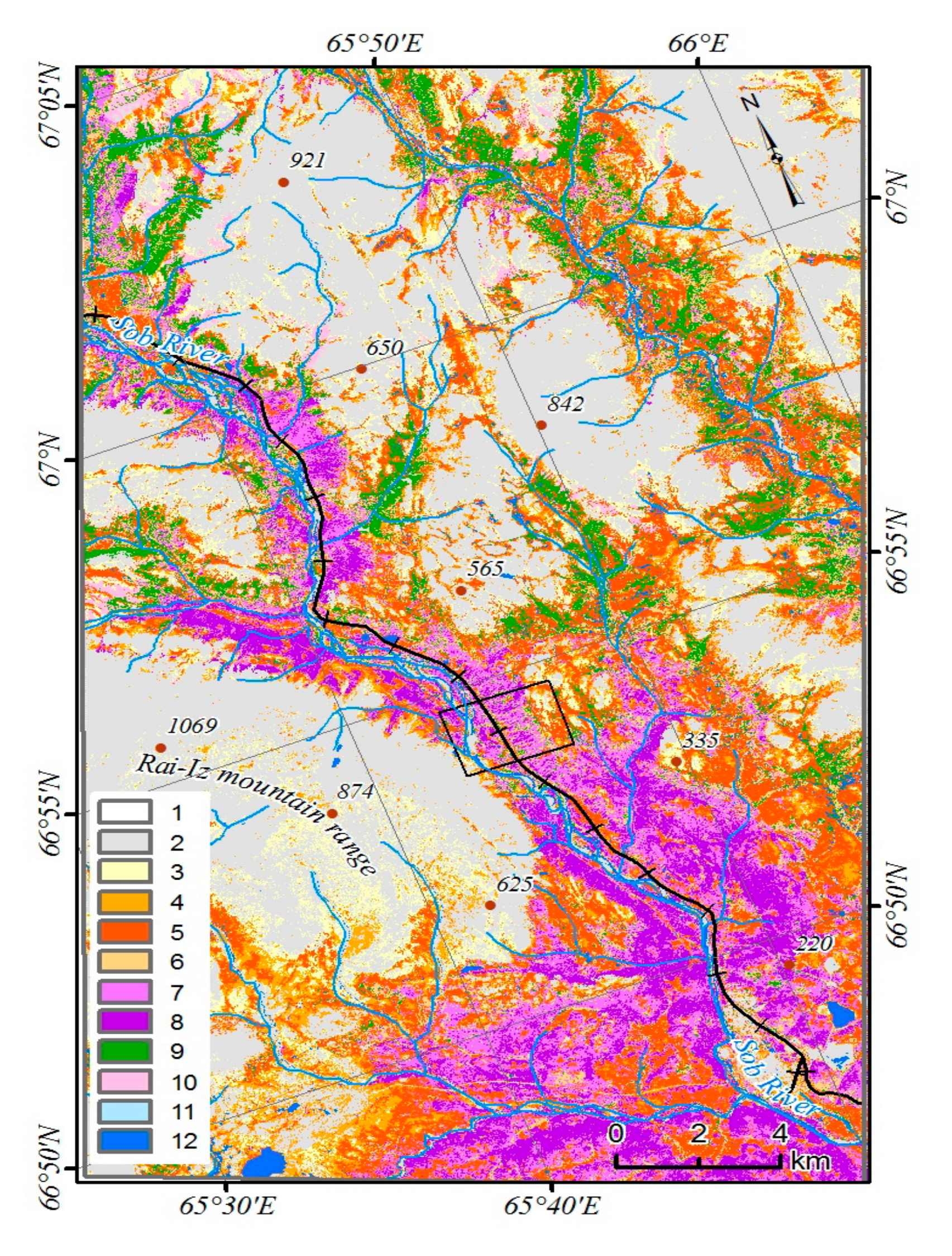
45]. Due to the low participation of tree species in the formation of the total reflection spectra, the open forest (with a canopy of less than 15%) was not considered. Of greatest interest are the changes in the class of woodlands (the canopy density of the stands is 15–30%). To characterize the dominant complexes of the vegetation cover, a supervised classification has been performed [
46]. In the study area, mountain-tundra, forest-tundra, and north boreal communities are represented (
Figure 3). Larch forests occupy the largest areas on the slopes of Mountains Slantsevaya and Rai-Iz while spruce forests occupy the Sob River valley. Above the forest boundary, bush communities are widespread with the predominance of
Betula nana and
Salix spp.
To assess the treeline dynamics (from 1956 to 2016), the following methodology is proposed. Earlier research on Mountain Konzhakovsky Kamen (Northern Urals, Russia) revealed that in 2005 the treeline of open forests (crown density is about 20%) was on average 18 m higher compared to the forest border displayed on the topographic map (M 1:25000) created in 1957 [
47]. We also compared the treeline contours from a number of modern topographic maps (M: 1:25000) with the maps of woody vegetation that we created for individual peaks of the Southern and Northern Urals based on modern high-resolution satellite images (less than 1 m
2/pixel). We found that the treeline contours of forest communities displayed on topographic maps coincide well with the boundaries of stands with a crown density of 35–40% [
48]. Based on this, we considered the treeline contours displayed in historical large-scale topographic maps of 1950–1960 and 1980–1990 as reliable for reconstruction of the upper border of stands with a crown density of 35–40%.
A quantitative assessment of changes of forest sites on the slopes of mountain ranges was carried out using the SAS. Planet 190707 program (
http://www.sasgis.org/). Using the ″Add Polygon″ tool from the tab of the ″Tags″ main menu, the contours of all sections were selected based on images in layers with modern (~2016) submeter resolution satellite imagery and topographic maps (~1956) of the State GisCenter (M: 1: 25000), where the density of stands was higher than 35–40% (closed forests). Information about the area and the length of the borders of the allocated plots in 1956 and 2016 was taken from the ″Label Information″ tab in the ″Label Management″ block. The information obtained was used to compare changes in the total area of closed forest plots in the research area from the end of the 1950s to the present.
2.4. Data Sampling and Processing for Dendrochronological Studies
All trial plots were installed in August 2015 and 2019. In order to study the current state and to reconstruct the phytomass reserves for different periods, a full recount of trees (
n = 263) was performed within six model plots of 20 × 20 m, located at 230 m a.s.l. in the forest. The trees were Siberian larch (
Larix sibirica Ledeb.) in association with Siberian spruce (
Picea obovata Ledeb.). The size of the total area of investigation was 1595 m
2. The dendrometric parameters (height, base diameter and crown projection) of each tree were measured with roulette tape (accuracy 0.5 cm), telescopic ruler (accuracy 1 cm) and digital rangefinder (accuracy 0.3 m) (
Table 1).
Each tree was cored to determine its age and tree ring width. Trees with a diameter > 2–3 cm were cored, using 400/5.15-mm increment borer (Haglöf, Sweden) at a height of up to 10 cm from the ground surface. Individuals with a diameter < 2 cm were cut at ground level. In total, 263 individuals were considered. Standing dead trees (< 3% of all trees) were also included to evaluate past stand dynamics. Further processing of the wood cores and discs was carried out according to standard dendrochronological techniques [
49,
50]. Tree-ring width was measured using a binocular microscope and a mechanic measure system with an accuracy of 0.01 mm (LINTAB, F. Rinn SA, Heidelberg, Germany). All samples were first cross-dated visually in TSAP programv4 (
http://www.rinntech.com/) and then checked using the software package COFECHA [
51]. Using tree-ring series and proportional method of geometric diameter determination [
52], the historical stem diameters of all examined trees were reconstructed for each 20 years of the 20th century.
The tree biomass estimates were conducted at the border of the trial-plots. So-called model trees (n=33) were felled and sectioned. The stems’ phytomass was determined with the hand scales with accuracy 50 grams. The weights of the leafless branches, needles, and generative organs were determined separately with a digital weight (accuracy 0.01 g). All subsamples were then oven-dried at 106 °C to stable weight. Drying time ranged from several hours to days. Then, the masses of all the fractions were summarized and allometric functions between the absolutely dry tree biomasses and the tree diameters at base height were conducted. Using the obtained dependence, the annual biomass accumulation of each tree was calculated. The calculations were made according to the following formula
where
Dn is the computed tree diameter in a certain year;
Rn is distance from the tree core to a certain annual ring;
Rfinal is the current tree radius; and
Dfinal is the current tree diameter.
Taking the fractional structure of the model trees into account, the reserves of individual phytomass fractions were reconstructed as well. Accounting the size of the trial plots, these values were converted into stand biomass values (t/ha). The change in phytomass of the two main forest trees was evaluated: Larix sibirica and Picea obovata, for 120 years, starting from 1900, dividing this period into six time intervals of 20 years, each (1900–1919, 1920–1939, 1940–1959, 1960–1979, 1980–1999, 2000–2019).
2.5. Fungal Sampling
Aphyllophoroid fungi are used as model group of macromycetes. Data from 60 years of mycological monitoring in the Sob river valley and on the slopes of Mountains Slantsevaya and Rai-Iz are included in the study [
53,
54,
55,
56]. Mycologists have been working here since 1961. The history of work can be divided into three periods, each lasting 20 years:
(1) 1960–1979: mycologists from the Institute of Plant and Animal Ecology of the Ural Branch of the Russian Academy of Sciences (Sverdlovsk), V.L. Komarov Botanical Institute (Leningrad), as well as from the Institute of Zoology and Botany, Estonia (Tartu);
(2) 1980–1999: numerous studies were conducted by employees of various scientific organizations in Russia and Europe. In 1996, the fifth international symposium on Arctic and Alpine mycology was held [
54], where mycologists from Finland, Denmark, Norway, Poland, Austria, and Italy participated. By the end of the 20th century, the eastern slope of the Polar Urals was the most studied region of the Russian Arctic in terms of the identified species richness of aphyllophoroid fungi [
34].
(3) 2000–2019: work focussing on aphyllophoroid fungi is carried out by employees of the Institute of Plant and Animal Ecology UrB RAS (Ekaterinburg), Finnish Environment Institute (Helsinki) and the Institute of Biology of the Komi Scientific Centre UrB RAS (Syktyvkar).
These three stages coincide with the periods for studying the dynamics of woody phytomass. A list of aphyllophoroids fungi analyzed in this work is given in [
55] and in (
Table S1 and Figure S1). Undoubtedly, one must not underestimate the insufficient exploration of the territory at different historical stages. The more specialists work, the more high-quality information is accumulated. The list of fungi includes information each species concerning:
(1) Fruitbody types of aphyllophoroid fungi include three species-rich groups (corticioids, poroids, and clavarioids), and some smaller groups, e.g., cantharelloids, thelephoroids, and stipitate hericioids. These groups are good indicators of bioclimatic conditions: in the tundra without native woody substrates, clavarioids are dominant, in shrub tundra with many different small twigs, corticioids on
Betula nana bushes as well as a lot of clavarioids on different grasses and herbs are dominating. In the boreal forests with many big coniferous and deciduous tree trunks, poroids have a big share of the aphyllophoroid species richness [
56,
57].
(2) Ecological strategies of aphyllophoroids include three basic types. Parasites (on alive trees, shrubs, grasses, and mosses), symbionts (mycorrhiza-forming and basidio-lichens), and saprobs (on deadwood, humus, and litter). Fungi growing on grass, leaves, needles, mixed decaying litter, twigs less than 2 cm in diameter, as well as on wood in its final decay stage 5 (representing individual fibers), or on a separate bark (for example, inside the birch bark) were defined as litter composers. A few fungi are mixotroph (e.g., genus Tomentella—mycorrhizal and litter saprobes, Osteina obducta (Berk.) Donk—wood and soil saprobs, Typhula micans (Pers.) Berthier—parasite and litter saprob, etc.). Therefore, the number of species in each of the time periods does not coincide with the total number of species identified in the corresponding time periods. To get a general picture, the shares were normalized to a common denominator (100%). In general, the ratio of these three groups varies significantly depending on the latitudinal-zonal position of the studied region.
(3) Height groups of fruitbodies are estimated only for clavarioids, cantharelloides, thelephoroids, and stipitate hydnoids, which have clearly distinguishable negative geotropic fruit bodies. They are divided into three groups: group I — fruit bodies up to 3 cm high, group II — up to 8 cm, and group III — more than 8 cm high. Small-fruiting species (I group) prevail in the arctic tundra, while in rich hemiboreal and nemoral forests with tall trunk trees, the proportion of large-fruiting species (III group) is high [
58].
(4) Chorological groups were established based on a study of the distribution of aphyllophoroid fungi along the Urals, including the tundra vegetation zone, the Arctic, mountains, nemoral forests, steppe, and temperate deserts [
55]. Four main groups are here proposed based on the confluence of fungal species in zonal units (not in the centre of the ecological optimum): Eurybionts — collected in most vegetation zones at the Urals, in forest areas, as well as in treeless areas like tundra, steppes or deserts. Arcto-alpine — growing in treeless alpine areas, as well as in treeless boreal habitats, like bogs and meadows. Boreal—found in the boreal regions, or boreal ‘islands’ in the nemoral zone. Forests — collected in most of the forest vegetation zones of the Urals. Aphyllophoroid fungal species are also analyzed by groups: northern — Arcto-alpine and eurybiontic (mainly represented in the tundra zone) and boreal-forest and boreal (most common in the taiga forests of the Urals).
Particular emphasis is placed on the discussion of newly appearing large-fruiting species that occurred in the third period of research (which could not have been missed earlier), for example, Osteina obducta, Trametes suaveolens (L.) Fr., Cantharellus cibarius Fr., etc. The collected fruit bodies were deposited in the following mycological collections: Estonian University of Life Sciences, Tartu (TAA); University of Helsinki (H); University of Copenhagen (K); Institute of Plant and Animal Ecology UrB RAS, Ekaterinburg (SVER); V.L. Komarov Botanical Institute, St. Petersburg RAS (LE); Siberian Institute of Plant Physiology SB RAS, Irkutsk (IRK); Institute of Biology Komi SC UrB RAS, Syktyvkar (SYKO); and Institute of North Development Problems SB RAS, Tyumen. Some specimens are deposited in the private collections of A. Shiryaev and H. Kotiranta.
3. Results
3.1. Climate Warming
Surface air temperatures increased by an average of 2–4 °С (Salekhard weather station), resulting in changes in plant growth phases and average soil temperatures. Steady intensive rise in temperatures (up to 6–11 °С from the average for a decade) was pronounced starting during the winter season of the first half of the1980s (
Figure 4). The increase in summer temperatures (excess of 10-day anomalies of 5–9 °С from the average) shifted towards the end of the 1980s: the most intense changes occurred in late May, the first half of summer. After a short-term decline in 1995–2000, also the snow-free season exhibited a smooth expansion of periods with increased temperature. After 2010, temperature indicators characteristically increase for all seasons of the year. The average annual and average seasonal (except for the spring period) air temperatures increased by 0.44 °С over 10 years (for spring 0.64 °С for 10 years). Over the past 60 years, the average summer air temperature (June–August) increased by 2.0 °С (to +12.2 °С), and average winter temperatures (November–March) by 1.8 °С (to −18.6 °С) in the area of investigation.
Along with warming, there was an increase in rainfall. For precipitation, the total annual precipitation increased by 10.8 mm over 10 years, but significant redistribution of indicators between seasons was observed: for winter, increase over 10 years was 2.2 mm, for spring 4.7 mm, for summer 2.1 mm, for autumn 1.2 mm. The amount of precipitation during summer and early autumn has short-term phases of increase during 1964–1968 and 1978–1980. Since the early 1990s, a precipitation increased steadily during all seasons, but with a shift to earlier periods of the year (
Figure 4). Since 1960, the average annual rainfall in summer and winter increased by 30 mm and 49 mm, respectively. The last decade was the warmest and wettest for the entire period of research.
Due to these climatic changes, the duration of the growing season increased by 6–7 days in the study area. Similar indicators were also found for the slopes of Mountain Chernaya, located at the southern slope of Mountain Rai-Iz [
35].
3.2. Changes in Litter Activity and Soil Thaw Depth
Due to climate warming and an increase in rainfall in the study area, the activity of soil and litter microbiota has significantly increased over the past 35 years (from 1978–1980 to 2013–2015). For the first (upper) level (230 m a.s.l.), where the upper border of the forest was located in 1900, the rate of cellulose degradation increased from 4.1% to 19.8%, i.e., almost five times (
Table 2). For the middle and lower levels, this parameter nearly duplicated. A similar study was carried in the Polar Urals, in the valley of the Sob River in the late 1960s [
39,
40]. They found that the decomposition rate of pure cellulose varied in average values of 10–19%, which is in accordance to our results (18.5%). Climate is playing a significant role on litter decomposition. Initial litter decomposition is determined by temperature in cold biomes, and for every 10 °C increase in temperature, a doubling of microbial decomposition is anticipated [
59]. Comparing our data with global estimates, it becomes evident that changes in decay rates are extraordinarily high in the Arctic.
The average depth of soil thaw at the first (upper) level was 1.5 m during 1978–1980, and in 2013–2015 it was 3.4 m (
Table 3). That means that over 35 years the soil was thawed 2.3 times deeper than at the beginning, reaching an average of 1.9 m at an altitude of 230 m a.s.l. At the second and third levels, the penetration depth became a meter deeper (1.4 times in both cases). Consequently, over the entire altitude profile, the soil began to thaw 1–2 meters deeper. For the late 1970s, a large difference in decay rates of cellulose was observed between three altitudinal belts, which was especially obvious between upper level ‘near the upper border of the forest’(soil thaw: 1.3–1.6 m) and ‘forest’(soil thaw: 2.3–2.8 m). At present, the soil thaw parameters for all three zones are close (varying in a narrow range of 3.2–4.0 m). It is worth noting that in the 2010s a similar increase in the thaw depth was reported for different types of soils in the vicinity of Labytnangi town (30 km east of Mountain Slantsevaya) [
60]. Moreover, soil thaw depth also increased in the tundra of the East European plain, located 50 km west of the study [
61]. Permafrost thawing results in increased emissions of all three important greenhouse gases—carbon dioxide, methane, and nitrous oxide [
62]. The release of these gases is often linked to increased microbiological activity: enzyme activity—e.g., soil glucosidase increases—dramatically in warming environmental conditions such as thawing. With Arctic permafrost transforming into active soil layers with climate warming, activity of these exoenzymes substantially increase rates of carbon breakdown [
63].
3.3. Vegetation Cover Dynamics
The treeline has risen in response to warming and an increased rainfall, deeper thaw of the soil, and increased activity of the soil-litter microbiota. A comparison of historical data (where the density of stands was higher than 35–40% according to topographic maps of 1956) with our satellite images takenin 2016 revealed that the total area of closed forests in the Sob River valley increased by 30%, i.e., by 15.6 km
2, starting from 52.1 km
2 to 67.7 km
2 (
Figure 5). This results in an increase in closed forest area of 0.095 km
2 per 1 km of the upper boundary since 1956.
Since 1956, the vertical shift of the treeline of forests with crown density of about 20% was 41 m (from 231 to 257 m a.s.l.) for closed forests 35 m (from 195 to 230 m a.s.l.). The horizontal shift for open forests was 290 m, for closed forests 520 m. These forest dynamics were confirmed comparison of photographs vegetation taken at different times in this area [
36,
47]. Almost all recent photographs show an increase in the productivity of pre-existing stands, which is expressed in an increase in the size of trees in height and in diameter, density of stands crowns [
36]. The young generation of larch, formed during a favourable climatic period, differs markedly in shape and growth rate from older generations, especially the overmature ones, which endured and survived severe climatic conditions for most of their life. Currently, the basis of the wood layer in the study area is made up of young and middle-aged generations of trees. Almost two-thirds of the trees did not exceed the age of 120 years (
Table S2).
Along with the increase in forest areas the growth of trees in height and in diameter has significantly increased during the last century [
35]. The average annual radial growth was 0.83 mm during the past 70–90 years, about 4 times more than during the previous 60–80 years (0.21 mm).
An increase in density indicators was noted for sites with woody vegetation during the period 1987–2018, with lowland dark coniferous forests exhibiting the most pronounced increase in closure (more than 15–20%) (
Figure 6). For larch, the most intensive renewal of larch growing on the south-eastern slope of the Mountain Rai-Iz massif occurred during the period 2004–2014, and was faster than detected for the previous period 1991–1999 [
35].
Repeated descriptions of trees on a constant altitudinal profile in the treeline ecotone were established in the early 1960s on the southern slope of Mountain Rai-Iz (Mountain Chernaya region). Changes in phytomass, number of trees, and stock, and stand density were monitored over the past 40 years [
64]. The obtained data showed that there was a 2–5-fold increase in phytomass stand density in nearly all sites. Exceptions were areas which were almost treeless in the 1960s, where phytomass and stand density increased even more. Siberian spruce became established under the canopy of larch stands, of the lower part of the treeline ecotone of mountain slopes in the Polar Urals, and advanced of its upper distribution border into higher areas. Young spruce trees usually have a high increase in diameter and, especially, in height. Here, spruce successfully displaced larch, first resulting in a two-tier spruce-larch forest, then into a larch-spruce forest of the north-boreal type.
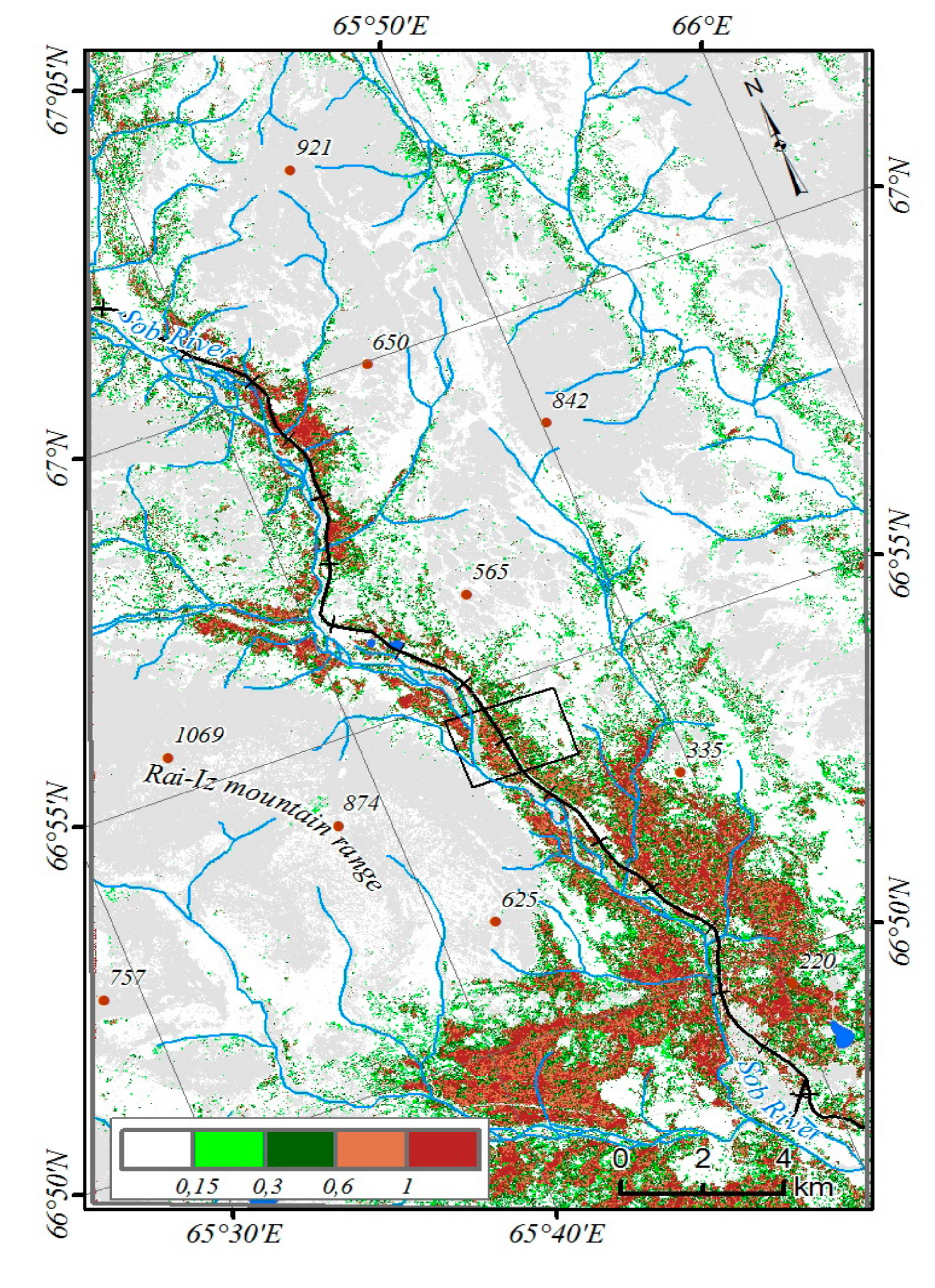
The NDVI generally increased in most of the studied territory. However, the total increase of NDVI values for all plant communities and the entire period 1988–2017 did not exceed 0.001 per year (
Figure 4). A similar study carried out in boreal forests of the area also reported a similar increase in NDVI values [
45,
46], and reported a considerable variability between different biome types, maximum values for larch covered areas being significantly correlated to July temperature [
63]. We found a distinct increase in NDVI for most plant communities addressed: e.g., the maximum increase in NDVI (up to 0.005 per year) was observed in spruce forests in the period 1988–1994, corresponding to the first phase of summer temperature increase (
Figure 4). However, the intensity of changes varied significantly in subsequent years, thus causing the low average value of total NDVI increase. Since 1988, the maximum total increase of NDVI (14–25%) occurred in spruce forests, as well as in willow thickets with northern green alder growing in the valley and on the slopes of the mountains bordering the Sob River valley (
Figure 7). The average of NDVI increased per model site was 15% over 26 years.
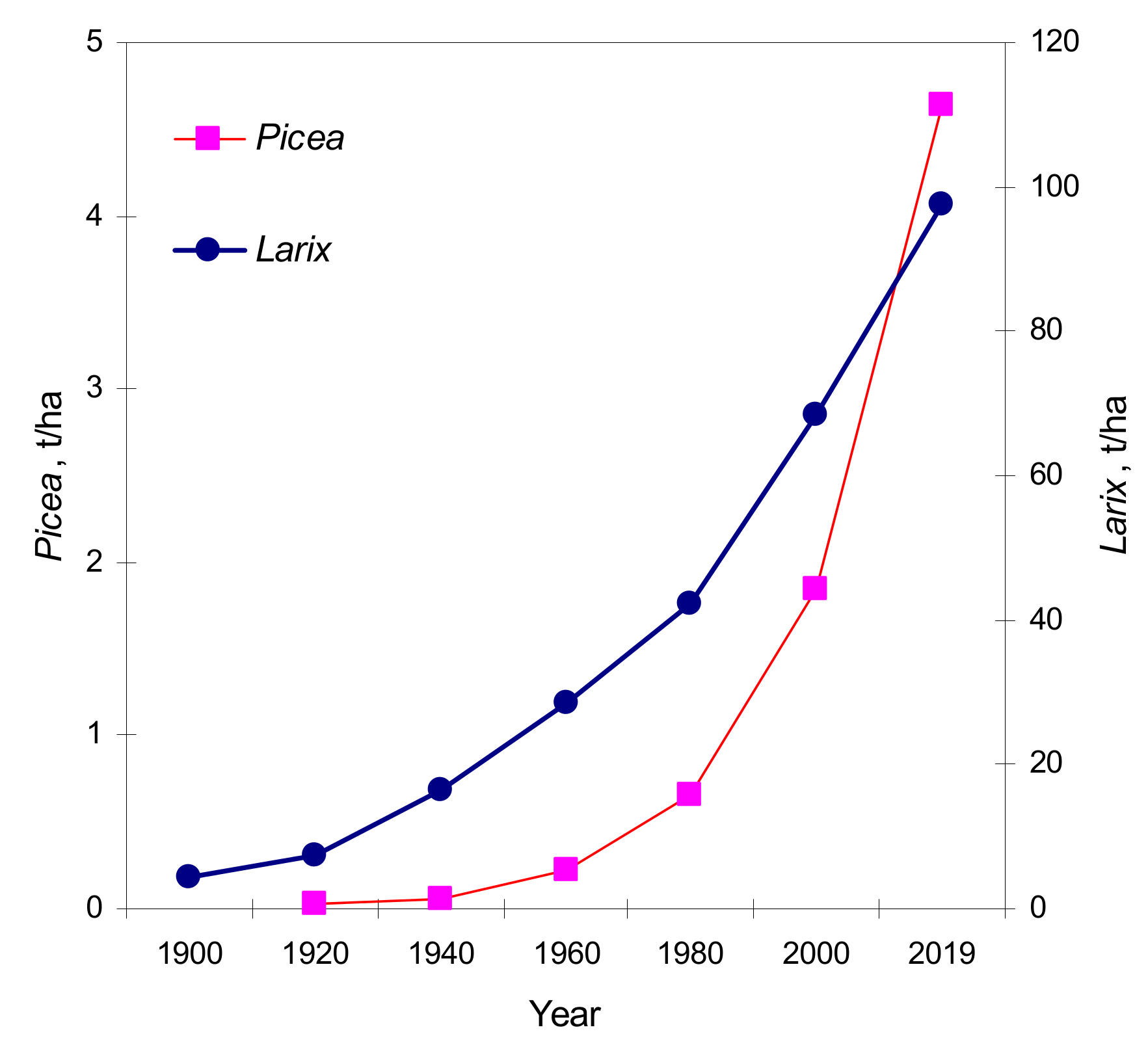
This proves a real greening effect of the region. On the slopes, the trees rise higher into the mountains, and low areas trees grow older, had higher crown densities and wider annual rings. This leads to an increase in phytomass volume of the main tree species (
Figure 8). Phytomass of
Larix and
Picea increased very much in the area, where the upper border of the forest was located in 1900 during the past 60 years:
Picea phytomass increased by a factor 21, from 0.22 to 4.64 t/ha, and
Larix phytomass increased by a factor 3.5, from 28.34 to 97.60 t/ha.
Phytomass dynamics was also studied for individual fractions of larch and spruce (trunks, branches, and needles). The trunk biomass was growing especially fast for spruce, accounting for 2.2 times the sum of the branches and needles (
Table 4). Larch trunks had 1.8 times more biomass than its branches and needles. In general, for 2019, the aboveground larch phytomass was 21 times higher than that of spruce. According to our data, the aboveground phytomass of all arboreal species (including green alder, willow, rowan, and birch) is currently 102.25 t/ha, whereas in 1960 it was 28.56 t/ha, and in 1900 only 4.77 t/ha. In closed forest stands, which started to form during the second part of 19th century, annual production of phytomass was 0.16 t/ha per year in 1900, but currently it is 10 times higher, with an average rate of 1.6 t/ha per year. Since 1900, the contribution of spruce to the total phytomass increased by only 4.5%, and spruce phytomass increased drastically (395 times). This is because in the 1900s like in 2019, more than 95% of the aboveground woody phytomass was formed by larch in this region (
Table S3).
The following tree range expansions have been observed in the floodplain of Ob River and the Ural Mountains: aspen (
Populus tremula L.), gray alder (
Alnus incana (L.) Moench), bird cherry (
Prunus padus L., or
Padus racemosa (Lam.) Gilib.), Siberian pine (
Pinus sibirica Du Tour), and European pine (
Pinus sylvestris L.). These trees are accompanied by coenotically related herbaceous plants. According to our observations, now the northern distributional border of some of them reaches just 120 km south of the towns Labytnangi, Salekhard, and the Arctic Circle, although 30 years ago it was located 300–350 km south. The vegetation of the study area was characterized as forest-tundra in the 1960s, and at the moment it has transformed into taiga [
36], and with the advent of above-mentioned trees it may become middle boreal. The Arctic Research Station of Labytnangi town has a Botanical Garden. In the 1970s, exotic tree species like aspens, Siberian pines, alders, and caraganas were planted there. Over the past 20 years, there has been a sharp activation of the growth of these trees. Currently, aspen and Siberian pines have spread throughout the town and are actively settling in the surrounding tundra and forest-tundra landscapes. This further confirms the fact that the climatic conditions of the region are becoming more comfortable for the ‘southern’ trees. Saprobial fungi have already adapted to the availability of these new substrates: e.g., in 2018, the clavarioid fungus
Typhula erythropus (Pers.) Fr. was detected on dead leaf of aspen in the tundra.
Along with warming in the region, precipitation, length of the growing season, soil thaw depth, and activity of soil microbiota increased. This resulted in rising of the timberline and increase in forest area and crown density, along with a maximal NDVI (
Table S4).
3.4. Mycobiota Dynamics
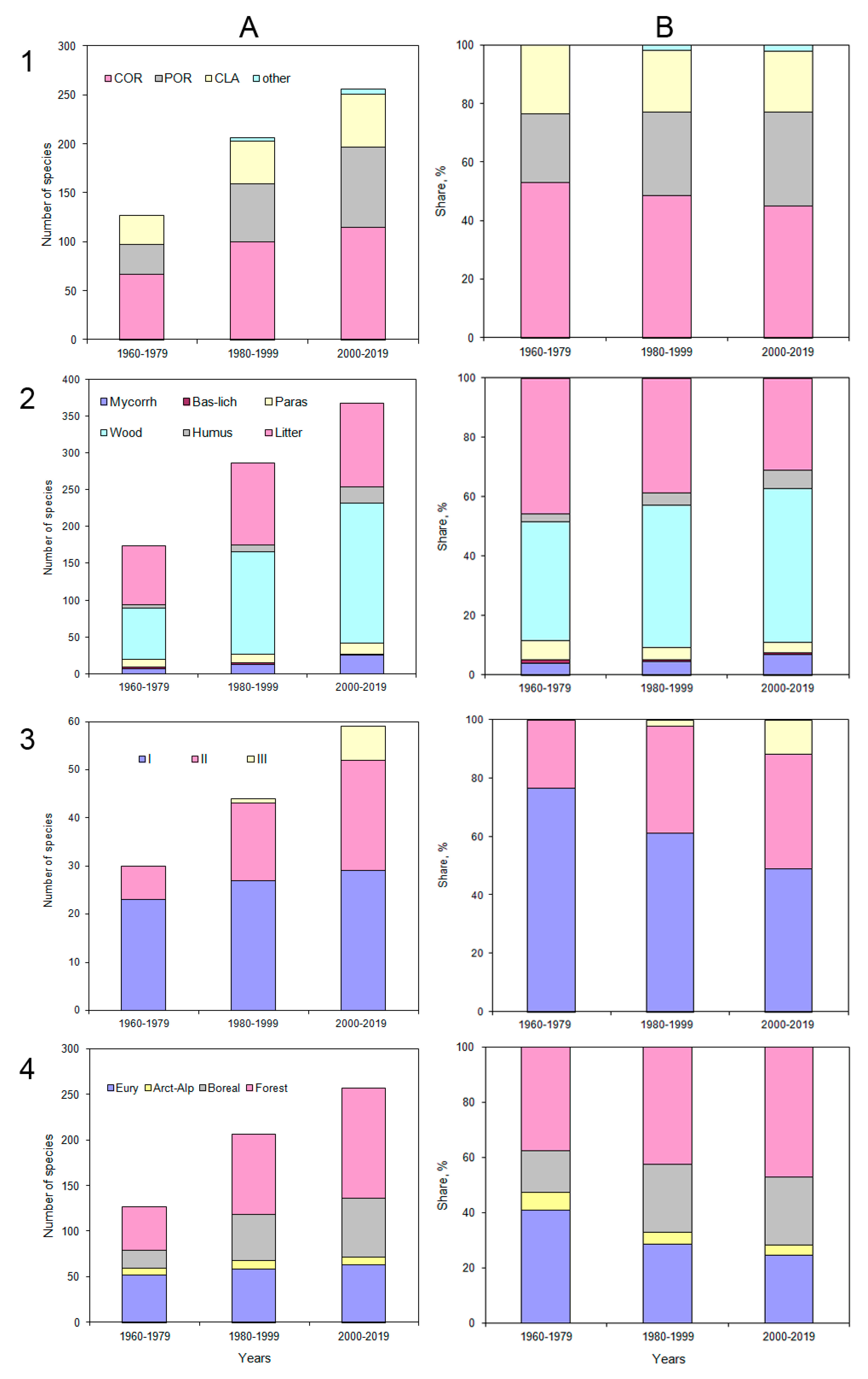
The number of species of aphyllophoroid fungi detected doubled: starting from 127 species detected in the first period of the study (1960–1979), it increased to 206 in the second (1980–1999), and 257 in the third period (2000–2019) (
Figure 9). A total of 263 species of fungi have been identified in the region over the entire period (
Table S1), which makes up 26.3% of the number of aphyllophoroid fungi known in the Urals [
53]. Each period includes aphyllophoroid species found only during this period.
The ratio of the main fruitbody types changed significantly. The Corticioids as most divers group decreasedfrom 52.8% to 44.7%, and also Clavarioids decreased (from 23.6% to 20.9%). In contrast thereto, Poroids increased from 23.6% to 32.1% (
Figure 9).
The number of aphyllophoroid fungal species forming fruit bodies on large deadwoodincreased from 69 to 190 (increasing from 39.9% to 51.9% of the total diversity).
During the last 20 years, many typical boreal polypores such
Osteina obducta, Laurilia sulcata (Burt) Pouzar,
Phellinus alni (Bondartsev) Parmasto,
P. ferrugineofuscus (P. Karst.) Bourdot & Galzin,
Polyporus arcularius (Batsch) Fr.,
Ramaria apiculata (Fr.) Donk,
Steccherinum luteoalbum (P. Karst.) Vesterh., and
Trametes suaveolens were first discovered in the area (
Figure S1 and Table S5).
Litter inhabiting species increased from 79 to 113 species. Over the past 20 years, the following species were first identified:
Clavaria fumosa Pers.,
Lentaria epichnoa (Fr.) Corner,
Macrotyphula tremula Berthier,
Ramariopsis biformis (G.F. Atk.) R.H. Petersen,
Ramaria subdecurrens (Coker) Corner (
Figure 9). An interesting discovery was the clavaroid fungus –
Clavariadelphus mucronatus V.L. Wells & Kempton: its main distributional range corresponds to North America. It was found in Eurasia only once, on the Kamchatka Peninsula before this record [
56]. Over the past 10 years, it was also reported fromparks and gardens of the Republics of Tatarstan and Udmurtia [
65,
66].
Only a limited number of ubiquitous aphyllophoroid fungi, which are widely distributed throughout the forest zones of the Urals, developed on deadwood, or on grass litter. Despite an increase in species richness, ubiquitous fungi decreased by almost 15% (from 45.7 to 30.9%).
Over 60 years, the proportion of mycorrhizal species has increased almost 2 times (from 4 to 7%), and humus saprobs 2.5 times (from 2.8 to 6.2%). Mycorrhizal fungi typical for boreal regions appeared for the first time, e.g.,
Cantharellus cibarius Fr.,
Ramaria botrytis (Pers.) Bourdot,
R. flavobrunnescens (G.F. Atk.) Corner,
Thelephora terrestris Ehrh. The most noticeable increase occurred during the third time period, when the soil was most thawed, and temperature, precipitation increased and woody phytomass increased most. Interestingly, the violet webcap,
Cortinarius violaceus (L.) Gray, a rare agaricoid mycorrhizal fungus, which is included in various regional Red Books of Russia [
67], occurred regularly on the slopes of Mountain Slantsevaya in 2019.
Unlike the mycorrhiza forming ones, the number and proportion of humus saprobs increased evenly throughout the study period. Fruit bodies of typically boreal fungi began to develop on the soil: Albatrellus ovinus (Schaeff.) Kotl. & Pouzar, Coltricia perennis (L.) Murrill, Cantharellus cibarius, Hydnum umbilicatum Peck, Clavariadelphus pistillaris (L.) Donk, etc. These species do not occur in the tundra and forest-tundra of Western Siberia and Eastern Europe, nor on the treeline or in the alpine areas of the Polar Urals.
With an increased diversity of herbaceous and woody plants species, the diversity of associated parasites increased (from 11 to 14 species). However, their proportion decreased almost halved, dropping from 6.4% to 3.5%. Diversity of moss parasites decreased (Clavaria sphagnicola Boud., Ramariopsis subarctica Pilát), tree parasites increased (on conifers Laricifomes officinalis (Vill.) Kotl. & Pouzar, Phellinus laricis (Jacz. ex Pilát) Pilát, Laetiporus sulphureus (Bull.) Murrill s.l.; on deciduous trees (Chondrostereum purpureum (Pers.) Pouzar, Inonotus obliquus (Fr.) Pilát), and grass parasites (Typhula incarnata Lasch) were detected for the first time. On willows, Phellinus igniarius (L.) Quél. expanded his territory from one single tree in the valley to 20% of all trees.
During the entire study period, only two basidio-lichens (Multiclavula vernalis (Schwein.) R.H. Petersen, M. corynoides (Peck) R.H. Petersen) were detected in the study area, and their proportion decreased from 1.2% to 0.5%.
For fungi with negatively geotropic basidiomata (Clavarioids, Chanterelles and stipitate Hydnoids), the number of species increased from 30 to 59 species. In the first period, more than 60% of all species belonged to size class 1 (less than 3 cm in height), while only 23.3% belonged to the second. By the third period, the proportion of small-body species decreased by almost 30%, and now makes up less than half of all species. On the other hand, for species of the second size class (from 3 to 8 cm in height) the share increased by 15% to 39%, and species of the third class newly appeared (11.9%).
Chorological groups changed during the last 60 years. The proportion of Arcto-alpine species was nearly halved (decreasing from 6.3% to 3.5%).
Ramariopsis subarctica was less and less found, and in dry and hot 2017, it was not collected at all throughout the valley, although it was regularly seen before [
56,
67]. Also,
Multiclavula corynoides and
M. vernalis were less and less found on open soil, and the Arсto-alpine species
Datronia scutellata (Schwein.) Gilb. & Ryvarden,
Peniophora aurantiaca (Bres.) Höhn. & Litsch.,
Plicatura nivea (Fr.) P. Karst. decreased in the alder thickets. The only exception was
Clavaria sphagnicola, another Arcto-alpine species growing in marshes, which did not reduce the number of fruiting bodies, even in hot and dry years.
Ubiquitous aphyllophoroids were the second largest group in the tundra. They diversity and proportions decreased by 16% (from 40.9 to 24.5%). Typical forest species increased in diversity from 37.8 to 47.1%, and their proportion increased from 15.0 to 24.9%. In general, 60 years ago the northern species accounted for almost half (47.2%), now only about four (28%) of aphyllophoroid species diversity. In contrast thereto, the proportion of taiga species increased by 25%.
4. Discussion
Fungi represent an important heterotrophic block of the ecosystem. The development of fungi sometimes requires specific sources of nutrients, and this needs to be considered when focusing on the effect of climate change on macromycetes. Based on our results, the diversity of aphyllophoroid fungi doubled during the last 60 years in the area. One first interpretation might be, that the first period (1960–1979) was understudied. A re-examination of samples collected during that period confirms that mainly widespread common species were identified. Upon careful examination, it turns out that none of these species grow on large-sized deadwood, on rich soil, or on mesophilic boreal herbs and grasses. The largest diversity of species was then detected on small deadwood, larch branches, shrubby willow, rowan and northern green alder. About 50–60 years ago, the study area was open larch forest tundra with a few spruce trees over poor permafrost soil. More than 80% of larches in area were less than 70 years old, and single spruce trees were 20–40 years old. This undoubtedly confirms that the fungi detected during the first period were adequately sampled, because due to shortage of large-sized deadwood, the habitat for species able to form large fruiting bodies was not present. The ubiquity of permafrost, with a thaw depth of only 1–1.5 m in summer, indicates that litter saprobial and mycorrhizal fungi were restricted under such conditions. Therefore, mycologists who worked in the 1960s in the Sob river valley and on the slopes of Mountain Slantsevaya were studying aphyllophoroid fungi at the arctic border of the forest. In this regard, the complex of fungi reported for the first period (1960–1979) is correct and reflects the bioclimatic conditions during that period.
More importantly, the ratio of the main fruitbody types changed significantly, and this can therefore be considered as an important indicator of bioclimatic conditions [
27]. Corticioids decreased significantly and poroids increased. The ratio of corticoids to poroids appears to be a parameter typical for forest types. This was also confirmed by a comparison with earlier results for the Ural latitudinal-zonal transect [
55,
68]. An increase in the volume of large wood contributes to an increase in the diversity and proportion of poroids. Many poroid species need large deadwood for their growth [
27,
68]. Changes in bioclimatic conditions, especially between periods 2 and 3 go along withsignificant changes in the structure of the vegetation cover. Larch and spruce phytomass increased, and trees became older. Therefore, also the number of dead trunks increased and they were generally thicker, which significantly affected the species composition of wood-destroying fungi. Suitable habitats for the growthof typical forest polypores are now present in the area. During the last 20-year period, many boreal fungi such as
Osteina obducta and
Laurilia sulcata (
Tables S2 and S5) were first discovered on large dead larch trunks.
Phellinus ferrugeneofuscus, Ramaria apiculata, Steccherinum luteoalbum, and
Mucronella calva (Alb. & Schwein.) Fr. appeared on the dead spruce wood. Birch (
Betula pendula) grows now higher than 12 m, thus enabling the fructification of
Polyporus arcularius. Phytomass produced in shrub belts also increased in the study area. The northern green alder (
Duschekia fruticosa (Rupr.) Pouzar, Syn.
Alnus fruticosa Rupr.), which is mainly responsible for such shrub thickets, can now reach up to 6–7 m in height [
69]. Now,
Phellinus alni can be regularly collected in these areas. Isolated findings were reported from 1980-1999, but now it occurs on a third of all
Duschekia bushes. The maximum NDVI also increased in floodplain willows, which start to be older, taller and fatter. Willows (
Salix caprea) up to 8 m high appeared along the Sob River, and in 2019, the poroid fungus
Trametes suaveolens was first collected on them. Thirty years ago, the northern border for the distribution of this species was 200 km south of the towns Labytnangi and Salekhard, in typical northern boreal forests. The occurrence of wood-decomposing fungi undoubtedly reflects changes in the structure of vegetation cover, and characterizes the recent mycobiota as typical for taiga, and not for forest-tundra anymore [
55,
56].
The phytomass of needles increased, leading to an increase in the diversity and richness of litter (
Table 3). The diversity of grassy litter has also increased. 30-50 years ago, many boreal herbaceous plants (
Aconitum excelsum Reichb.,
Athyrium filix-femina (L.) Roth ex Mert.,
Veratrum lobelianum Bernh.,
Anthriscus sylvestris (L.) Hoffm.) were found only at the valley bottom. Now, due to warming, these species have expanded their territory to upland habitats and to the treeline. European blueberry (
Vaccinium myrtillus L.) acts as an indicator of borealization of bioclimatic conditions in the region. In the 1960s, it was not present at all on Mountain Slantsevaya (E. Parmasto, pers. comm.), and in the 1980s it was found only at the valley bottom, but not the slopes of Mountain Slantsevaya (S. Obolevski, pers. comm.). Compositional and quantitative changes in the vegetation permitted for a significant increase in diversity of litter inhabiting fungi (
Figure 9). Moreover, an increase in the soil thaw depth and concomitant increase in the activity of the soil microbiota also contributed to the appearance of Cantharelles and stipitate Hydnoids in such high-latitude permafrost regions [
55]. The detected proportion of litter species is characteristic for complexes of aphyllophoroid fungi growing in the taiga.
Eurybiont aphyllophoroid fungi decreased by almost 15% during the last 60 years. A lower proportion of ubiquitous species is typical for boreal forests, as confirmed by similar carried out in the Urals [
55,
68].
An increase in phytomass and diversity of woody plants, together with deeper soil thaw and increase in litter activity, contributed to the increase of typical forest fungi forming mycorrhiza. Also, the proportion of mycorrhized roots increased in the study area due to warming [
41]. The proportion of mycorrhizal fungi almost doubled during the last 60 years, and the species composition reflects atypical boreal mycobiota [
55,
58].
With an increased diversity of herbaceous and woody plants species, also the diversity of associated parasites increased, but their proportion decreased. Only parasites on trees and mosses were reported in previous periods, but now grass parasites appeared for the first time (
Typhula incarnata). Grass parasites on are generally expanding their territory along the floodplain of the Ob River, and
T. ishikariensis has already been reported from Salekhard in 2018. An increase in the number of tree and grass parasitic species was also reported from in other regions of the Arctic [
70].
Basidio-lichenshave theirpeak of diversity in the tundra, especially in Arctic deserts [
71]. Only two species were detected, and their proportion was <1%. This is typical for northern boreal forests [
55,
72].
Aphyllophoroid fungi with larger fruit bodies appeared during the last 60 years, replacing species with small fruit bodies. In the tundra and Arctic deserts, there are generally no species of class three (above 8 cm in height), and species belonging to class two are very rare. Six out of seven species forming large fruit bodies appeared in the third period. These were predominantly mycorrhizal
Ramaria species which typically occur in hemiboreal forests. Litter saprobic species have usually smaller fruit bodies [
73], and they decreased towards period three. An increase in the proportion of fungal species with large fruit bodies was also reported for clavarioid fungi of middle boreal forests [
55].
Over the three studied periods, the proportions of the chorological groups of fungi significantly changed. Arcto-alpine fungi are species whose distributional peaks are in the arctic and alpine regions. Arcto-alpine fungi are usually widespread in the tundra and arctic deserts [
56,
71]. A reduction in the range of Arcto-alpine species became evident during the last 60 years and is widely discussed [
14]. The winners were typical forest species, which significantly increased in diversity. This consolidates our observation that the mycobiota transformed from a forest-tundra into a northern taiga type during the studied period [
58], thus reflecting changes in the vegetation cover and in soil- or litter conditions. However, several typical boreal fungi have not yet been reported from the region. Among them are ordinary inhabitants of coniferous forest like
Fomitopsis rosea (Alb. & Schwein.) P. Karst.,
F. cajanderi (P. Karst.) Kotl. & Pouzar,
Ganoderma lucidum (Curtis) P. Karst.,
Leptoporus mollis (Pers.) Quél.,
Gloeoporus dichrous (Fr.) Bres.,
Amylocystis lapponica (Romell) Bondartsev & Singer,
Phaeolus schweinitzii (Fr.) Pat., and
Dichomitus squalens (P. Karst.) D.A. Reid. Also, some usual boreal species growing on large birches and willows have not yet been detected:
Datronia mollis (Sommerf.) Donk,
Hericium coralloides (Scop.) Pers.,
Trametes trogii Berk.,
Oxyporus corticola (Fr.) Ryvarden,
O. populinus (Schumach.) Donk, and
Trichaptum biforme (Fr.) Ryvarden. (
Table S5). It is not clear, whether the absence of these species is due to environmental conditions (e.g., substrate volume), or rather due to insufficient sampling.
Boreal aphyllophoroid fungi forming large fruiting bodies can be considered as indicators for the ongoing borealization in the Arctic, such species developing on large trunks in the Polar Urals are Laricifomes officinalis, Laurilia sulcata, Datronia scutellata, Skeletocutis stellae, and Trametes suaveolens. Litter saprobial species capable of forming fruiting bodies only on deeply thawed soil (Clavariadelphus pistillaris, Coltricia perennis), or mycorrhiza-forming species (Cantharellus cibarius, Hydnum umbilicatum, Ramaria botrytis) could also be considered as indicators. The reduced range and abundance of many Arcto-alpine fungi further confirms an ongoing borealization, because these species are indicators for tundra-alpine communities.
There is no economic activity in the study area. However, the Yamal-Nenets Autonomous District is currently the region with the most intensive development of infrastructure in the Russian Arctic. A railway passing through the Sob River valley connects the district with the European part of the country. The railway line is to be expanded, to increase freight traffic. This will be associated with deforestation and destruction of swamps close to the railway, and adversely affect the fragile high-latitudinal ecosystem in the region. Unfortunately, the Sob River valley and the slope of Mountain Slantsevaya are not within protected natural areas, although one of the northernmost forests of the Urals is located here. The slope of Mountain Rai-Iz is encircling the Sob River valley from the west and is already located inside the Polyarnouralsky Natural Park. We therefore recommend that the entire valley of the Sob River, including Mountain Slantsevaya, should be included in the boundaries of Polyarnouralsky Natural Park.
5. Conclusions
Over the past 60 years, noticeable rapid changes in the main bioclimatic parameters have occurred on the eastern macroscope of the Polar Urals, in the Sob River valley, as well as on Mountains Slantsevaya and Rai-Iz. Summer air temperatures increased by 2 °C and winter air temperatures by 1.8 °C. Summer precipitation increased by 30 mm and winter precipitation by 49 mm, and the growing season lasted 6–7 days longer. The permafrost thawed 1–2 m deeper, and cellulose decomposition increased 2–5 times. All these factors favoured tree growth, as detected by rising of the timberline (35–41 m) and increase in total forest area (30%),in radial growth of trees (4 times), in crown density (15–20%) and in phytomass (tenfold for all trees). The diversity and proportion of boreal herbaceous mesophilic plants increased in the river valley and on mountain slopes. These data are confirmed by an average increase in NDVI by 15%, in spruce and shrub vegetation by even 15–25%. This proves ongoing borealization and greening of the Polar Ural’s vegetation.
Over 60 years, the number of species of aphyllophoroid fungi doubled. Fungi depend on the availability of suitable substrate, which is now available due to climate warming. The trees became older, and large-sized deadwood appeared, now enabling the growth of species forming large fruit bodies on such a substrate (e.g., Poroids which increased by 12%). The increase in phytomass of needles, small twigs, and the emergence of new mesophilic boreal plants led to a sharp increase in the number of litter saprobes, and to the appearance of boreal fungal species. Furthermore, a deeper soil thaw contributed to the emergence of humus saprobs and mycorrhiza forming species, and their proportion doubled. Large-body fungal species generally increased, thus clearly reflecting a transition of the in mycobiota from forest-tundra to taiga type. Many of the large-bodied wood and humus saprobial, as well as mycorrhiza-forming fungi, can be considered as indicators of the ongoing borealization of the Arctic.
The flip side of warming and greening is the weakening of native Arcto-alpine bryophilic species, which now try to survive in the state of neo-relicts in a decreasing number of available habitats, such as in Sphagnum bogs and Alpine vegetation on mountaintops. Basidio-lichen species are also sharply reduced. These species retain their position only above treeline.
New parasitic fungi appeared in the region. Grass parasites causing enormous damage in agriculture (snow mold disease) in the taiga zone have appeared over the past 10 years. Growing of various crops becomes now possible due to milder climate, and it can be assumed that corresponding parasitic fungi will soon appear. Also, wood-destroying fungi are expected to cause economical loss in the near future. The advancement of most species of fungi to the north is not limited by climatic conditions, but by the lack of the necessary substrate [
71]. Aphyllophoroids are not only indicators of bioclimatic changes at high latitudes, but also for substrate availability. In this regard, they will take an active part in the transformation of the economic infrastructure of the region.