Consumption Performance of Five Detritivore Species Feeding on Alnus glutinosa L. Leaf Litter in a Microcosm Experiment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Plant
2.2. Animals
2.3. Experimental Design
2.4. Plant Litter, Animal Weight, and Excrement
2.5. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lajtha, K.; Bowden, R.D.; Crow, S.; Fekete, I.; Kotroczó, Z.; Plante, A.; Simpson, M.J.; Nadelhoffer, K.J. The detrital input and removal treatment (DIRT) network: Insights into soil carbon stabilization. Sci. Total Environ. 2018, 640–641, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Djukic, I.; Kepfer-Rojas, S.; Schmidt, I.K.; Larsen, K.S.; Beier, C.; Berg, B.; Verheyen, K. Early stage litter decomposition across biomes. Sci. Total Environ. 2018, 628–629, 1369–1394. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M. Spatiotemporal effects of invertebrates on soil processes. Biol. Fert. Soils 1988, 6, 216–227. [Google Scholar] [CrossRef]
- Coulis, M.; Hättenschwiler, S.; Coq, S.; David, J.F. Leaf litter consumption by macroarthropods and burial of their faeces enhance decomposition in a Mediterranean ecosystem. Ecosystems 2016, 19, 1104–1115. [Google Scholar] [CrossRef]
- García-Palacios, P.; Maestre, F.T.; Kattge, J.; Wall, D.H. Climate and litter quality differently modulate the effects of soil fauna on litter decomposition across biomes. Ecol. Lett. 2013, 16, 1045–1053. [Google Scholar] [CrossRef]
- Lindahl, B.O.; Taylor, A.F.S.; Finlay, R.D. Defining nutritional constraints on carbon cycling in boreal forests-towards a less ‘phytocentric’ perspective. Plant Soil 2002, 242, 123–135. [Google Scholar] [CrossRef]
- Berg, B.; McClaugherty, C. Plant Litter: Decomposition, Humus Formation, Carbon Sequestration; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Lavelle, P.; Spain, A.V. Soil Ecology; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001. [Google Scholar]
- Wardle, D.A. Communities and Ecosystems-Linking the Aboveground and Belowground Components; Princeton University Press: Princeton, NJ, USA, 2002. [Google Scholar]
- Loureiro, S.; Sampaio, A.; Brandao, A.; Nogueira, A.J.A.; Soares, A.M.V.M. Feeding behaviour of the terrestrial isopod Porcellionides pruinosus Brandt, 1833 (Crustacea, Isopoda) in response to changes in food quality and contamination. Sci. Total Environ. 2006, 369, 119–128. [Google Scholar] [CrossRef]
- Crowther, T.W.; Boddy, L.; Jones, T.H. Functional and ecological consequences of saprotrophic fungus-grazer interactions. ISME J. 2012, 6, 1992–2001. [Google Scholar] [CrossRef]
- Kooijman, S.A.L.M. Dynamic Energy Budget Theory for Metabolic Organisation, 3rd ed.; Cambridge University Press: Cambridge, UK, 2010; 490p. [Google Scholar]
- Nisbet, R.M.; Muller, E.B.; Lika, K.; Kooijman, S.A.L.M. From molecules to ecosystems through dynamic energy budget models. J. Anim. Ecol. 2000, 69, 913–926. [Google Scholar] [CrossRef]
- Kooijman, S.A.L.M.; Sousa, T.; Pecquerie, L.; van der Meer, J.; Jager, T. From food-dependent statistics to metabolic parameters, a practical guide to the use of dynamic energy budget theory. Biol. Rev. 2008, 83, 533–552. [Google Scholar] [CrossRef]
- David, J.F.; Handa, I.T. The ecology of saprophagous macroarthropods (millipedes, woodlice) in the context of global change. Biol. Rev. 2010, 85, 881–895. [Google Scholar] [CrossRef] [PubMed]
- Vos, V.C.A.; van Ruijven, J.; Berg, M.P.; Peeters, E.T.; Berendse, F. Macro-detritivore identity drives leaf litter diversity effects. Oikos 2011, 120, 1092–1098. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setälä, H.; van der Putten, W.H.; Wall, D.H. Ecological linkages between aboveground and belowground biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef]
- David, J.F. The role of litter-feeding macroarthropods in decomposition processes: A reappraisal of common views. Soil Biol. Biochem. 2014, 76, 109–118. [Google Scholar] [CrossRef]
- Lavelle, P.; Bignell, D.; Lepage, M.; Wolters, W.; Roger, P.; Ineson, P.; Heal, O.; Dhillion, S. Soil function in a changing world: The role of invertebrate ecosystem engineers. Eur. J. Soil Biol. 1997, 33, 159–193. [Google Scholar]
- Hättenschwiler, S.; Tiunov, A.V.; Scheu, S. Biodiversity and litter decomposition in terrestrial ecosystems. Ann. Rev. Ecol. Evol. Syst. 2005, 36, 191–218. [Google Scholar] [CrossRef]
- Mullins, D.E.; Cochran, D.G. Nutritional ecology of cockroaches. In Nutritional Ecology of Insects, Mites, Spiders and Related Invertebrates; Slansky, F., Rodriguez, J.G., Eds.; Wiley: New York, NY, USA, 1986; pp. 885–902. [Google Scholar]
- Ristok, C.; Leppert, K.N.; Scherer-Lorenzen, M.; Niklaus, P.A.; Bruelheide, H. Soil macrofauna and leaf functional traits drive the decomposition of secondary metabolites in leaf litter. Soil Biol. Biochem. 2019, 135, 429–437. [Google Scholar] [CrossRef]
- Taylor, B.R.; Parkinson, D.; Parsons, W.F.J. Nitrogen and lignin content as predictors of litter decay rates: A microcosm test. Ecology 1989, 70, 97–104. [Google Scholar] [CrossRef]
- Warren, M.W.; Zou, X. Soil macrofauna and litter nutrients in three tropical tree plantations on a disturbed site in Puerto Rico. For. Ecol. Manag. 2002, 170, 161–171. [Google Scholar] [CrossRef]
- Loranger-Merciris, G.; Imbert, D.; Bernhard-Reversat, F.; Lavelle, P.; Ponge, J.F. Litter N-content influences soil millipede abundance, species richness and feeding preferences in a semi-evergreen dry forest of Guadeloupe (Lesser Antilles). Biol. Fert. Soils 2008, 45, 93–98. [Google Scholar] [CrossRef]
- Zimmer, M.; Pennings, S.C.; Buck, T.L.; Carefoot, T.H. Species-specific patterns of litter processing by terrestrial isopods (Isopoda: Oniscidea) in high intertidal salt marshes and coastal forests. Funct. Ecol. 2002, 16, 596–607. [Google Scholar] [CrossRef]
- Quadros, A.F.; Zimmer, M.; Araujo, P.B.; Kray, J.G. Litter traits and palatability to detritivores: A case study acrossbio-geographical boundaries. Nauplius 2014, 22, 103–111. [Google Scholar] [CrossRef]
- Frouz, J.; Špaldoňová, A.; Lhotáková, Z.; Cajthaml, T. Major mechanisms contributing to the macrofauna-mediated slow down of litter decomposition. Soil Biol. Biochem. 2015, 91, 23–31. [Google Scholar] [CrossRef]
- Pey, B.; Trân, C.; Cruz, P.; Hedde, M.; Jouany, C.; Laplanche, C.; Nahmani, J.; Chauvet, E.; Lecerf, A. Nutritive value and physical and chemical deterrents of forage grass litter explain feeding performances of two soil macrodetritivores. Appl. Soil Ecol. 2019, 133, 81–88. [Google Scholar] [CrossRef]
- Tajovský, K. Feeding biology of the millipede Glomeris hexasticha. Ber. Naturwiss.-Med. Ver. Innsb. 1992, 10, 302–311. [Google Scholar]
- David, J.F.; Gillon, D. Annual feeding rate of the millipede Glomeris marginata on holm oak (Quercus ilex) leaf litter under Mediterranean conditions. Pedobiologia 2002, 46, 42–52. [Google Scholar] [CrossRef]
- David, J.F.; Malet, N.; Coûteaux, M.M.; Roy, J. Feeding rates of the woodlouse Armadillidium vulgare on herb litters produced at two levels of atmospheric CO2. Oecologia 2001, 127, 343–349. [Google Scholar] [CrossRef]
- Quadros, A.F.; Araujo, P.B. An assemblage of terrestrial isopods (Crustacea) in southern Brazil and its contribution to leaf litter processing. Rev. Braz. Zool. 2008, 25, 58–66. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Bühler, S.; Körner, C. Quality, decomposition and isopod consumption of tree litter produced under elevated CO2. Oikos 1999, 85, 271–281. [Google Scholar] [CrossRef]
- Rushton, S.P.; Hassall, M. Food and feeding rates of the terrestrial isopod Armadillidium vulgare (Latreille). Oecologia 1983, 57, 415–419. [Google Scholar] [CrossRef]
- Soma, K.; Saito, T. Ecological studies of soil organisms with references to the decomposition of pine needles II. Litter feeding and breakdown by the woodlouse, Porcellio scaber. Plant Soil 1983, 75, 139–151. [Google Scholar] [CrossRef]
- Striganova, B.R.; Prishutova, Z.G. Food requirements of diplopods in the dry steppe subzone of the USSR. Pedobiologia 1990, 34, 37–41. [Google Scholar]
- Beck, L.; Friebe, B. Recycluing of carbohydrates for Oniscus asellus (Isopoda) and Polydesmus angustus (Diplopoda). Pedobiologia 1981, 21, 19–29. [Google Scholar]
- Ihnen, K.; Zimmer, M. Selective consumption and digestion of litter microbes by Porcellio scaber (Isopoda: Oniscidea). Pedobiologia 2008, 51, 335–342. [Google Scholar] [CrossRef]
- Kukor, J.J.; Martin, M.M. The effect of acquired microbial enzymes on assimilation efficiency in the common woodlouse Tracheoniscus rathkei. Oecologia 1986, 69, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, M. Nutrition in terrestrial isopods (Isopoda: Oniscidea): An evolutionary–ecological approach. Biol. Rev. 2002, 77, 455–493. [Google Scholar] [CrossRef]
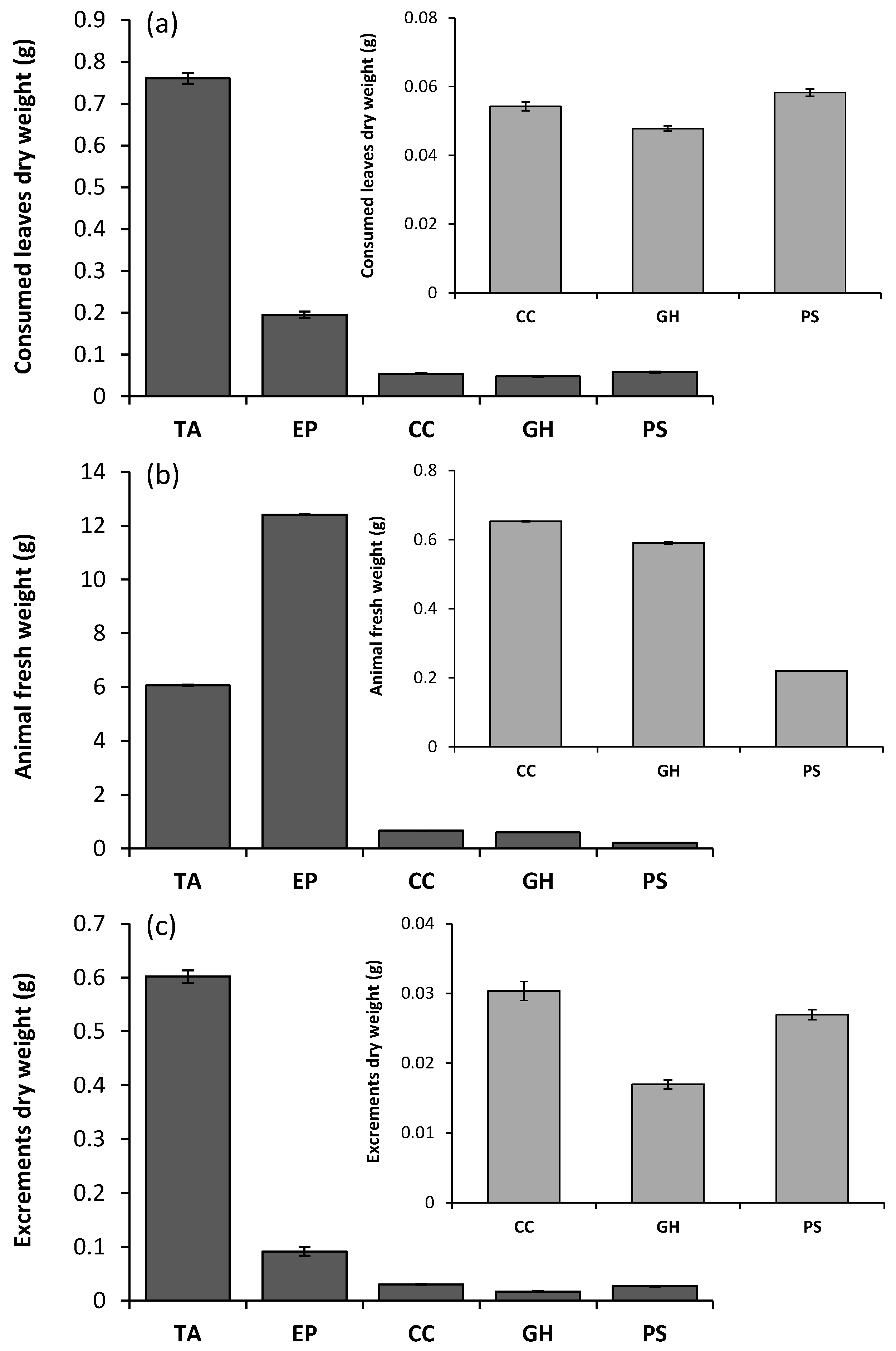
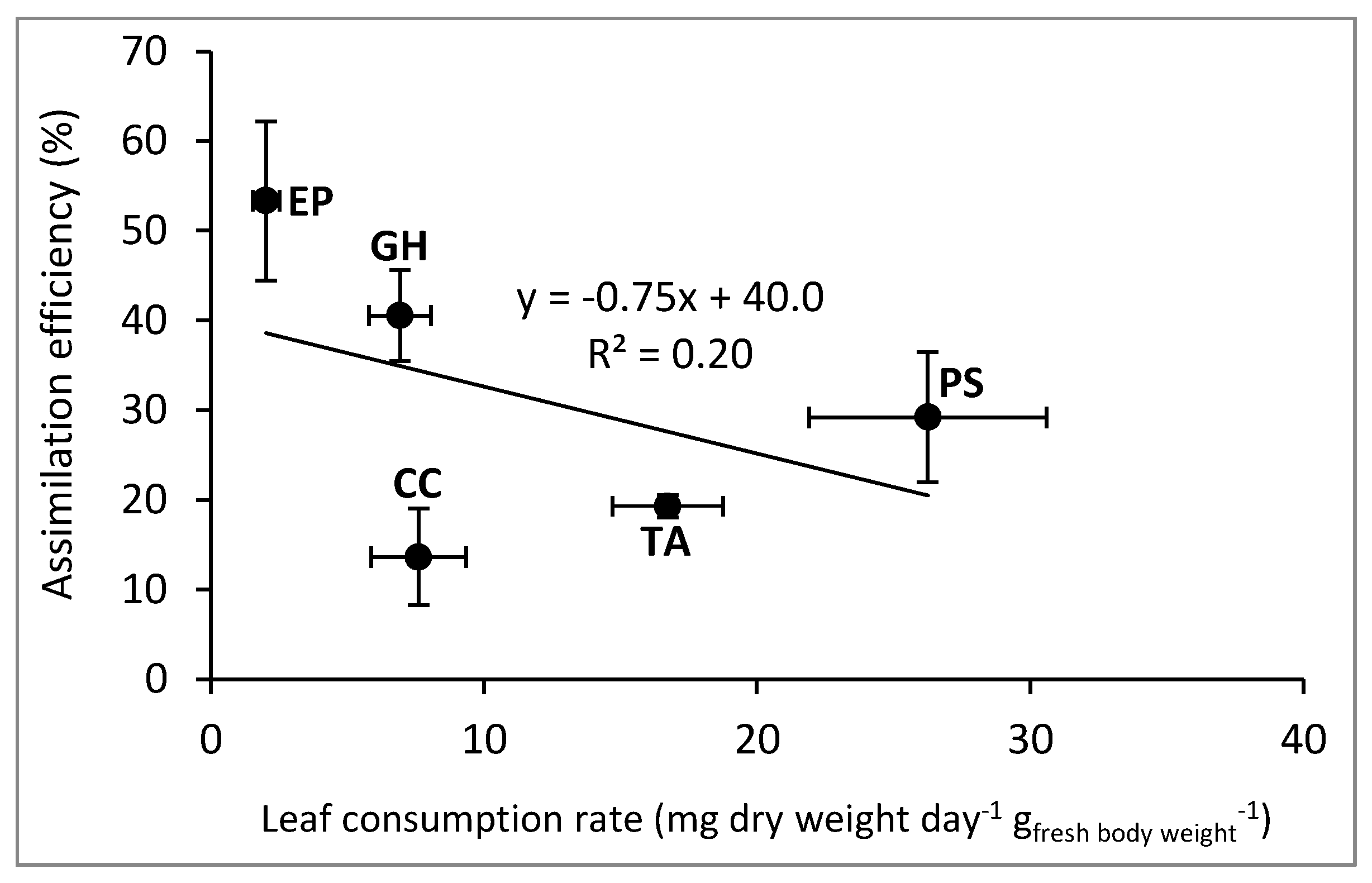
| Animal Species | Time | Telodeinopus aoutii | Epibolus pulchripes | Cylindroiulus caeruleocinctus | Glomeris hexasticha | Porcellio scaber |
|---|---|---|---|---|---|---|
| Litter consumption rate | Interval 1 | 30.9 | 8.25 | 1.50 | 1.32 | 1.34 |
| Interval 2 | 25.6 | 4.14 | 1.53 | 0.46 | 1.65 | |
| Interval 3 | 19.3 | 4.34 | 0.72 | 0.70 | 1.05 | |
| Interval 4 | 18.2 | 4.68 | 0.78 | 0.83 | 0.92 | |
| Interval 5 | 12.3 | 3.72 | 0.47 | 0.78 | 0.61 | |
| Animal growth rate | Interval 1 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 |
| Interval 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Interval 3 | 0.06 | 0.00 | 0.00 | 0.06 | 0.01 | |
| Interval 4 | 0.00 | 0.79 | 0.18 | 0.07 | 0.03 | |
| Interval 5 | 0.00 | 0.31 | 0.00 | 0.04 | 0.00 | |
| Excrement production rate | Interval 1 | 25.3 | 6.60 | 1.13 | 0.71 | 0.99 |
| Interval 2 | 21.1 | 2.01 | 1.13 | 0.26 | 1.04 | |
| Interval 3 | 15.6 | 1.28 | 0.69 | 0.31 | 0.76 | |
| Interval 4 | 13.8 | 1.68 | 0.67 | 0.56 | 0.48 | |
| Interval 5 | 10.1 | 1.48 | 0.71 | 0.57 | 0.58 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
M. Ardestani, M.; Šustr, V.; Frouz, J. Consumption Performance of Five Detritivore Species Feeding on Alnus glutinosa L. Leaf Litter in a Microcosm Experiment. Forests 2019, 10, 1080. https://doi.org/10.3390/f10121080
M. Ardestani M, Šustr V, Frouz J. Consumption Performance of Five Detritivore Species Feeding on Alnus glutinosa L. Leaf Litter in a Microcosm Experiment. Forests. 2019; 10(12):1080. https://doi.org/10.3390/f10121080
Chicago/Turabian StyleM. Ardestani, Masoud, Vladimír Šustr, and Jan Frouz. 2019. "Consumption Performance of Five Detritivore Species Feeding on Alnus glutinosa L. Leaf Litter in a Microcosm Experiment" Forests 10, no. 12: 1080. https://doi.org/10.3390/f10121080
APA StyleM. Ardestani, M., Šustr, V., & Frouz, J. (2019). Consumption Performance of Five Detritivore Species Feeding on Alnus glutinosa L. Leaf Litter in a Microcosm Experiment. Forests, 10(12), 1080. https://doi.org/10.3390/f10121080





