Plasticity of Plant N Uptake in Two Native Species in Response to Invasive Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Target Species
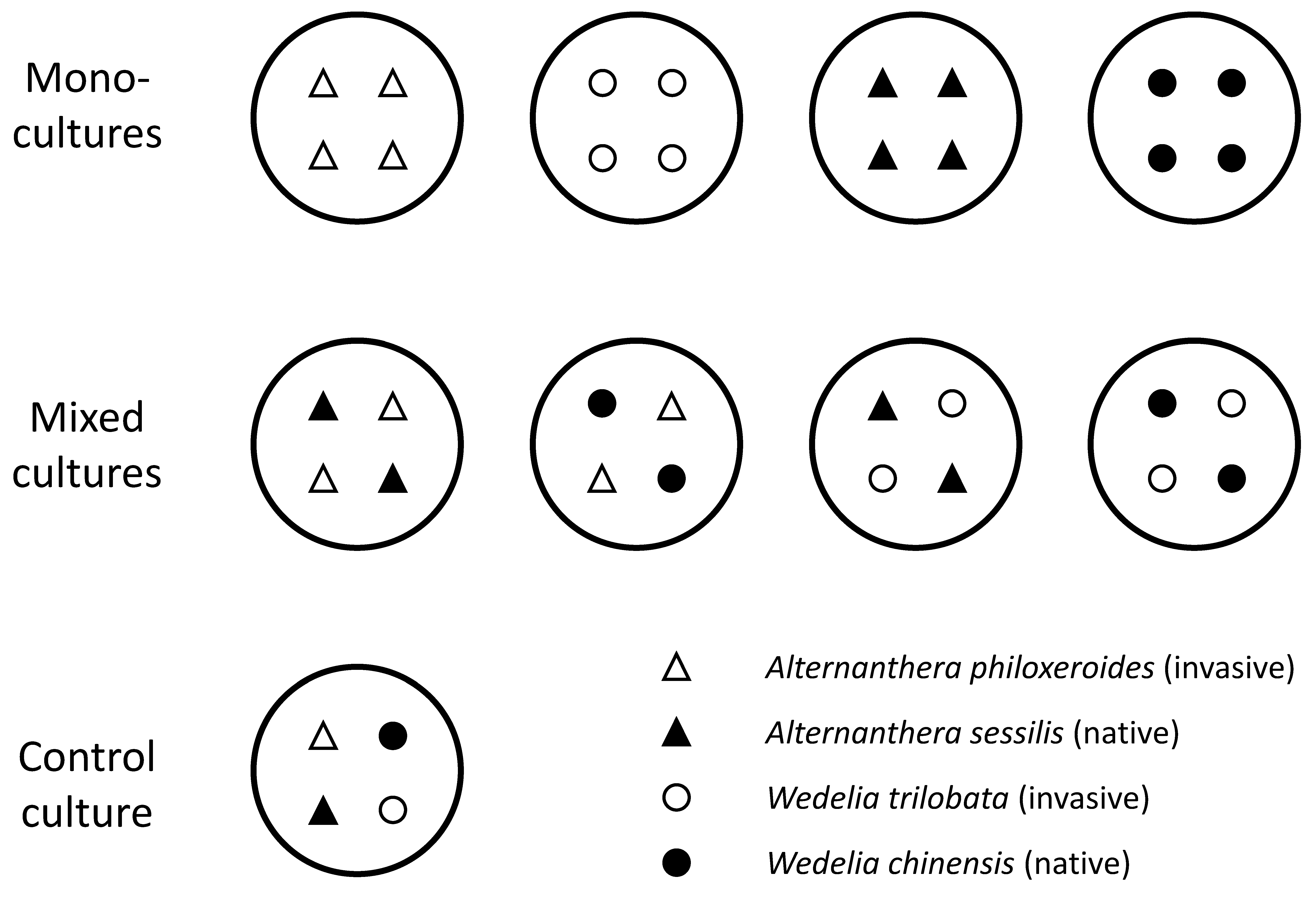
2.2. Experimental Design
2.3. Sampling and Isotope Analysis
2.4. Data Analysis
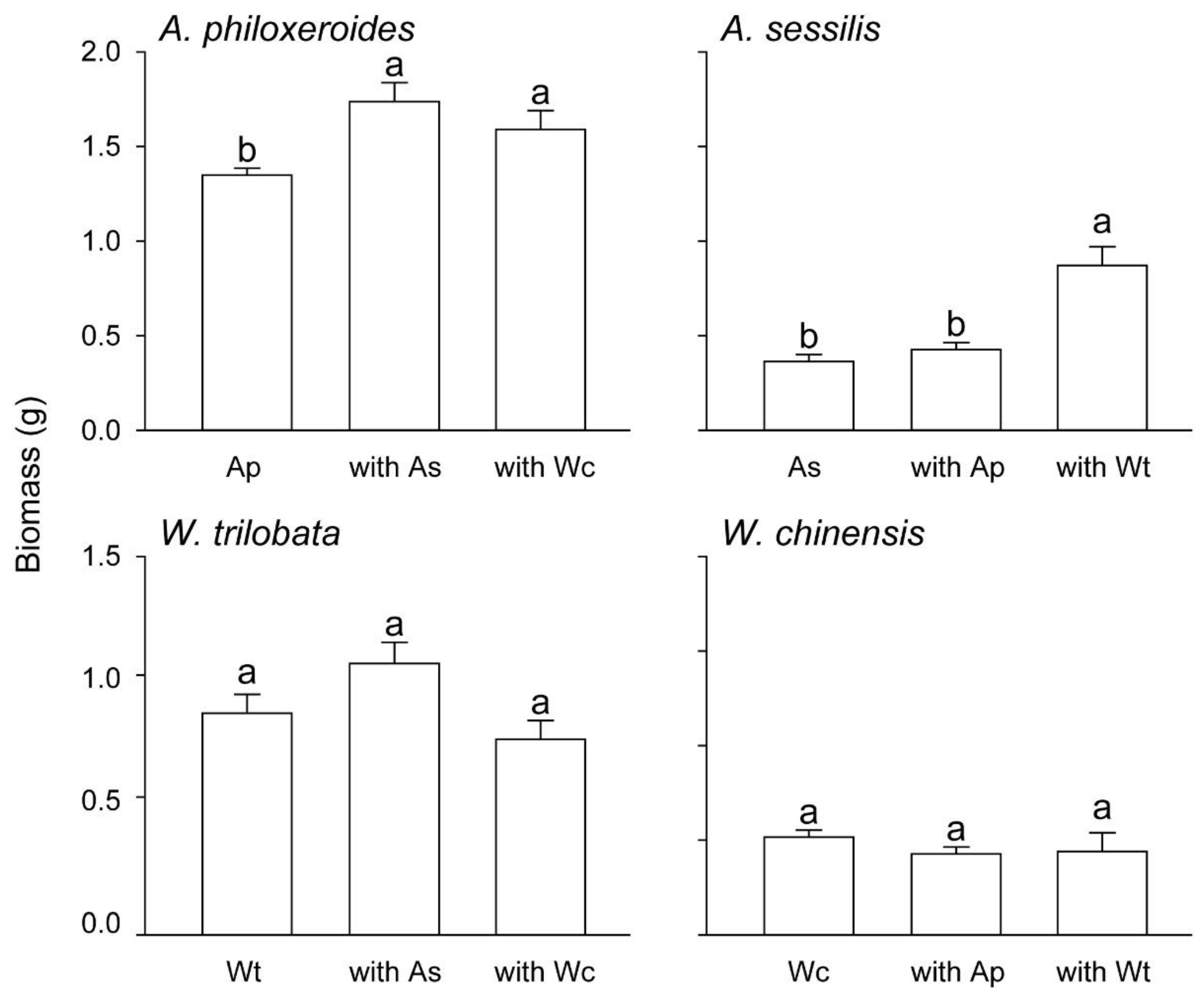
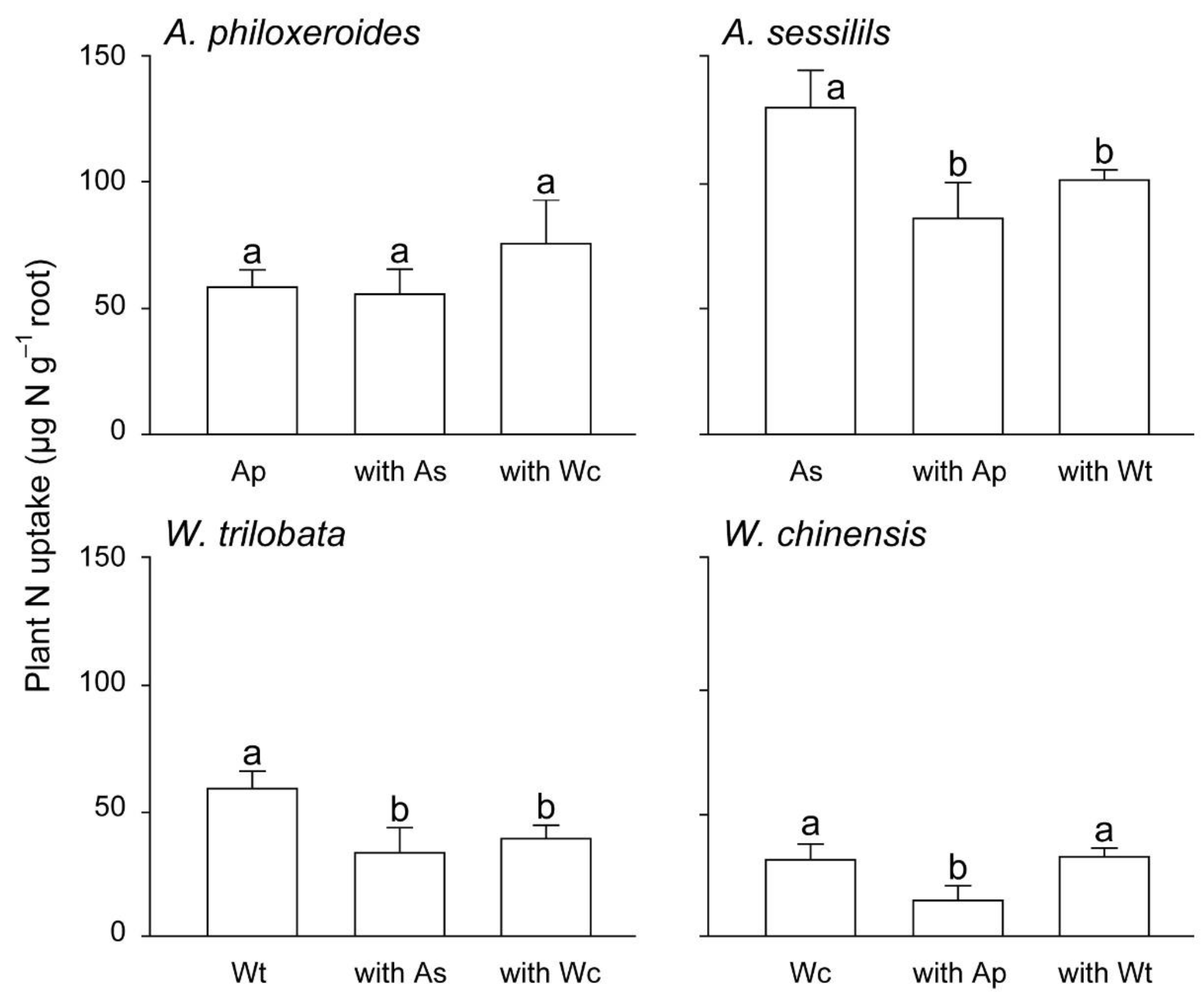
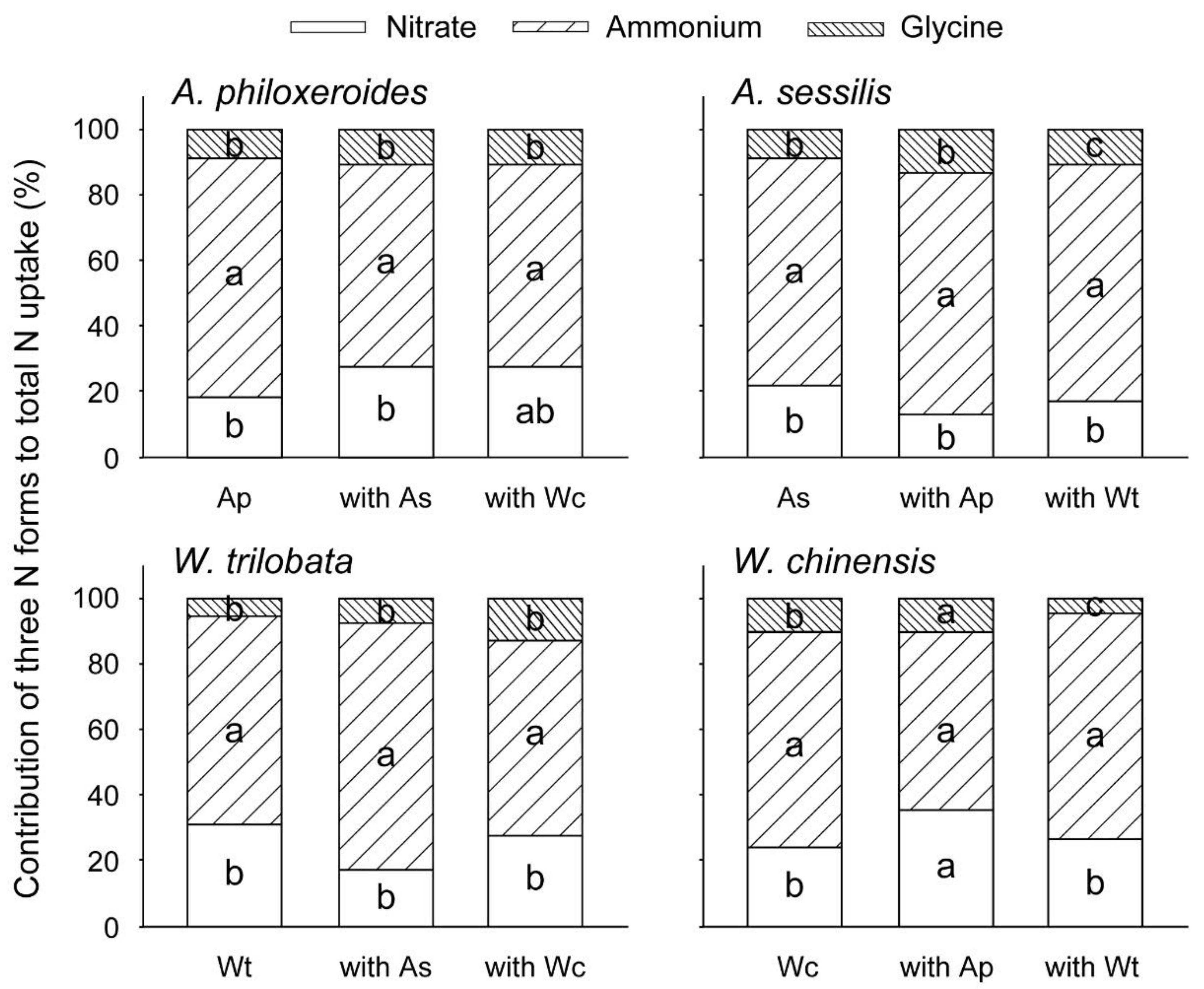
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Biswas, S.R.; Biswas, P.L.; Limon, S.H.; Yan, E.; Xu, M.; Khan, M.S.I. Plant invasion in mangrove forests worldwide. For. Ecol. Manag. 2018, 429, 480–492. [Google Scholar] [CrossRef]
- Flory, S.L.; Clay, K. Non-native grass invasion alters native plant composition in experimental communities. Biol. Invasions 2010, 12, 1285–1294. [Google Scholar] [CrossRef]
- Murphy, G.E.P.; Romanuk, T.N. A meta-analysis of declines in local species richness from human disturbances. Ecol. Evol. 2014, 4, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, L.J.; Suding, K.N. Applying competition theory to invasion: Resource impacts indicate invasion mechanisms in California shrublands. Biol. Invasions 2014, 16, 191–203. [Google Scholar] [CrossRef]
- Reinhart, K.O. The role of facilitative interactions in tree invasions. New Phytol. 2010, 187, 559–562. [Google Scholar] [CrossRef]
- Peltzer, D.A.; Kurokawa, H.; Wardle, D.A. Soil fertility and disturbance interact to drive contrasting responses of co-occurring native and nonnative species. Ecology 2016, 97, 515–529. [Google Scholar] [CrossRef]
- Gavilán, R.G.; Sánchez-Mata, D.; Gaudencio, M.; Gutiérrez-Girón, A.; Vilches, B. Impact of the non-indigenous shrub species Spartium junceum (Fabaceae) on native vegetation in central Spain. J. Plant Ecol. 2016, 9, 195–202. [Google Scholar] [CrossRef]
- Perry, L.G.; Galatowitsch, S.M.; Rosen, C.J. Competitive control of invasive vegetation: A native wetland sedge suppresses Phalaris arundinacea in carbon-enriched soil. J. Appl. Ecol. 2004, 41, 151–162. [Google Scholar] [CrossRef]
- Canfield, D.E.; Glazer, A.N.; Falkowski, P.G. The Evolution and Future of Earth’s Nitrogen Cycle. Science 2010, 330, 192–196. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Hattenschwiler, S.; Olander, L.; Allison, S. Nitrogen and nature. AMBIO 2002, 31, 97–101. [Google Scholar] [CrossRef]
- Xu, X.; Bai, J.; Ouyang, H. Advances in Studies on Organic Nitrogen Uptake by Terrestrial Plants. J. Nat. Resour. 2011, 26, 715–724. [Google Scholar]
- Kraiser, T.; Gras, D.E.; Gutierrez, A.G.; Gonzalez, B.; Gutierrez, R.A. A holistic view of nitrogen acquisition in plants. J. Exp. Bot. 2011, 62, 1455–1466. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ouyang, H.; Kuzyakov, Y.; Richter, A.; Wanek, W. Significance of organic nitrogen acquisition for dominant plant species in an alpine meadow on the Tibet plateau, China. Plant Soil 2006, 285, 221–231. [Google Scholar] [CrossRef]
- Mangla, S.; Sheley, R.L.; James, J.J.; Radosevich, S.R. Intra and interspecific competition among invasive and native species during early stages of plant growth. Plant Ecol. 2011, 212, 531–542. [Google Scholar] [CrossRef]
- Miller, A.E.; Bowman, W.D.; Suding, K.N. Plant uptake of inorganic and organic nitrogen: Neighbor identity matters. Ecology 2007, 88, 1832–1840. [Google Scholar] [CrossRef]
- Miller, A.E.; Bowman, W.D. Alpine plants show species-level differences in the uptake of organic and inorganic nitrogen. Plant Soil 2003, 250, 283–292. [Google Scholar] [CrossRef]
- Yong, T.; Liu, X.; Yang, F.; Song, C.; Wang, X.; Liu, W.; Su, B.; Zhou, L.; Yang, W. Characteristics of Nitrogen Uptake, Use and Transfer in a Wheat-Maize-Soybean Relay Intercropping System. Plant Prod. Sci. 2015, 18, 388–397. [Google Scholar] [CrossRef]
- Ashton, I.W.; Miller, A.E.; Bowman, W.D.; Suding, K.N. Nitrogen preferences and plant-soil feedbacks as influenced by neighbors in the alpine tundra. Oecologia 2008, 156, 625–636. [Google Scholar] [CrossRef]
- Xiao, Y.B.; Li, L.; Zhang, F.S. Effect of root contact on interspecific competition and N transfer between wheat and fababean using direct and indirect N-15 techniques. Plant Soil 2004, 262, 45–54. [Google Scholar] [CrossRef]
- Shen, H.; Dong, S.; Li, S.; Xiao, J.; Han, Y.; Yang, M.; Zhang, J.; Gao, X.; Xu, Y.; Li, Y.; et al. Effects of simulated N deposition on photosynthesis and productivity of key plants from different functional groups of alpine meadow on Qinghai-Tibetan plateau. Environ. Pollut. 2019, 251, 731–737. [Google Scholar] [CrossRef]
- Shen, H.; Dong, S.; Li, S.; Xiao, J.; Han, Y.; Yang, M.; Zhang, J.; Gao, X.; Xu, Y.; Li, Y.; et al. Grazing enhances plant photosynthetic capacity by altering soil nitrogen in alpine grasslands on the Qinghai-Tibetan plateau. Agric. Ecosyst. Environ. 2019, 280, 161–168. [Google Scholar] [CrossRef]
- Trinder, C.; Brooker, R.; Davidson, H.; Robinson, D. Dynamic trajectories of growth and nitrogen capture by competing plants. New Phytol. 2012, 193, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Corre-Hellou, G.; Fustec, J.; Crozat, Y. Interspecific competition for soil N and its interaction with N-2 fixation, leaf expansion and crop growth in pea-barley intercrops. Plant Soil 2006, 282, 195–208. [Google Scholar] [CrossRef]
- Julien, M.H.; Skarratt, B.; Maywald, G.F. Potential geographical distribution of alligator weed and its biological control by Agasicles hygrophila. J. Aquat. Plant Manag. 1995, 33, 55–60. [Google Scholar]
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species: A Selection from the Global Invasive Species Database; Invasive Species Specialist Group: Auckland, New Zealand, 2000; Volume 12. [Google Scholar]
- Jones, D.L.; Kielland, K. Soil amino acid turnover dominates the nitrogen flux in permafrost-dominated taiga forest soils. Soil Biol. Biochem. 2002, 34, 209–219. [Google Scholar] [CrossRef]
- Pang, R.; Sun, Y.; Xu, X.; Song, M.; Ouyang, H. Effects of clipping and shading on 15NO3− and 15NH4+ recovery by plants in grazed and ungrazed temperate grasslands. Plant Soil 2018, 433, 339–352. [Google Scholar] [CrossRef]
- Liu, M.; Qiao, N.; Zhang, Q.; Xu, X. Cropping regimes affect NO3− versus NH4+ uptake by Zea mays and Glycine max. Plant Soil 2018, 426, 241–251. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, L.; Gao, J.; Ding, Y. Effects of interspecific competition on the growth of invasive and native species. Chin. J. Ecol. 2016. [Google Scholar] [CrossRef]
- Quan, W.M.; Han, J.D.; Shen, A.L.; Ping, X.Y.; Qian, P.L.; Li, C.J.; Shi, L.Y.; Chen, Y.Q. Uptake and distribution of N, P and heavy metals in three dominant salt marsh macrophytes from Yangtze River estuary, China. Mar. Environ. Res. 2007, 64, 21–37. [Google Scholar] [CrossRef]
- Fickbohm, S.S.; Zhu, W.X. Exotic purple loosestrife invasion of native cattail freshwater wetlands: Effects on organic matter distribution and soil nitrogen cycling. Appl. Soil Ecol. 2006, 32, 123–131. [Google Scholar] [CrossRef]
- Mozdzer, T.J.; Brisson, J.; Hazelton, E.L.G. Physiological ecology and functional traits of North American native and Eurasian introduced Phragmites australis lineages. AoB Plants 2013, 5. [Google Scholar] [CrossRef]
- Leffler, A.J.; James, J.J.; Monaco, T.A. Temperature and functional traits influence differences in nitrogen uptake capacity between native and invasive grasses. Oecologia 2013, 171, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Matzek, V. Superior performance and nutrient-use efficiency of invasive plants over non-invasive congeners in a resource-limited environment. Biol. Invasions 2011, 13, 3005–3014. [Google Scholar] [CrossRef]
- Qing, H.; Cai, Y.; Xiao, Y.; Yao, Y.; An, S. Nitrogen Uptake and Use Efficiency of Invasive Spartina alterniflora and Native Phragmites australis: Effect of Nitrogen Supply. Clean Soil Air Water 2015, 43, 305–311. [Google Scholar] [CrossRef]
- Schimel, J.P.; Bennett, J. Nitrogen mineralization: Challenges of a changing paradigm. Ecology 2004, 85, 591–602. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, S. Effects of nitrogen forms on nutrient uptake and growth of trees. Chin. J. Appl. Ecol. 2003, 14, 2044–2048. [Google Scholar]
- Javaid, A. Arbuscular mycorrhizal mediated nutrition in plants. J. Plant Nutr. 2009, 32, 1595–1618. [Google Scholar] [CrossRef]
- Andrews, M.; Raven, J.A.; Lea, P.J. Do plants need nitrate? The mechanisms by which nitrogen form affects plants. Ann. Appl. Biol. 2013, 163, 174–199. [Google Scholar] [CrossRef]
- Hewins, D.B.; Hyatt, L.A. Flexible N uptake and assimilation mechanisms may assist biological invasion by Alliaria petiolata. Biol. Invasions 2010, 12, 2639–2647. [Google Scholar] [CrossRef]
- Hawkes, C.V.; Wren, I.F.; Herman, D.J.; Firestone, M.K. Plant invasion alters nitrogen cycling by modifying the soil nitrifying community. Ecol. Lett. 2005, 8, 976–985. [Google Scholar] [CrossRef]
- Reynolds, H.L.; Packer, A.; Bever, J.D.; Clay, K. Grassroots ecology: Plant-microbe-soil interactions as drivers of plant community structure and dynamics. Ecology 2003, 84, 2281–2291. [Google Scholar] [CrossRef]
- Jackson, R.B.; Caldwell, M.M. Integrating Resource Heterogeneity and Plant Plasticity: Modelling Nitrate and Phosphate Uptake in a Patchy Soil Environment. J. Ecol. 1996, 84, 891–903. [Google Scholar] [CrossRef]
- McKane, R.B.; Johnson, L.C.; Shaver, G.R.; Nadelhoffer, K.J.; Rastetter, E.B.; Fry, B.; Giblin, A.E.; Kielland, K.; Kwiatkowski, B.L.; Laundre, J.A.; et al. Resource-based niches provide a basis for plant species diversity and dominance in arctic tundra. Nature 2002, 415, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Kielland, K. Amino acid absorption by arctic plants: Implications for plant nutrition and nitrogen cycling. Ecology 1994, 75, 2373–2383. [Google Scholar] [CrossRef]
- Chapin III, F.S.; Moilanen, L.; Kielland, K. Preferential use of organic nitrogen for growth by a non-mycorrhizal arctic sedge. Nature 1993, 361, 150. [Google Scholar] [CrossRef]
| Family | Species | Status | Native Range | Habitat |
|---|---|---|---|---|
| Amaranthaceae | Alternanthera philoxeroides | Invasive | South America | Canals, wetlands, nearby fields |
| Alternanthera sessilis | Native | Asia, Africa | Moist habitats | |
| Asteraceae | Wedelia trilobata | Invasive | North and South America | Moist grasslands, edges of canals, roadsides |
| Wedelia chinensis | Native | Asia | Moist grasslands, edges of canals, roadsides, crop fields |
| Species | NH4+ | NO3− | ||
|---|---|---|---|---|
| F3,36 | p | F | p | |
| A. philoxeroides | 1.44 | 0.249 | 1.18 | 0.330 |
| W. trilobata | 0.60 | 0.617 | 0.96 | 0.424 |
| A. sessilis | 1.43 | 0.249 | 0.83 | 0.485 |
| W. chinensis | 0.68 | 0.570 | 1.15 | 0.341 |
| Species | Species Identity | N Form | Interaction | |||
|---|---|---|---|---|---|---|
| F2,18 | p | F | p | F | p | |
| A. philoxeroides | 2.0 | 0.164 | 27.6 | <0.001 | 0.7 | 0.608 |
| W. trilobata | 4.4* | 0.028 | 49.0 | <0.001 | 1.8 | 0.167 |
| A. sessilis | 1.1 | 0.372 | 74.5 | <0.001 | 0.6 | 0.656 |
| W. chinensis | 1.5 | 0.248 | 15.0 | <0.001 | 0.8 | 0.519 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.-H.; Zhou, Y.-L.; Gao, J.-Q.; Zhang, X.-Y.; Song, M.-H.; Xu, X.-L. Plasticity of Plant N Uptake in Two Native Species in Response to Invasive Species. Forests 2019, 10, 1075. https://doi.org/10.3390/f10121075
Hu Y-H, Zhou Y-L, Gao J-Q, Zhang X-Y, Song M-H, Xu X-L. Plasticity of Plant N Uptake in Two Native Species in Response to Invasive Species. Forests. 2019; 10(12):1075. https://doi.org/10.3390/f10121075
Chicago/Turabian StyleHu, Yi-Heng, Yu-Lu Zhou, Jun-Qin Gao, Xiao-Ya Zhang, Ming-Hua Song, and Xing-Liang Xu. 2019. "Plasticity of Plant N Uptake in Two Native Species in Response to Invasive Species" Forests 10, no. 12: 1075. https://doi.org/10.3390/f10121075
APA StyleHu, Y.-H., Zhou, Y.-L., Gao, J.-Q., Zhang, X.-Y., Song, M.-H., & Xu, X.-L. (2019). Plasticity of Plant N Uptake in Two Native Species in Response to Invasive Species. Forests, 10(12), 1075. https://doi.org/10.3390/f10121075




