Species Association of the Dominant Tree Species in an Old-Growth Forest and Implications for Enrichment Planting for the Restoration of Natural Degraded Forest in Subtropical China
Abstract
:1. Introduction
2. Materials and Methods
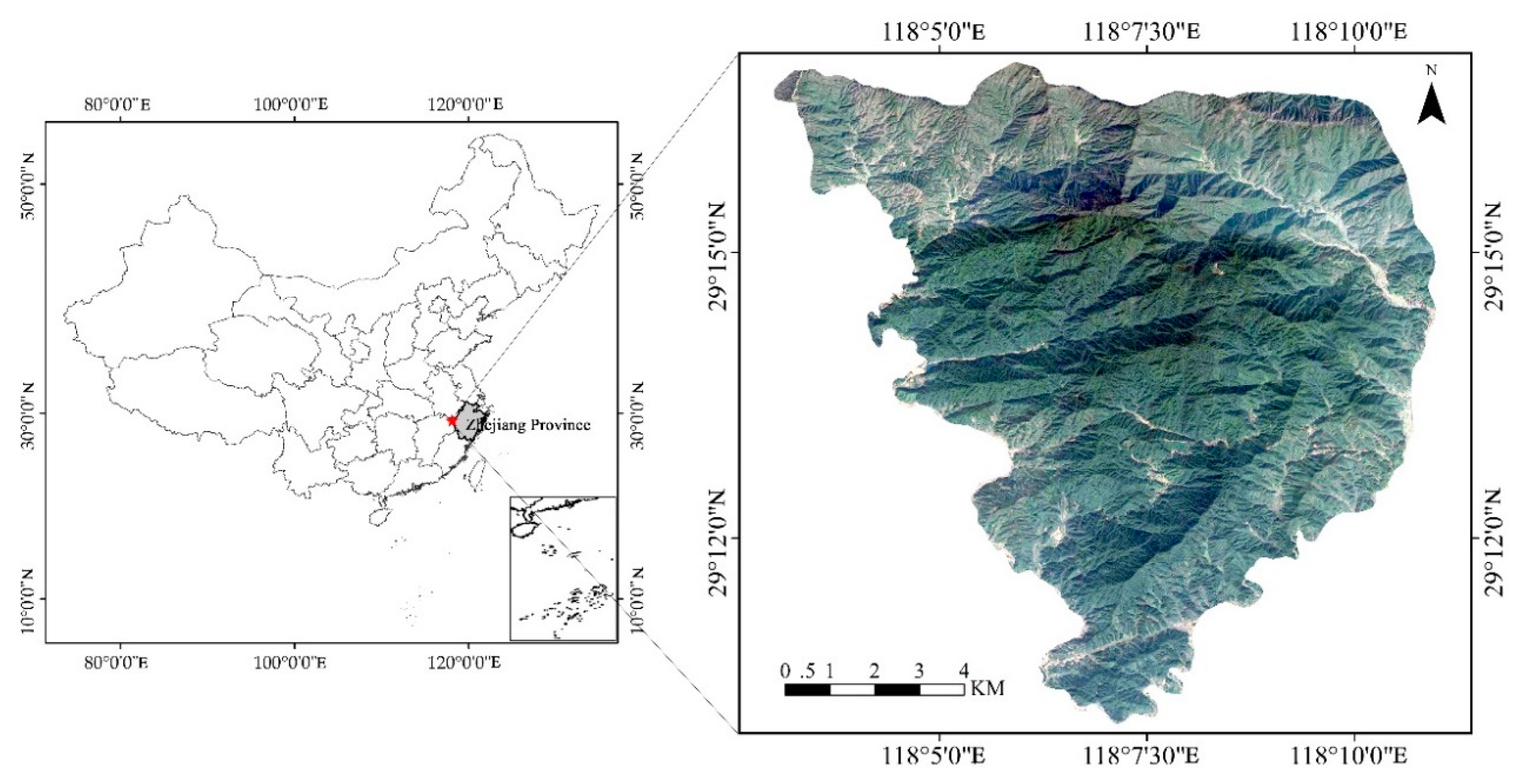
2.1. Study Area
2.2. Plot Establishment and Data Collection
2.3. Importance Value
2.4. Overall Species Association
2.5. Pairwise Species Association
2.5.1. The χ2 Test
2.5.2. Dice Index (Di)
2.5.3. Spearman’s Rank Correlation Coefficient (SRCC)
3. Results
3.1. Species Composition and Stand Structure
3.2. Overall Associations of Dominant Species
3.3. Pairwise Species Associations of Dominant Species
3.3.1. Test of Pairwise Species Associations
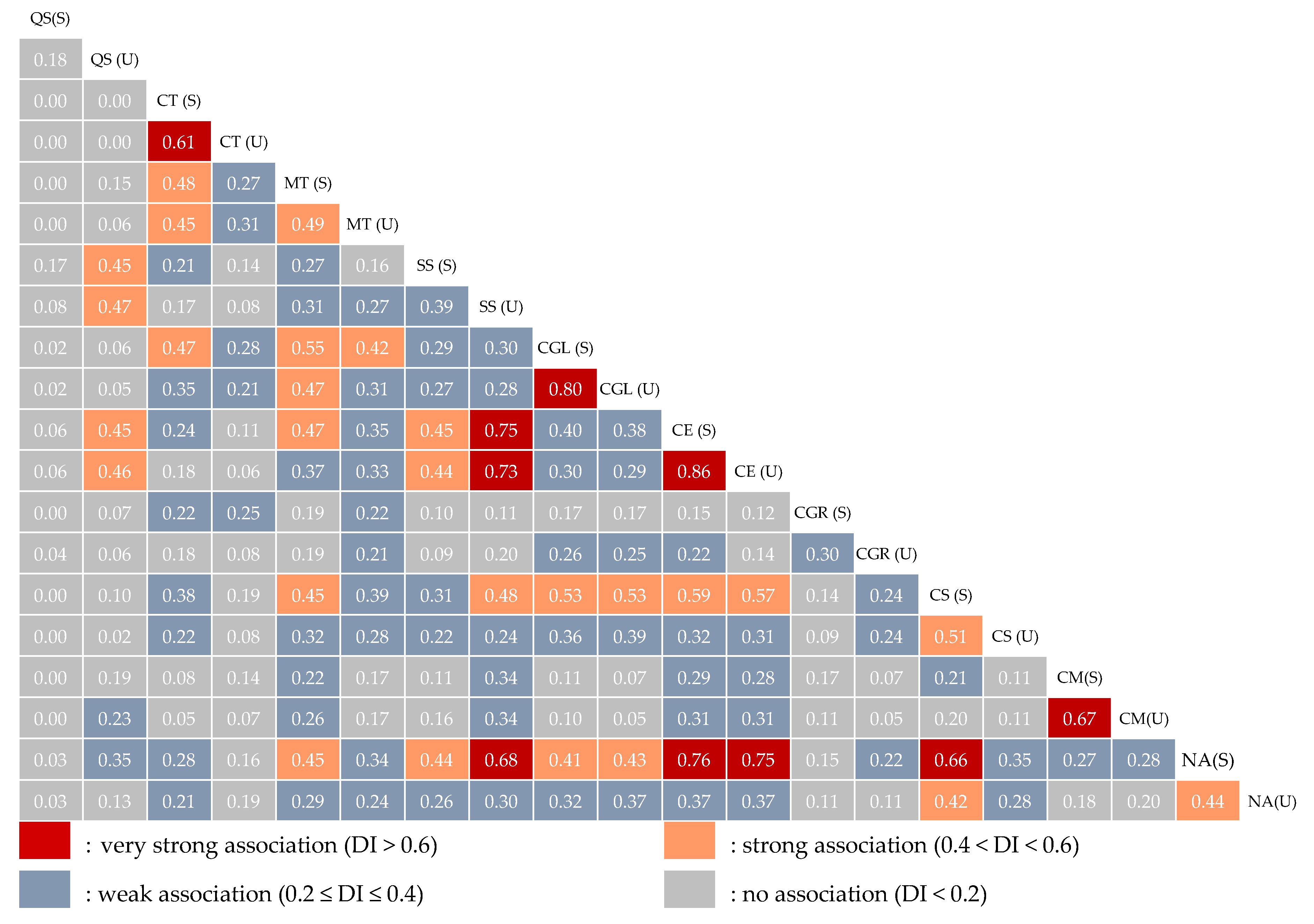
3.3.2. Measure of Pairwise Species Associations
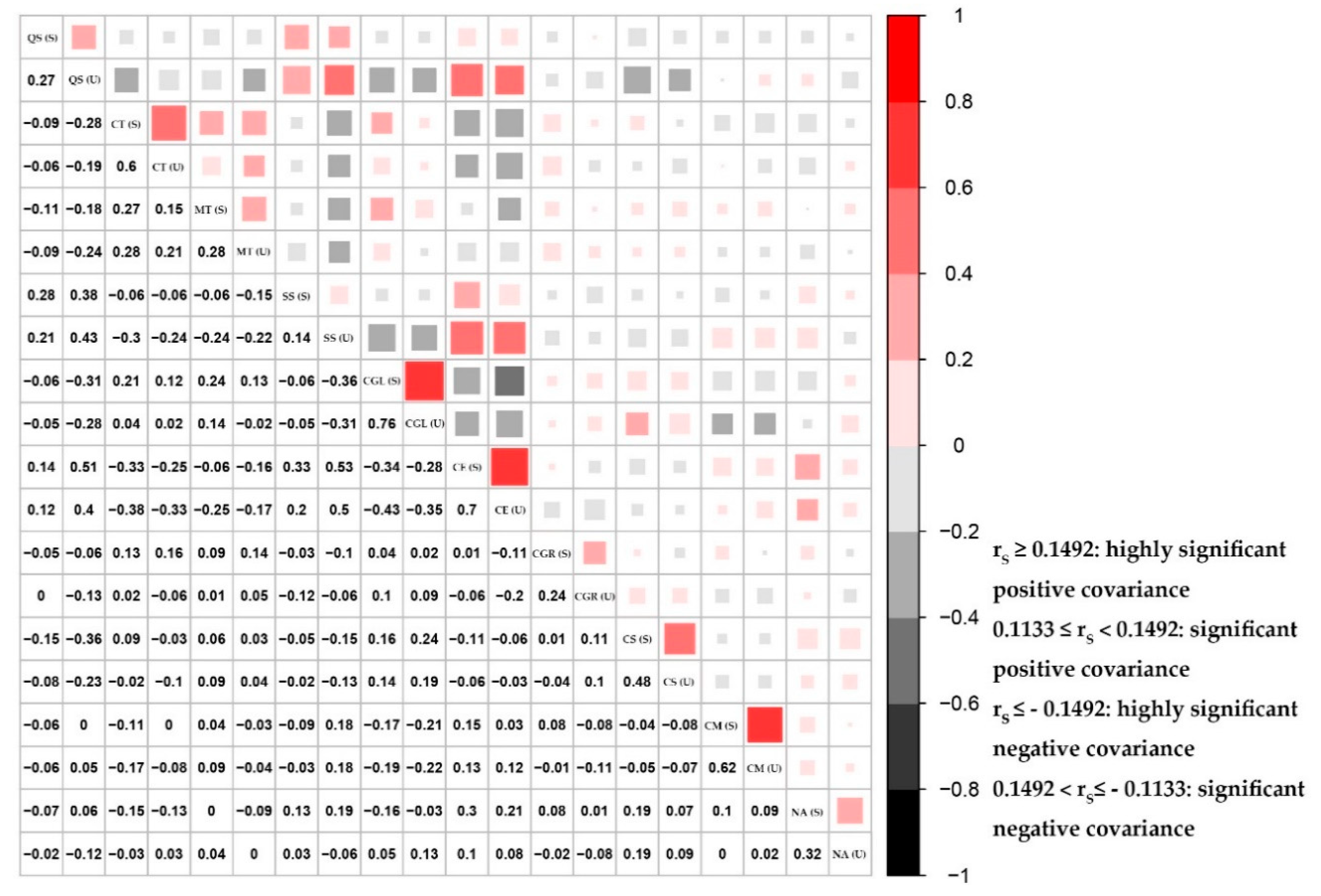
3.3.3. Measure of Pairwise Species Covariance
4. Discussion
4.1. Final Determination of Species Associations
4.2. Possible Reasons for the Observed Species Associations
4.3. Implications for Enrichment Planting
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Administration, S.F. China Forest Resource Report (2014–2018); China Forestry Press: Beijing, China, 2019. [Google Scholar]
- Yang, X.F.; Bauhus, J.; Both, S.; Fang, T.; Härdtle, W.; Kröber, W.; Ma, K.P.; Nadrowski, K.; Pei, K.Q.; Scherer-Lorenzen, M.; et al. Establishment success in a forest biodiversity and ecosystem functioning experiment in subtropical china (BEF-China). Eur. J. For. Res. 2013, 132, 593–606. [Google Scholar] [CrossRef]
- Veloso, M.G.; Dieckow, J.; Zanatta, J.A.; Bayer, C.; Higa, R.C.; Brevilieri, R.C.; Comerford, N.B.; Stoppe, A.M. Reforestation with loblolly pine can restore the initial soil carbon stock relative to a subtropical natural forest after 30 years. Eur. J. For. Res. 2018, 137, 593–604. [Google Scholar] [CrossRef]
- Chen, C.R.; Xu, Z.H.; Mathers, N.J. Soil carbon pools in adjacent natural and plantation forests of subtropical australia. Soil Sci. Soc. Am. J. 2004, 68, 282–291. [Google Scholar] [CrossRef]
- Liu, S.R.; Shi, Z.M.; Ma, J.M.; Zhao, C.M.; Zhang, Y.D.; Liu, X.L. Ecological strategies for restoration and reconstruction of degraded natural forests on the upper reaches of the yangtze river. Sci. Silvae Sin. 2009, 45, 120–124. [Google Scholar]
- Liu, S.R. Ecological Restoration Principle and Techniques of Natural Forest; China Forestry Publishing House: Beijing, China, 2011. [Google Scholar]
- Liu, S.R.; Ma, J.M.; Miao, N. Achievements in natural forest protection, ecological restoration, and sustainable management in china. Acta Ecol. Sin. 2015, 35, 212–218. [Google Scholar]
- Daily, G.C. Restoring value to the world’s degraded lands. Science 1995, 269, 350–354. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, G.; Tang, Y.S.; Zhang, W.Y.; Zang, R.G. Zoning of degraded natural forests in mountain areas of southwest china. For. Res. 2010, 23, 612–616. [Google Scholar]
- Silvestrini, M.; Cysneiro, A.D.D.; Lima, A.L.; Veiga, L.G.; Isernhagen, I.; Tamashiro, J.Y.; Gandolfi, S.; Rodrigues, R.R. Natural regeneration in abandoned fields following intensive agricultural land use in an atlantic forest island, brazil. Rev. Árvore 2012, 36, 659–671. [Google Scholar] [CrossRef]
- Silvestrini, M.; Dos Santos, F.A.M. Variation in the population structure between a natural and a human-modified forest for a pioneer tropical tree species not restricted to large gaps. Ecol. Evol. 2015, 5, 2420–2432. [Google Scholar] [CrossRef] [PubMed]
- Herrera, J.M.; Garcia, D. Effects of forest fragmentation on seed dispersal and seedling establishment in ornithochorous trees. Conserv. Biol. 2010, 24, 1089–1098. [Google Scholar] [CrossRef]
- Lamb, D.; Erskine, P.D.; Parrotta, J.A. Restoration of degraded tropical forest landscapes. Science 2005, 310, 1628–1632. [Google Scholar] [CrossRef] [PubMed]
- Blay, D. Tropical secondary forest management in humid Africa: Reality and perspectives. In Proceedings of the Fao Workshop on Tropical Secondary Forest Management in Africa, Nairobi, Kenya, 9–13 December 2002. [Google Scholar]
- Hoffmann, S.; Jaeger, D.; Shuirong, W. Adapting chinese forest operations to socio-economic developments: What is the potential of plantations for strengthening domestic wood supply? Sustainability 2018, 10, 1042. [Google Scholar] [CrossRef]
- Delang, C.O. China’s Soil Pollution and Degradation Problems; Routledge: Abingdon, UK, 2017. [Google Scholar]
- Sun, X.F.; Canby, K.; Liu, L.J. China’s Logging Ban in Natural Forests: Impacts of Extended Policy at Home and Abroad; Forest Trends Association: Washington, DC, USA, 2016. [Google Scholar]
- Wilson, E. Multiaged silviculture: Managing for complex forest stand structures. Int. For. Rev. 2015, 17, 115–116. [Google Scholar]
- Puettmann, K.J.; Wilson, S.M.; Baker, S.C.; Donoso, P.J.; Drössler, L.; Amente, G.; Harvey, B.D.; Knoke, T.; Lu, Y.C.; Nocentini, S. Silvicultural alternatives to conventional even-aged forest management-what limits global adoption? For. Ecosyst. 2015, 2, 8. [Google Scholar] [CrossRef]
- O’Hara, K.L. What is close-to-nature silviculture in a changing world? For. Int. J. For. Res. 2016, 89, 1–6. [Google Scholar] [CrossRef]
- Favre, L.A.; Oberson, J.M. 111 années d’application de la méthode du contrôle à la forêt de couvet|111 years of application of the method of control in the community forest of couvet. Schweiz. Z. Forstwes. 2002, 153, 298–313. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Hu, G.; Zhu, J.D.; Luo, D.H.; Ni, J. Spatial patterns and interspecific associations of dominant tree species in two old-growth karst forests, SW China. Ecol. Res. 2010, 25, 1151–1160. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Kang, X.G.; Guo, Z.Q.; Yang, H.; Xu, M. Species interactions in spruce–fir mixed stands and implications for enrichment planting in the Changbai Mountains, China. Mt. Res. Dev. 2012, 32, 187–197. [Google Scholar] [CrossRef]
- Jiang, J.; Lu, Y.C.; Pang, L.F. Analysis on interspecific association and management modes of the tree species for plantation in southern subtropical zone, China. For. Resour. Manag. 2014, 6, 79–84. [Google Scholar]
- Hao, Z.Q.; Zhang, J.; Song, B.; Ye, J.; Li, B.H. Vertical structure and spatial associations of dominant tree species in an old-growth temperate forest. For. Ecol. Manag. 2007, 252, 1–11. [Google Scholar] [CrossRef]
- Chai, Z.Z.; Sun, C.L.; Wang, D.X.; Liu, W.Z. Interspecific associations of dominant tree populations in a virgin old-growth oak forest in the Qinling Mountains, China. Bot. Stud. 2016, 57, 23. [Google Scholar] [CrossRef] [PubMed]
- Greig-Smith, P. Quantitative Plant Ecology; University of California Press: Berkeley, CA, USA, 1983. [Google Scholar]
- Cole, L.C. The measurement of interspecific associaton. Ecology 1949, 30, 411–424. [Google Scholar] [CrossRef]
- Li, G.; Zhu, Z.H.; Wang, X.A.; Guo, H. Interspecific association of trees species in quercus wutaiensis communities in ziwu mountain related to quadrat size. Chin. J. Ecol. 2008, 27, 689–696. [Google Scholar]
- Zhao, Z.H.; Zu, Y.G.; Yang, F.J.; Cong, P.T. Study on the sampling technique of interspecific association of ligneous plant in quercus liaotungensis forest in Dongling Mountain. Acta Phytoecol. Sin. 2003, 27, 396–403. [Google Scholar]
- Xie, T.; Ju, T.Z.; Shi, H.X.; Fan, Z.H.; Yang, G.K.; Zhang, S.Z. Interspecific association of rare and endangered pinus bungeana community in Xiaolongshan of Gansu. Chin. J. Ecol. 2010, 29, 448–453. [Google Scholar]
- Yang, C.Y.; Liu, S.F.; Yu, L.F. Interspecific associations of dominant tree species with restoration of a karst forest. J. Zhejiang For. Coll. 2010, 27, 44–50. [Google Scholar]
- Paluch, J.G.; Bartkowicz, L.E. Spatial interactions between scots pine (Pinus sylvestris L.), common oak (Quercus robur L.) and silver birch (Betula pendula Roth.) as investigated in stratified stands in mesotrophic site conditions. For. Ecol. Manag. 2004, 192, 229–240. [Google Scholar] [CrossRef]
- White, D.L.; Lloyd, F.T. Defining old growth: Implications for management. Presentation at the 8th Biennial Southern Silvicultural Research Conference, Auburn, AL, USA, 1–3 November 1994. [Google Scholar]
- Lund, H.G. Definitions of Old Growth, Pristine, Climax, Ancient Forests, Degradation, Desertification, Forest Fragmentation, and Simiar Terms (Definitions of Forest State, Stage, and Origin); Forest Information Services: Gainesville, VA, USA, 2005. [Google Scholar]
- Abrams, M.D.; Copenheaver, C.A. Temporal variation in species recruitment and dendroecology of an old-growth white oak forest in the virginia piedmont, USA. For. Ecol. Manag. 1999, 124, 275–284. [Google Scholar] [CrossRef]
- Nagel, T.A.; Zenner, E.K.; Brang, P. Research in old-growth forests and forest reserves: Implications for integrated forest management. In Integrative Approaches as an Opportunity for the Conservation of Forest Biodiversity; European Forest Institute: Freiburg, Germany, 2013; pp. 44–50. [Google Scholar]
- Du, Y.J.; Mi, X.C.; Liu, X.J.; Chen, L.; Ma, K.P. Seed dispersal phenology and dispersal syndromes in a subtropical broad-leaved forest of china. For. Ecol. Manag. 2009, 258, 1147–1152. [Google Scholar] [CrossRef]
- Feng, G.; Zhang, J.L.; Pei, N.C.; Rao, M.D.; Mi, X.C.; Ren, H.B.; Ma, K.P. Comparison of phylobetadiversity indices based on community data from gutianshan forest plot. Chin. Sci. Bull. 2012, 57, 623–630. [Google Scholar] [CrossRef]
- Hu, Z.H.; Yu, M.J.; Ding, B.Y.; Fang, T.; Qian, H.Y.; Chen, Q.C. Types of evergreen broad-leaved forests and their species diversity in gutian mountain national nature reserve. Chin. J. Appl. Environ. Biol. 2003, 9, 341–345. [Google Scholar]
- Lou, L.H.; Jin, S.H. Spermatophyta flora of gutianshan nature reserve in Zhejiang. J. Beijing For. Univ. 2000, 22, 33–39. [Google Scholar]
- Legendre, P.; Legendre, L. Developments in Numerical Ecology; Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar]
- Lamont, B.B.; Downes, S.; Fox, J.E. Importance–value curves and diversity indices applied to a species-rich heathland in Western Australia. Nature 1977, 265, 438–441. [Google Scholar] [CrossRef]
- Wiryani, E. The Abundance and Importance Value of Tree in “Sendang Kalimah Toyyibah” Surrounding and its Implication to the Spring; IOP Publishing: Semarang, Indonesia, 2018. [Google Scholar]
- Schluter, D. A variance test for detecting species associations, with some example applications. Ecology 1984, 65, 998–1005. [Google Scholar] [CrossRef]
- Chow, K.V.; Denning, K.C. A simple multiple variance ratio test. J. Econom. 1993, 58, 385–401. [Google Scholar] [CrossRef]
- Ludwig, J.A.; Quartet, L.; Reynolds, J.F. Statistical Ecology: A Primer in Methods and Computing; John Wiley & Sons: New York, NY, USA, 1988. [Google Scholar]
- Hong, W.; Chen, M.X. Calculation and application of interspecific association of broad-leaved trees in the North Fujian. Sci. Silvae Sin. 1990, 26, 175–181. [Google Scholar]
- Zar, J.H. Biostatistical Analysis; Prentice-Hall: Upper Saddle River, NJ, USA, 1974. [Google Scholar]
- Su, S.J.; Liu, J.F.; He, Z.S.; Zheng, S.Q.; Hong, W.; Xu, D.W. Ecological species groups and interspecific association of dominant tree species in daiyun mountain national nature reserve. J. Mt. Sci. 2015, 12, 637–646. [Google Scholar] [CrossRef]
- Gu, L.; Gong, Z.W.; Li, W.Z. Niches and interspecific associations of dominant populations in three changed stages of natural secondary forests on loess plateau, PR China. Sci. Rep. 2017, 7, 6604. [Google Scholar] [CrossRef]
- Gautheir, T.D. Detecting trends using spearman’s rank correlation coefficient. Environ. Forensics 2001, 2, 359–362. [Google Scholar] [CrossRef]
- Hurlbert, S.H. A coefficient of interspecific assciation. Ecology 1969, 50, 1–9. [Google Scholar] [CrossRef]
- Bishara, A.J.; Hittner, J.B. Testing the significance of a correlation with nonnormal data: Comparison of pearson, spearman, transformation, and resampling approaches. Psychol. Methods 2012, 17, 399–417. [Google Scholar] [CrossRef]
- Team, R.C. A Language and Environment for Statistical Computing; R Project; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Zhang, J.L. Spaa: Species Association Analysis, R Package version 0.2.2; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Hadley, W. The Split-apply-combine strategy for data analysis. J. Stat. Softw. 2011, 40, 1–29. [Google Scholar]
- Wei, T.; Simko, V. R package “corrplot”: Visualization of a Correlation Matrix (Version 0.84). Available online: https://github.com/taiyun/corrplot (accessed on 1 August 2017).
- Xue, O.; Wei, T.X. Interspecific association among understory species of the low-efficiency plantation in the jiufeng national forest park. Bull. Bot. Res. 2016, 36, 34–42. [Google Scholar]
- Li, J.; Xie, F.; Chen, C.; Zhang, S.; Xiao, R.; Zhang, D. Interspecif ic association of dominant species in Betula luminifera natural forest communities of Shaowu, Fujian Province. Chin. J. Appl. Ecol. 2001, 12, 168–170. [Google Scholar]
- Mundry, R.; Fischer, J. Use of statistical programs for nonparametric tests of small samples often leads to incorrect pvalues: Examples from animal behaviour. Anim. Behav. 1998, 56, 256–259. [Google Scholar] [CrossRef]
- Dezfuli, B.; Giari, L.; De Biaggi, S.; Poulin, R. Associations and interactions among intestinal helminths of the brown trout, salmo trutta, in northern Italy. J. Helminthol. 2001, 75, 331–336. [Google Scholar] [CrossRef]
- Lun, Z.; Hai, Y.; Kun, C. Relationship between microcystin in drinking water and colorectal cancer. Biomed. Environ. Sci. 2002, 15, 166–171. [Google Scholar]
- Chen, Q.; Chen, J.; Zhong, J.J.; Ji, L.T.; Kang, B. Interspecific association and functional group classification of the dominant populations in shrub layer in secondary forest of pinus tabuliformis in Qinling Mountain, China. J. Appl. Ecol. 2018, 29, 1736–1744. [Google Scholar]
- Kobe, R.K.; Vriesendorp, C.F. Conspecific density dependence in seedlings varies with species shade tolerance in a wet tropical forest. Ecol. Lett. 2011, 14, 503–510. [Google Scholar] [CrossRef]
- Yang, H.; Wu, Y. Species composition, age structure and regeneration strategies in broad-leaved Korean pine mixed forest in Changbai Mountain. Sci. Silvae Sin. 1988, 24, 18–27. [Google Scholar]
- Du, Z.; Kang, X.; Meng, J.; Kong, L.; Guo, W.; Yue, G. Spatial distribution pattern and association of dominant tree species in poplar-birch secondary forest stand in Changbai Mountains. J. Northeast For. Univ. 2013, 41, 36–42. [Google Scholar]
- Grubb, P.J. The maintenance of species-richness in plant communities: The importance of the regeneration niche. Biol. Rev. 1977, 52, 107–145. [Google Scholar] [CrossRef]
- Liu, J.H.; Hong, W.; Fan, H.B.; Lin, R.F. Study on the inter-specific association of species in the vegetation layer in castanopsis kawakamii forest. Sci. Silvae Sin. 2001, 37, 117–123. [Google Scholar]
- Wu, C.; Wang, Z.; Fan, Z. Significance of senescence study on tree roots and its advances. J. Appl. Ecol. 2004, 15, 1276–1280. [Google Scholar]
- Ma, L.Q. Relations of photosynthetic area of crown leaves and potential use of solar energy to crown profile area. J. Ecol. 1986, 5, 23–26. [Google Scholar]
- Chazdon, R.L. Second Growth: The Promise of Tropical Forest Regeneration in an Age of Deforestation; University of Chicago Press: Chicago, IL, USA, 2014. [Google Scholar]
- Aide, T.M.; Zimmerman, J.K.; Pascarella, J.B.; Rivera, L.; Humfredo, M.V. Forest regeneration in a chronosequence of tropical abandoned pastures: Implications for restoration ecology. Restor. Ecol. 2000, 8, 328–338. [Google Scholar] [CrossRef]
- Oliver, C.D.; Larson, B.C. Forest Stand Dynamics; Wiley: New York, NY, USA, 1996. [Google Scholar]
- Gu, L.; O’Hara, K.L.; Li, W.Z.; Gong, Z.W. Spatial patterns and interspecific associations among trees at different stand development stages in the natural secondary forests on the Loess Plateau, China. Ecol. Evol. 2019. [Google Scholar] [CrossRef]
- Lamprecht, H. Tropical forest ecosystems and their tree species. In Siliviculture in Tropics, Possibilities and Methods for Their Long-Term Utilization; Institute of for Siliviculture of the University of Gottingen, Technical Cooperation of Federal Republic of Germany: Berlin, Germany, 1989. [Google Scholar]
- Swaine, M.D.; Hall, J.B. Early succession on cleared forest land in Ghana. J. Ecol. 1983, 71, 601–627. [Google Scholar] [CrossRef]
- Poorter, L. Biodiversity of West African Forests: An Ecological Atlas of Woody Plant Species; CABI: Wallingford, UK, 2004. [Google Scholar]
- Hill, J.L.; Curran, P.J. Area, shape and isolation of tropical forest fragments: Effects on tree species diversity and implications for conservation. J. Biogeogr. 2003, 30, 1391–1403. [Google Scholar] [CrossRef]
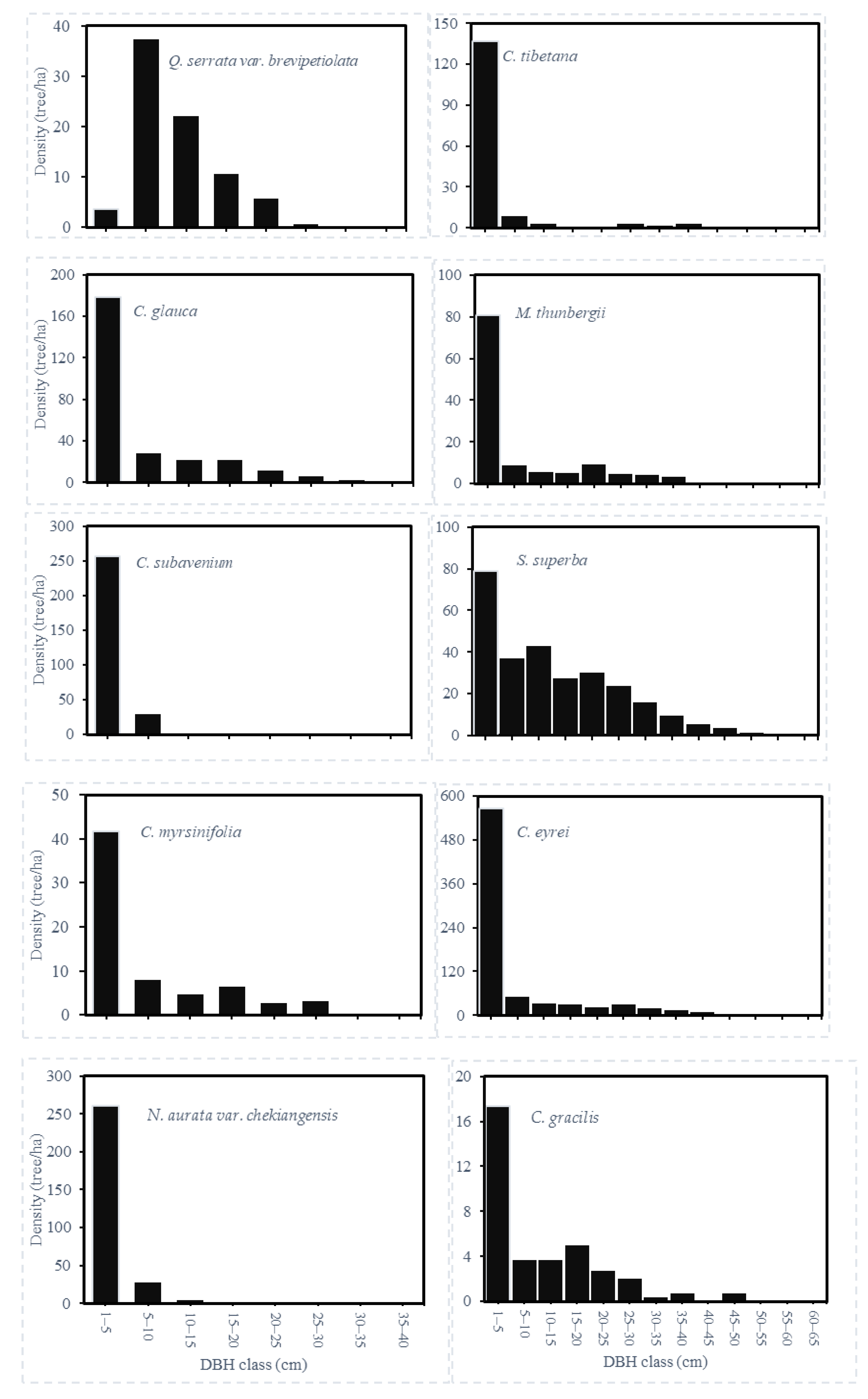

| Latin Name | Acronym | Density (Trees ha–1) | Mean DBH (cm) | Basal Area (m2 ha–1) | Light Requirement 1 | Relative Abundance | Relative Frequency | Relative Dominance | Importance Value |
|---|---|---|---|---|---|---|---|---|---|
| Castanopsis eyrei | CE | 213 | 20.8 | 0.0121 | OP | 23.04 | 32.39 | 14.59 | 23.34 |
| Schima superba | SS | 197 | 20.1 | 0.0367 | SI | 21.30 | 27.08 | 12.33 | 20.24 |
| Cyclobalanopsis glauca | CGL | 90 | 14.5 | 0.0408 | ST | 9.73 | 6.17 | 8.46 | 8.12 |
| Machilus thunbergii | MT | 41 | 20.3 | 0.0408 | ST | 4.43 | 5.63 | 6.69 | 5.58 |
| Quercus serrata var. brevipetiolata | QS | 76 | 11.4 | 0.0204 | SI | 8.26 | 3.11 | 4.83 | 5.40 |
| Neolitsea aurata var. chekiangensis | NA | 32 | 7.2 | 0.0451 | ST | 3.42 | 0.48 | 5.40 | 3.10 |
| Cinnamomum subavenium | CS | 31 | 7.2 | 0.0340 | ST | 3.39 | 0.45 | 4.92 | 2.92 |
| Cyclobalanopsis myrsinifolia | CM | 25 | 14.9 | 0.0043 | ST | 2.70 | 1.82 | 3.63 | 2.72 |
| Castanopsis tibetana | CT | 20 | 17.6 | 0.0216 | OP | 2.16 | 2.47 | 3.06 | 2.57 |
| Cyclobalanopsis gracilis | CGR | 18 | 18.4 | 0.0045 | ST | 2.02 | 2.14 | 3.22 | 2.46 |
| Significant Positive Associations | Significant Negative Associations | |
|---|---|---|
| First priority | Q. serrata (U)–S. superba (S), Q. serrata (U)–S. superba (U), Q. serrata (U)–C. eyrei (S), Q. serrata (U)–C. eyrei (U), C. tibetana (S)–C. tibetana (U), C. tibetana (S)–M. thunbergii (S), C. tibetana (S)–M. thunbergii (U), C. tibetana (S)–C. glauca (S), M. thunbergii (S)–M. thunbergii (U), M. thunbergii (S)–C. glauca (S), M. thunbergii (S)–C. glauca (U), S. superba (S)–C. eyrei (S), S. superba (S)–C. eyrei (U), S. superba (S)–N. aurata var. chekiangensis (S), S. superba (U)–C. eyrei (S), S. superba (U)–C. eyrei (U), S. superba (U)–N. aurata var. chekiangensis (S), C. glauca (S)–C. glauca (U), C. glauca (U)–C. subavenium (S), C. eyrei (S)–C. eyrei (U), C. eyrei (S)–N. aurata var. chekiangensis (S), C. eyrei (U)–N. aurata var. chekiangensis (S), C. subavenium (S)–C. subavenium (U), C. subavenium (S)–N. aurata var. chekiangensis (S), C. subavenium (S)–N. aurata var. chekiangensis (U), C. myrsinifolia (S)–C. myrsinifolia (U), N. aurata var. chekiangensis (S)–N. aurata var. chekiangensis (U), M. thunbergii (U)–C. glauca (S), C. glauca (S)–C. subavenium (S) | Q. serrata (U)–C. tibetana (S), Q. serrata (U)–C. tibetana (U), Q. serrata (U)–M. thunbergii (U), Q. serrata (U)–C. glauca (S), Q. serrata (U)–C. glauca (U), Q. serrata (U)–C. subavenium (S), Q. serrata (U)–C. subavenium (U), C. tibetana (S)–S. superba (U), C. tibetana (S)–C. eyrei (S), C. tibetana (S)–C. eyrei (U), C. tibetana (U)–S. superba (U), C. tibetana (U)–C. eyrei (S), C. tibetana (U)–C. eyrei (U), C. tibetana (S)–C. myrsinifolia (U), M. thunbergii (S)–S. superba (U), M. thunbergii (S)–C. eyrei (U), S. superba (U)–C. glauca (S), S. superba (U)–C. glauca (U), C. glauca (S)–C. eyrei (S), C. glauca (S)–C. eyrei (U), C. glauca (S)–C. myrsinifolia (S), C. glauca (S)–C. myrsinifolia (U), C. glauca (S)–N. aurata var. chekiangensis (S), C. glauca (U)–C. eyrei (S), C. glauca (U)–C. eyrei (U), C. glauca (U)–C. myrsinifolia (S), C. glauca (U)–C. myrsinifolia (U), C. eyrei (U)–C. gracilis (U), Q. serrata (S)–C. subavenium (S), Q. serrata (U)–M. thunbergii (S), C. tibetana (S)–C. myrsinifolia (S), C. tibetana (S)–N. aurata var. chekiangensis (S), M. thunbergii (U)–S. superba (S), M. thunbergii (U)–S. superba (U) |
| Second priority | Q. serrata (S)–Q. serrata (U), Q. serrata (S)–S. superba (S), C. tibetana (U)–M. thunbergii (S), C. tibetana (U)–M. thunbergii (U), C. tibetana (U)–C. gracilis (S), S. superba (U)–C. myrsinifolia (S), S. superba (U)–C. myrsinifolia (U), C. glauca (U)–C. subavenium (U), C. eyrei (S)–C. myrsinifolia (U), C. gracilis (S)–C. gracilis (U), C. tibetana (S)–C. gracilis (S), C. tibetana (U)–C. glauca (S), M. thunbergii (U)–C. gracilis (S), C. glauca (S)–C. subavenium (U), C. glauca (U)–N. aurata var. chekiangensis (U), C. eyrei (U)–C. myrsinifolia (U) | - |
| Third priority | - | S. superba (U)–C. subavenium (S) |
| Fourth priority | Q. serrata (S)–S. superba (U), C. eyrei (S)–C. myrsinifolia (S), Q. serrata (S)–C. eyrei (S), Q. serrata (S)–C. eyrei (U), S. superba (S)–S. superba (U), C. gracilis (U)–C. subavenium (S) | M. thunbergii (U)–C. eyrei (S), M. thunbergii (U)–C. eyrei (U), Q. serrata (U)–C. gracilis (U), Q. serrata (U)–N. aurata var. chekiangensis (U), C. tibetana (U)–N. aurata var. chekiangensis (S), S. superba (S)–C. gracilis (U), S. superba (U)–C. subavenium (U) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Zhu, Y.; Wang, J.; Ma, W.; Meng, J. Species Association of the Dominant Tree Species in an Old-Growth Forest and Implications for Enrichment Planting for the Restoration of Natural Degraded Forest in Subtropical China. Forests 2019, 10, 957. https://doi.org/10.3390/f10110957
Liu Z, Zhu Y, Wang J, Ma W, Meng J. Species Association of the Dominant Tree Species in an Old-Growth Forest and Implications for Enrichment Planting for the Restoration of Natural Degraded Forest in Subtropical China. Forests. 2019; 10(11):957. https://doi.org/10.3390/f10110957
Chicago/Turabian StyleLiu, Ziwei, Yan Zhu, Jianjun Wang, Wu Ma, and Jinghui Meng. 2019. "Species Association of the Dominant Tree Species in an Old-Growth Forest and Implications for Enrichment Planting for the Restoration of Natural Degraded Forest in Subtropical China" Forests 10, no. 11: 957. https://doi.org/10.3390/f10110957
APA StyleLiu, Z., Zhu, Y., Wang, J., Ma, W., & Meng, J. (2019). Species Association of the Dominant Tree Species in an Old-Growth Forest and Implications for Enrichment Planting for the Restoration of Natural Degraded Forest in Subtropical China. Forests, 10(11), 957. https://doi.org/10.3390/f10110957





