Selection of Poplar Genotypes for Adapting to Climate Change
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Study Design
2.2. Measurement of Gas Exchange Parameters
2.3. Measurement of Morphological Characteristics
2.4. Statistical Analyses
3. Results
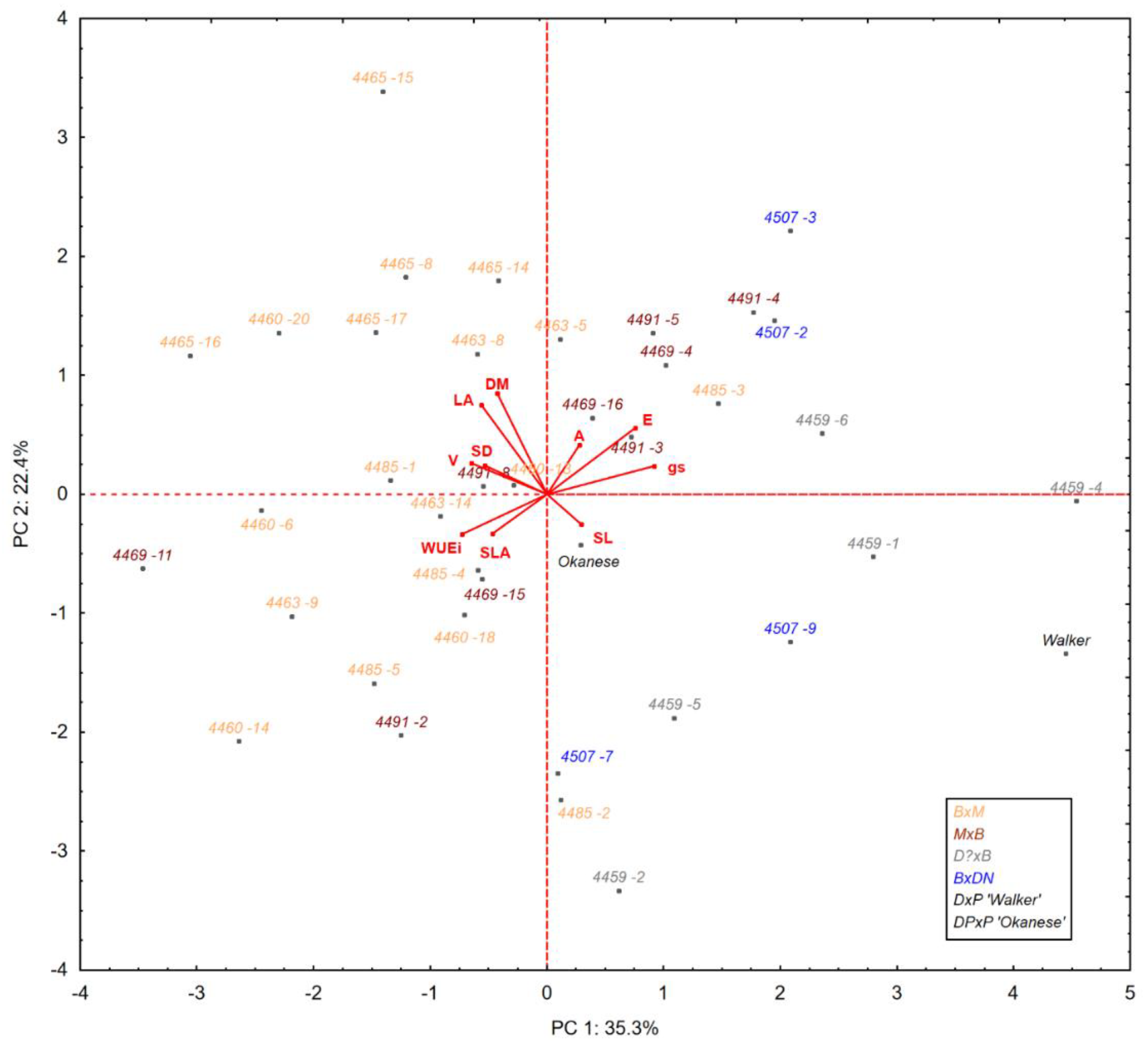
3.1. Relationships between Traits
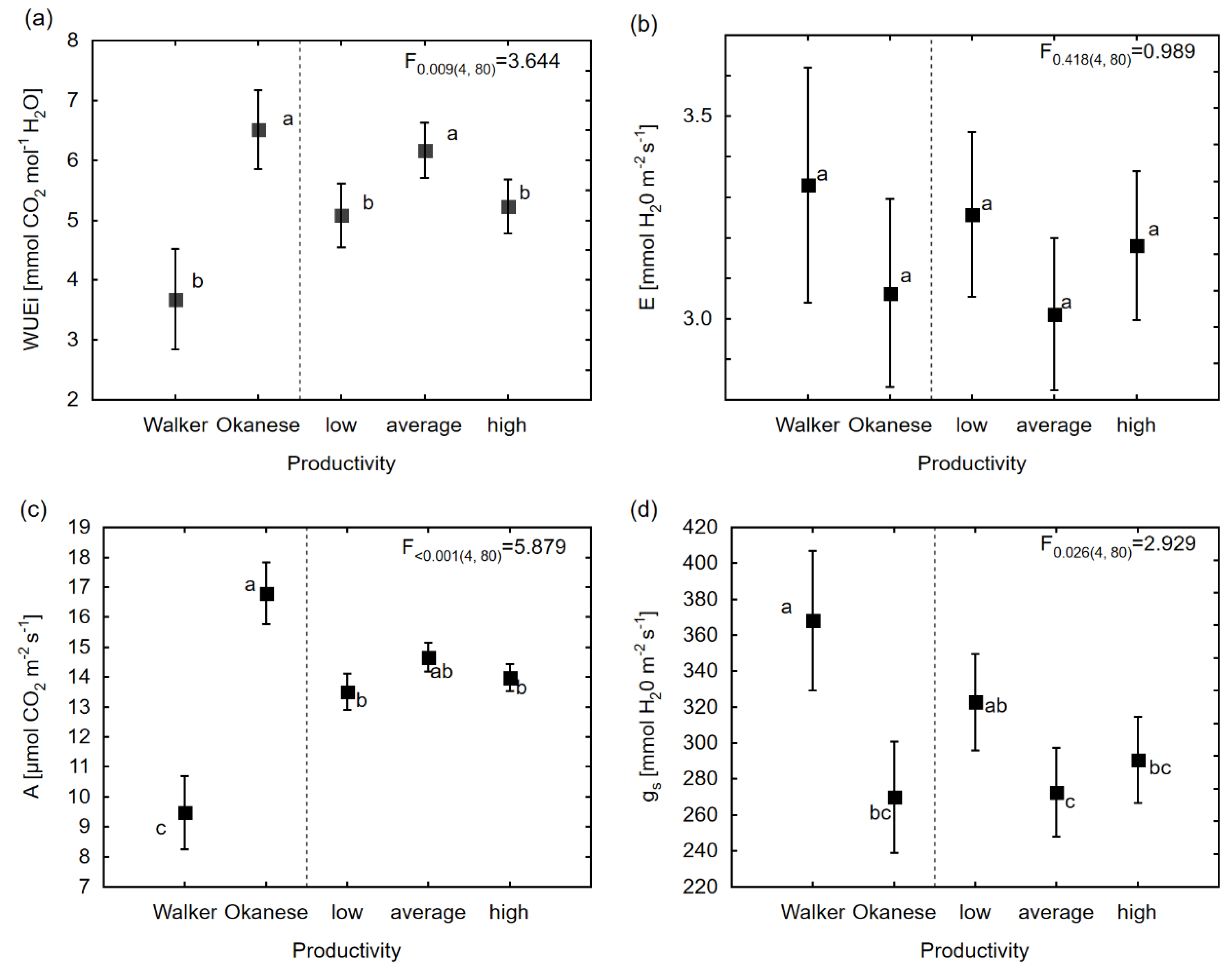
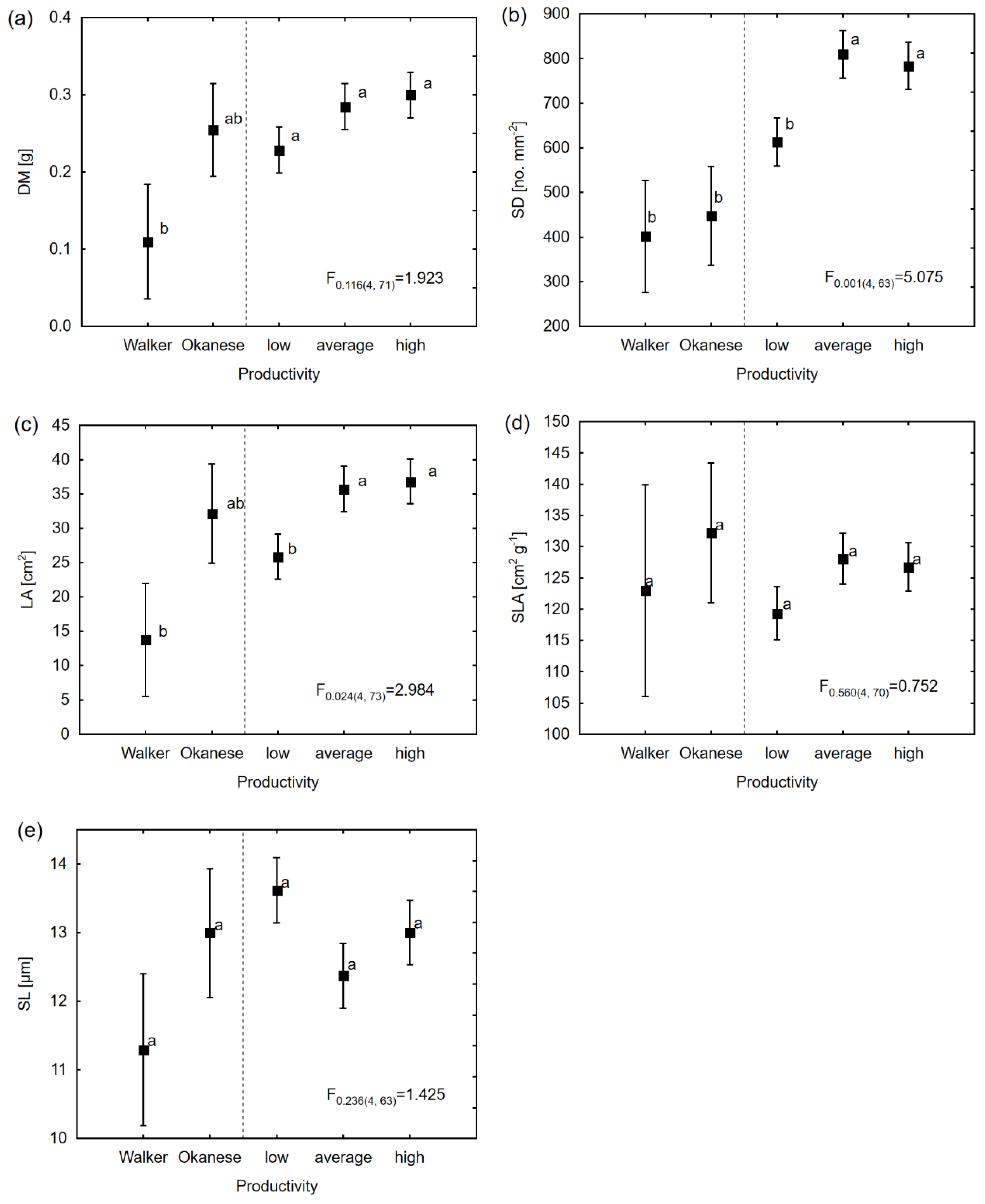
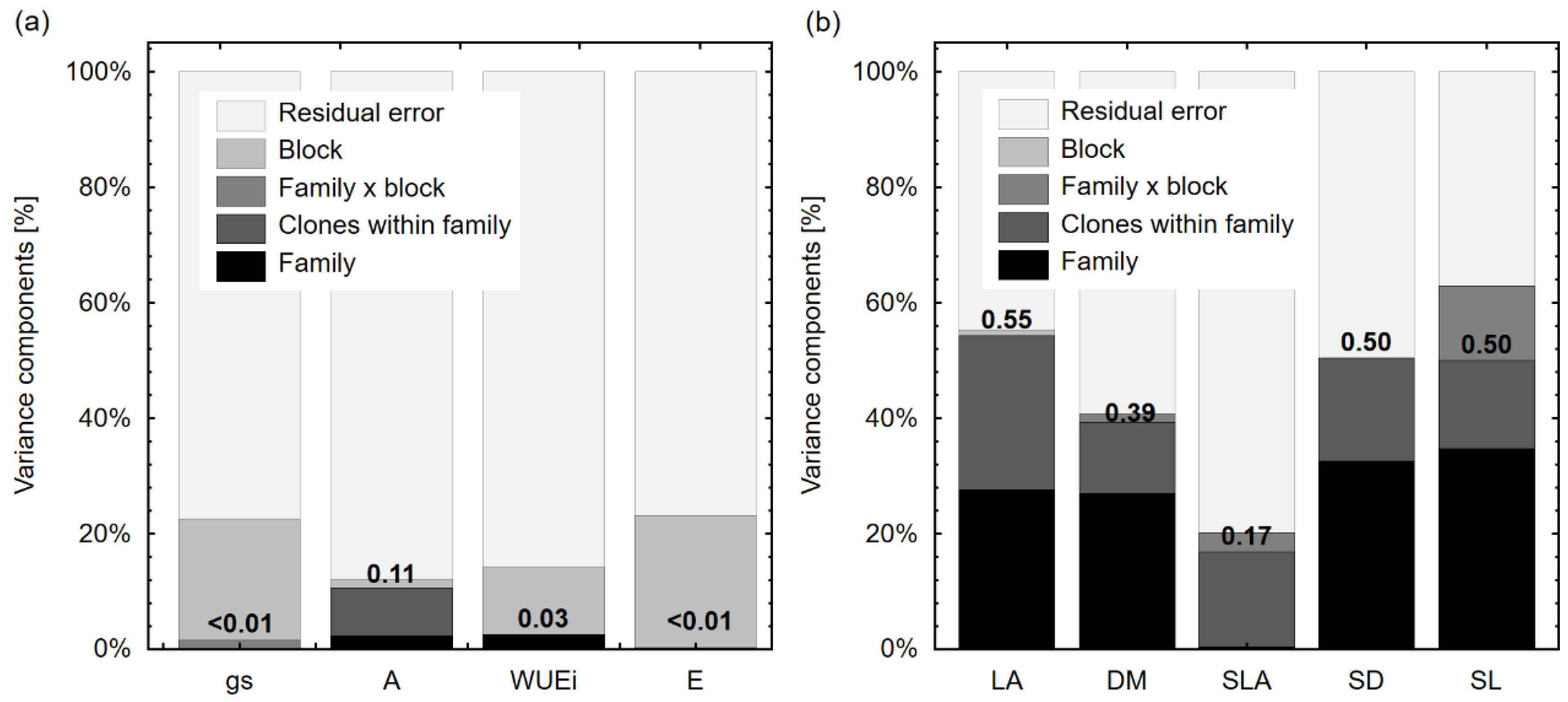
3.2. Genetic Variation in Water Use Physiological and Morphological Characteristics Among Different Productivity Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moran, E.; Lauder, J.; Musser, C.; Stathos, A.; Shu, M. The genetics of drought tolerance in conifers. New Phytol. 2017, 216, 1034–1048. [Google Scholar] [CrossRef]
- Bacelar, E.L.V.A.; Moutinho-Pereira, J.M.; Gonçalves, B.M.C.; Brito, C.V.Q.; Gomes-Laranjo, J.; Ferreira, H.M.F.; Correia, C.M. Water use strategies of plants under drought conditions. In Plant Responses to Drought Stress: From Morphological to Molecular Features; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 145–170. [Google Scholar]
- Hogg, E.H.; Michaelian, M.; Hook, T.I.; Undershultz, M.E. Recent climatic drying leads to age-independent growth reductions of white spruce stands in western Canada. Glob. Chang. Biol. 2017, 23, 5297–5308. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hogg, E.H.; Price, D.T.; Edwards, J.; Williamson, T. Past and projected future changes in moisture conditions in the Canadian boreal forest. Houst. Chron. 2014, 90, 678–691. [Google Scholar] [CrossRef]
- Richardson, J.; Isebrands, J.G.; Ball, J.B. Ecology and physiology of poplars and willows. In Poplars and Willows: Trees for Society and the Environment; Isebrands, J.G., Richardson, J., Eds.; CAB International and FAO: Rome, Italy, 2014; pp. 92–123. [Google Scholar]
- Żelawski, W. Gaseous exchange and water relations. In Topole populus, L.; Białobok, S., Ed.; Państwowe Wydawnictwo Naukowe: Warsaw, Poland, 1973; pp. 204–235. [Google Scholar]
- Stanton, B.J.; Serapiglia, M.J.; Smart, L.B. The domestication and conservation of Populus and Salix genetic resources. In Poplars and Willows: Trees for Society and the Environment; Isebrands, J.G., Richardson, J., Eds.; CAB International and FAO: Rome, Italy, 2014; pp. 124–199. [Google Scholar]
- Stettler, R.F.; Zsuffa, L.; Wu, R. The role of hybridization in the genetic manipulation of Populus. In Biology of Populus; Stettler, R.F., Bradshaw, H.D., Heilmann, P.E., Hinckley, T.M., Eds.; NRC Research Press: Ottawa, ON, Canada, 1996; pp. 87–112. [Google Scholar]
- Hu, Y.; Thomas, B.R. Hormones and heterosis in hybrid balsam poplar (Populus balsamifera L.). Forests 2019, 10, 143. [Google Scholar] [CrossRef]
- Goff, S.A. A unifying theory for general multigenic heterosis: Energy efficiency, protein metabolism, and implications for molecular breeding. New Phytol. 2011, 189, 923–937. [Google Scholar] [CrossRef] [PubMed]
- Blake, T.J.; Tschaplinski, T.J.; Eastham, A. Stomatal control of water use efficiency in poplar clones and hybrids. Can. J. Bot. 1984, 62, 1344–1351. [Google Scholar] [CrossRef]
- Condon, A.G.; Richards, R.A.; Rebetzke, G.J.; Farquhar, G.D. Breeding for high water-use efficiency. J. Exp. Bot. 2004, 55, 2447–2460. [Google Scholar] [CrossRef] [PubMed]
- Medrano, H.; Tomás, M.; Martorell, S.; Flexas, J.; Hernández, E.; Rosselló, J.; Pou, A.; Escalona, J.M.; Bota, J. From leaf to whole-plant water use efficiency (WUE) in complex canopies: Limitations of leaf WUE as a selection target. Crop J. 2015, 3, 220–228. [Google Scholar] [CrossRef]
- Tschaplinski, T.J.; Blake, T.J. Water-stress tolerance and late-season organic solute accumulation in hybrid poplar. Can. J. Bot. 1989, 67, 1681–1688. [Google Scholar] [CrossRef]
- Tschaplinski, T.J.; Tuskan, G.A.; Gunderson, C.A. Water-stress tolerance of black cottonwood and eastern cottonwood clones and four of their hybrid progeny. I. Growth, water relations and gas exchange. Can. J. Res. 1994, 24, 364–371. [Google Scholar] [CrossRef]
- Marron, N.; Villar, M.; Dreyer, E.; Delay, D.; Boudouresque, E.; Petit, J.M.; Delmotte, F.M.; Guehl, J.M.; Brignolas, F. Diversity of leaf traits related to productivity in 31 Populus deltoides x Populus nigra clones. Tree Physiol. 2005, 25, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.R.; Macdonald, S.E.; Dancik, B.P. Variance components, heritabilities and gain estimates for growth chamber and field performance of Populus tremuloides: Gas exchange parameters. Silvae Genet. 1997, 46, 309–317. [Google Scholar]
- Condon, A.G.; Richards, R.A.; Rebetzke, G.J.; Farquhar, G.D. Improving intrinsic water-use efficiency and crop yield. Crop Sci. 2002, 42, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Al Afas, N.; Marron, N.; Ceulemans, R. Clonal variation in stomatal characteristics related to biomass production of 12 poplar (Populus) clones in a short rotation coppice culture. Environ. Exp. Bot. 2006, 58, 279–286. [Google Scholar] [CrossRef]
- Pearce, D.W.; Millard, S.; Bray, D.F.; Rood, S.B. Stomatal characteristics of riparian poplar species in a semi-arid environment. Tree Physiol. 2006, 26, 211–218. [Google Scholar] [CrossRef]
- Arshad, M.; Biswas, K.; Bisgrove, S.; Schroeder, W.R.; Thomas, B.R.; Mansfield, S.D.; Mattsson, J.; Plant, A. Differences in drought resistance in nine North American hybrid poplars. Trees 2019, 33, 1111–1128. [Google Scholar] [CrossRef]
- Condon, A.G.; Farquhar, G.D.; Richards, R.A. Genotypic variation in carbon isotope discrimination and transpiration efficiency in wheat. Leaf gas exchange and whole plant studies. Aust. J. Plant Physiol. 1990, 17, 9–22. [Google Scholar] [CrossRef]
- Ceulemans, R. Genetic Variation in Functional and Structural Productivity Determinants in Poplar; Thesis Publishers: Amsterdam, The Netherlands, 1990; p. 99. [Google Scholar]
- Brendel, O.; Pot, D.; Plomion, C.; Rozenberg, P.; Guehl, J.-M. Genetic parameters and QTL analysis of δ13C and ring width in maritime pine. Plant Cell Environ. 2002, 25, 945–953. [Google Scholar] [CrossRef]
- Pallardy, S.G.; Kozlowski, T.T. Frequency and length of stomata of 21 Populus clones. Can. J. Bot. 1979, 57, 2519–2523. [Google Scholar] [CrossRef]
- Ceulemans, R.; Impens, I.; Steenackers, V. Stomatal and anatomical leaf characteristics of 10 Populus clones. Can. J. Bot. 1984, 62, 513–518. [Google Scholar] [CrossRef]
- Dunlap, J.M.; Stettler, R.F. Variation in leaf epidermal and stomatal traits of Populus trichocarpa from two transects across the Washington Cascades. Can. J. Bot. 2001, 7, 528–536. [Google Scholar] [CrossRef]
- Alberta Climate Information Service. Available online: https://agriculture.alberta.ca/acis/ (accessed on 1 October 2019).
- Niemczyk, M.; Thomas, B.R. Multi-trait selection of hybrid poplars for northern climates. Ann. For. Sci. under review.
- Lindquist, C.H.; Cram, W.H.; Howe, J.A.G. Walker Poplar. Can. J. Plant Sci. 1977, 57, 1019. [Google Scholar] [CrossRef]
- Schroeder, W.; Soolanayakanahally, R.; Lindquist, C. Okanese poplar. Can. J. For. Res. 2013, 93, 1281–1283. [Google Scholar] [CrossRef]
- Ceulemans, R.; van Praet, L.; Jiang, X.N. Effects of CO2 enrichment, leaf position and clone on stomatal index and epidermal cell density in poplar (Populus). New Phytol. 1995, 131, 99–107. [Google Scholar] [CrossRef]
- Fetter, K.C.; Eberhardt, S.; Barclay, R.S.; Wing, S.; Keller, S.R. StomataCounter: A neural network for automatic stomata identification and counting. New Phytol. 2019, 223, 1671–1681. [Google Scholar] [CrossRef]
- Searle, S.R.; Casella, G.; McCulloch, C.E. Variance Components; John Wiley and Sons Inc.: New York, NY, USA, 1992; p. 501. [Google Scholar]
- Zamudio, F.; Wolfinger, R.; Stanton, B.; Guerra, F. The use of linear mixed model theory for the genetic analysis of repeated measures from clonal tests of forest trees. I. A focus on spatially repeated data. Tree Genet. Genomes 2008, 4, 299–313. [Google Scholar] [CrossRef]
- Mbogga, M.S.; Hamann, A.; Wang, T. Historical and projected climate data for natural resource management in western Canada. Agric. Meteorol. 2009, 149, 881–890. [Google Scholar] [CrossRef]
- Niemczyk, M.; Przybysz, P.; Przybysz, K.; Kaliszewski, A.; Wojda, T.; Liesebach, M. Productivity, growth patterns, and cellulosic pulp properties of hybrid aspen clones. Forests 2019, 10, 450. [Google Scholar] [CrossRef]
- Peterson, E.B.; Peterson, N.M. Ecology, Management, and Use of Aspen and Balsam Poplar in the Prairie Provinces, Canada; Northern Forestry Center: Edmonton, AB, Canada, 1992; 252p. [Google Scholar]
- Hamilton, M.A.; Murray, B.R.; Cadotte, M.W.; Hose, G.C.; Baker, A.C.; Harris, C.J.; Licari, D. Life-history correlates of plant invasiveness at regional and continental scales. Ecol. Lett. 2005, 8, 1066–1074. [Google Scholar] [CrossRef]
- Lee, D.W.; Graham, R. Leaf optical properties of rainforest sun and extreme shade plants. Am. J. Bot. 1986, 73, 1100–1108. [Google Scholar] [CrossRef]
- Grotkopp, E.; Rejmánek, M. High seedling relative growth rate and specific leaf area are traits of invasive species: Phylogenetically independent contrasts of woody angiosperms. Am. J. Bot. 2007, 94, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Lake, J.C.; Leishman, M.R. Invasion success of exotic plants in natural ecosystems: The role of disturbance, plant attributes and freedom from herbivores. Biol. Conserv. 2004, 117, 215–226. [Google Scholar] [CrossRef]
- Erice, G.; Sanz-Sáez, A.; Aranjuelo, I.; Irigoyen, J.J.; Sánchez-Díaz, M. Future Environmental Conditions will Limit Yield in N2 Fixing Alfalfa. In Plant Responses to Drought Stress: From Morphological to Molecular Features; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 363–382. [Google Scholar]
- Foster, J.R.; Smith, W.K. Stomatal conductance patterns and environment in high elevation phreatophytes of Wyoming. Can. J. Bot. 1991, 69, 647–655. [Google Scholar] [CrossRef]
- Sparks, J.P.; Black, R.A. Regulation of water loss in populations of Populus trichocarpa: The role of stomatal control in preventing xylem cavitation. Tree Physiol. 1999, 19, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Hetherington, A.M.; Woodward, F.I. The role of stomata in sensing and driving environmental change. Nature 2003, 424, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Bugała, W. Systematyka i zmienność. In Topole populus L.; Białobok, S., Ed.; Państwowe Wydawnictwo Naukowe: Warsaw, Poland, 1973; pp. 9–136. [Google Scholar]
- Braatne, J.H.; Hinckley, T.M.; Stettler, R.F. Influence of soil water on the physiological and morphological components of plant water balance in Populus trichocarpa, Populus deltoides and their F1 hybrids. Tree Physiol. 1992, 11, 325–339. [Google Scholar] [CrossRef]
- Rood, S.B.; Mahoney, J.M. River damming and riparian cottonwoods along the Marias River, Montana. Rivers 1995, 5, 195–207. [Google Scholar] [CrossRef]
- Royer, D.L. Stomatal density and stomatal index as indicators of paleoatmospheric CO2 concentration. Rev. Palaeobot. Palynol. 2001, 114, 1–28. [Google Scholar] [CrossRef]
- Larcher, L.; Hara-Nishimura, I.; Sternberg, L. Effects of stomatal density and leaf water content on the 18O enrichment of leaf water. New Phytol. 2015, 206, 141–151. [Google Scholar] [CrossRef]
- Aasamaa, K.; Sõber, A.; Rahi, M. Leaf anatomical characteristics associated with shoot hydraulic conductance, stomatal conductance and stomatal sensitivity to changes of leaf water status in temperate deciduous trees. Funct. Plant Biol. 2001, 28, 765–774. [Google Scholar] [CrossRef]
- Desrochers, A.; van den Driessche, R.; Thomas, B.R. The interaction between nitrogen source, soil pH, and drought in the growth and physiology of three poplar clones. Botany 2007, 85, 1046–1057. [Google Scholar] [CrossRef]
- Arango-Velez, A.; Zwiazek, J.J.; Thomas, B.R.; Tyree, M.T. Stomatal factors and vulnerability of stem xylem to cavitation in poplars. Physiol. Plant. 2011, 143, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Pliura, A.; Suchockas, V.; Sarsekova, D.; Gudynaite, V. Genotypic variation and heritability of growth and adaptive traits, and adaptation of young poplar hybrids at northern margins of natural distribution of Populus nigra in Europe. Biomass Bioenergy 2014, 70, 513–529. [Google Scholar] [CrossRef]
- Henkel-Johnson, D.; Macdonald, S.E.; Bork, E.W.; Thomas, B.R. Influence of weed composition, abundance, and spatial proximity on growth in young hybrid poplar plantations. Ecol. Manag. 2016, 362, 55–68. [Google Scholar] [CrossRef]
- Goehing, J.; Thomas, B.R.; Macdonald, S.E.; Bork, E.W. Effects of alternative establishment systems on resource availability, understorey composition and tree performance in juvenile hybrid poplar plantations. Forestry 2017, 90, 515–529. [Google Scholar] [CrossRef]
- Jiang, Z.-H.; Wang, X.-Q.; Fei, B.-H.; Ren, H.-Q.; Liu, X.-E. Effect of stand and tree attributes on growth and wood quality characteristics from a spacing trial with Popul Xiaohei. Ann. Sci. 2007, 64, 807–814. [Google Scholar] [CrossRef]
| Productivity Group | Female Parent | Male Parent | Cross Type | Family Codes | Clone Numbers |
|---|---|---|---|---|---|
| high | P. balsamifera | P. maximowiczii | B × M | 4465 | AP 4465 -8; AP 4465 -14; AP 4465 -15; AP 4465 -16; AP 4465 -17 |
| 4485 | AP 4485 -1; AP 4485 -2; AP 4485 -3; AP 4485 -4; AP 4485 -5 | ||||
| P. maximowiczii | P. balsamifera | M × B | 4491 | AP 4491 -2; AP 4491 -3; AP 4491 -4; AP 4491 -5; AP 4491 -8 | |
| average | P. balsamifera | P. maximowiczii | B × M | 4460 | AP 4460 -6; AP 4460 -13; AP 4460 -14; AP 4460 -18; AP 4460 -20 |
| 4463 | AP 4463 -2; AP 4463 -5; AP 4463 -8; AP 4463 -9; AP 4463 -14 | ||||
| P. maximowiczii | P. balsamifera | M × B | 4469 | AP 4469 -4; AP 4469 -11; AP 4469 -15; AP 4469 -16; | |
| low | (P. deltoides × P. nigra) × P. balsamifera | P. balsamifera | (DN)B × B | 4483 | AP 4483 -1; AP 4483 -4; AP 4483 -5; AP 4483 -7; AP 4483 -8 |
| P. balsamifera | P. deltoides × P. nigra | B × DN | 4459 | AP 4459 -1; AP 4459 -2; AP 4459 -4; AP 4459 -5; AP 4459 -6 | |
| P. deltoides | P. balsamifera | D? × B * | 4507 | AP 4507 -2; AP 4507 -3; AP 4507 -7; AP 4507 -9 | |
| Okanese | P. deltoides × P. × petrowskyana (“Walker”) | P. × petrowskyana | DP × P | 2403 | AP 2403 |
| Walker | P. deltoides | P. × petrowskyana | D × P | 24 | AP 24 |
| Trait 1 | Mean | CV [%] | gs | A | WUEi | E | V | LA | DM | SLA | SD |
|---|---|---|---|---|---|---|---|---|---|---|---|
| gs | 291.66 | 25.97 | |||||||||
| A | 13.97 | 13.87 | 0.39 | ||||||||
| WUEi | 5.51 | 22.27 | −0.75 | 0.06 | |||||||
| E | 3.15 | 12.36 | 0.85 | 0.37 | −0.79 | ||||||
| V | 65.58 | 62.83 | −0.44 | 0.01 | 0.31 | −0.26 | |||||
| LA | 32.87 | 28.75 | −0.32 | 0.16 | 0.17 | 0.03 | 0.53 | ||||
| DM | 0.27 | 28.42 | −0.23 | 0.16 | 0.04 | 0.11 | 0.35 | 0.91 | |||
| SLA | 125.37 | 10.98 | −0.36 | −0.23 | 0.35 | −0.37 | 0.39 | 0.21 | −0.15 | ||
| SD | 734.85 | 21.66 | −0.48 | −0.20 | 0.16 | −0.27 | 0.25 | 0.34 | 0.39 | −0.05 | |
| SL | 12.82 | 7.93 | 0.18 | 0.21 | −0.05 | 0.11 | −0.17 | −0.22 | −0.29 | 0.07 | −0.14 |
| Trait 1 | PC 1 | PC 2 | R2 |
|---|---|---|---|
| gs | 0.92 | 0.24 | 0.90 |
| DM | −0.42 | 0.85 | 0.90 |
| E | 0.76 | 0.56 | 0.89 |
| LA | −0.56 | 0.75 | 0.88 |
| WUEi | −0.73 | −0.33 | 0.64 |
| V | −0.65 | 0.26 | 0.49 |
| SD | −0.53 | 0.24 | 0.34 |
| SLA | −0.47 | −0.33 | 0.32 |
| A | 0.28 | 0.42 | 0.25 |
| SL | 0.29 | −0.25 | 0.15 |
| Trait 2 | Random Effects | Fixed Effect | Covariate | |||||
|---|---|---|---|---|---|---|---|---|
| Variance Components ± SE 1 | F | |||||||
| Productivity | VPD | |||||||
| Physiological | gs | <0.001 ± <0.001 | <0.001 ± <0.001 | 131.93 ± 576.18 | 1787.55 ± 1620.79 | 6596.62 ± 907.41 * | 2.93 * | 149.79 * |
| A | 0.15 ± 0.41 | 0.51 ± 0.59 | <0.001 ± <0.001 | 0.09 ± 0.19 | 5.44 ± 0.74 * | 5.88 * | 103.85 * | |
| E | <0.001 ± <0.001 | <0.001 ± <0.001 | 0.001±0.027 | 0.108±0.097 | 0.364 ± 0.049 * | 0.99 | 24.39 * | |
| WUEi | 0.10 ± 0.20 | <0.001 ± <0.001 | <0.001 ± <0.001 | 0.44 ± 0.44 | 3.21 ± 0.39 * | 3.64 * | 30.88 * | |
| Morphological | LA | 24.17 ± 18.33 | 23.31 ± 9.38 * | <0.001 ± <0.001 | 0.79 ± 1.54 | 39.11 ± 5.58 * | 2.98 * | - |
| DM | 2.11 × 10−3 ± 1.55 × 10−3 | 9.56 × 10−4 ± 6.86 × 10−4 * | 1.16 × 10−4 ± 3.78 × 10−4 | <0.001 ± <0.001 | 4.61× 10−4 ± 7.29 × 10−4 * | 1.92 | ||
| SLA | 1.92 ± 32.43 | 77.98 ± 49.57 | 15.72 ± 34.62 | <0.001 ± <0.001 | 379.18 ± 60.74 * | 0.75 | ||
| SD | 6855.76 ± 4833.98 | 3758.52 ± 1905.00 * | <0.001 ± <0.001 | <0.001 ± <0.001 | 10425.4 ± 1531.30 | 5.07* | ||
| SL | 0.52 ± 0.38 | 0.23 ± 0.11 * | 0.19 ± 0.11 * | <0.001 ± <0.001 | 0.56 ± 0.1 * | 1.42 | ||
| V | <0.001 ± <0.001 | 737.62 ± 217.91 * | 22.18 ± 54.07 | 7.35 ± 23.98 | 640.03 ± 100.23 * | 8.8 3* | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niemczyk, M.; Hu, Y.; Thomas, B.R. Selection of Poplar Genotypes for Adapting to Climate Change. Forests 2019, 10, 1041. https://doi.org/10.3390/f10111041
Niemczyk M, Hu Y, Thomas BR. Selection of Poplar Genotypes for Adapting to Climate Change. Forests. 2019; 10(11):1041. https://doi.org/10.3390/f10111041
Chicago/Turabian StyleNiemczyk, Marzena, Yue Hu, and Barb R. Thomas. 2019. "Selection of Poplar Genotypes for Adapting to Climate Change" Forests 10, no. 11: 1041. https://doi.org/10.3390/f10111041
APA StyleNiemczyk, M., Hu, Y., & Thomas, B. R. (2019). Selection of Poplar Genotypes for Adapting to Climate Change. Forests, 10(11), 1041. https://doi.org/10.3390/f10111041





