Effect of Soil Fauna on Home-Field Advantages of Litter Mass Loss and Nutrient Release in Different Temperate Broad-Leaved Forests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Set Up
2.3. Statistical Analyses
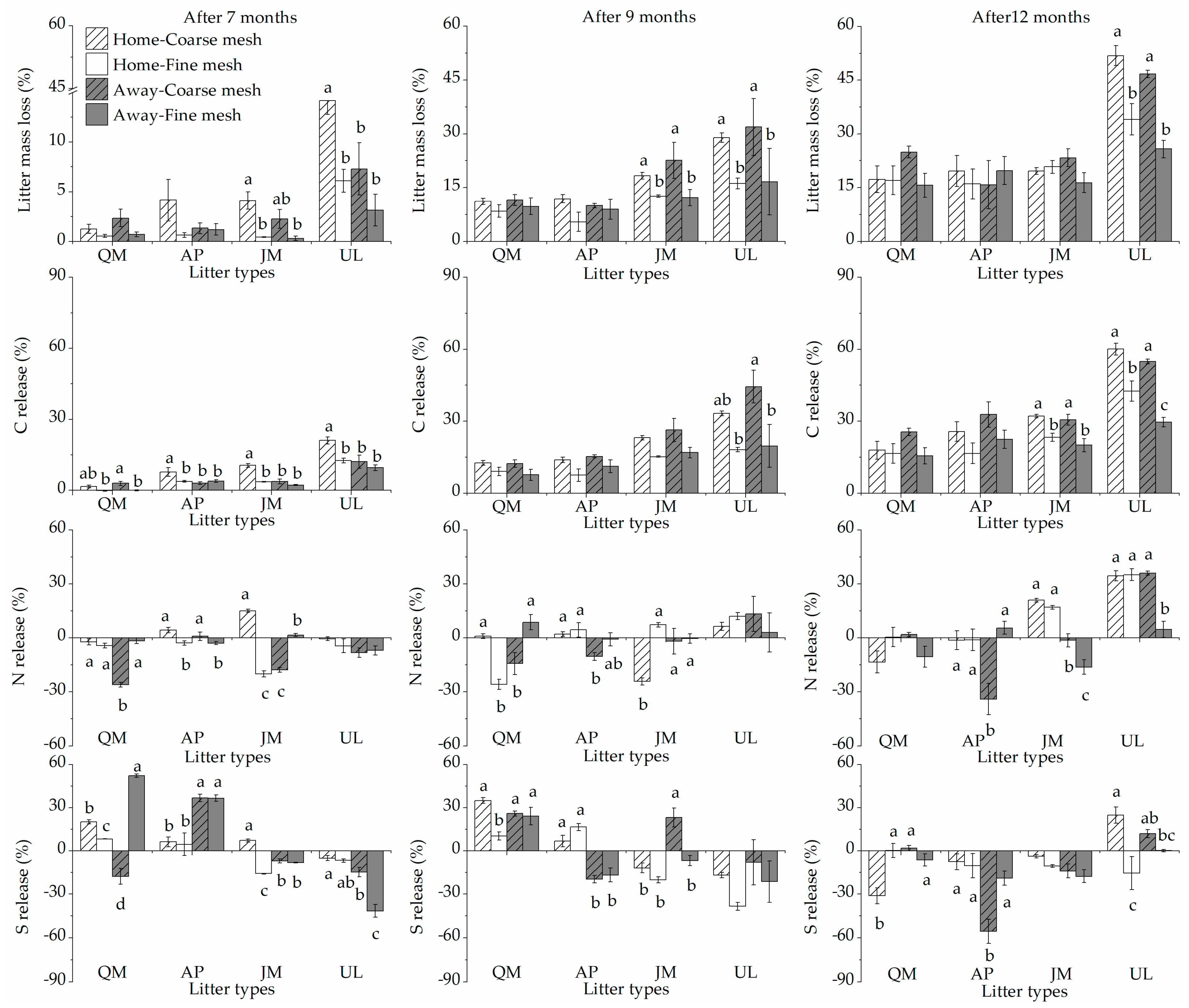
3. Results
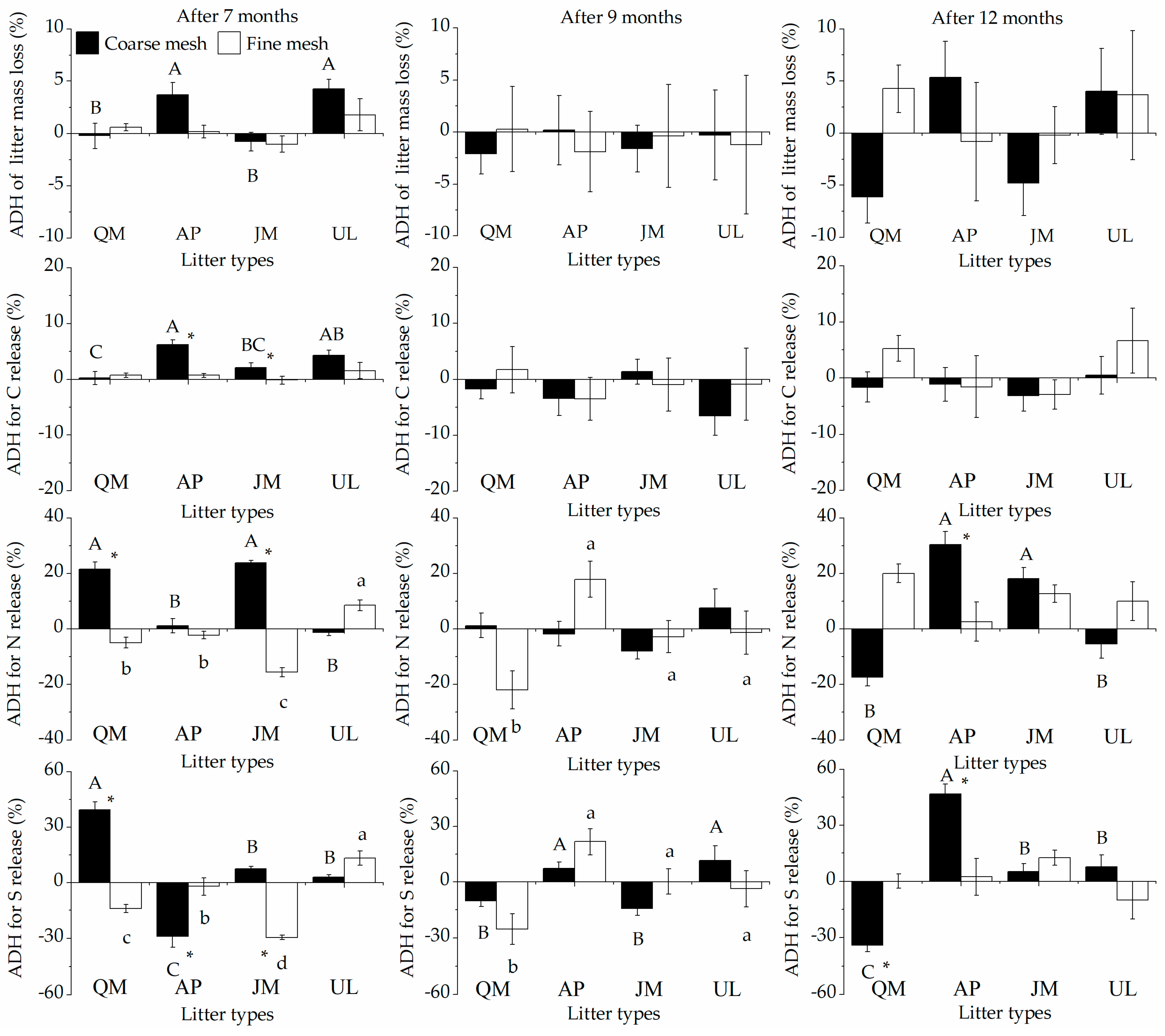
3.1. Litter Mass Losses and HFA
3.2. Litter Nutrient Release and HFA
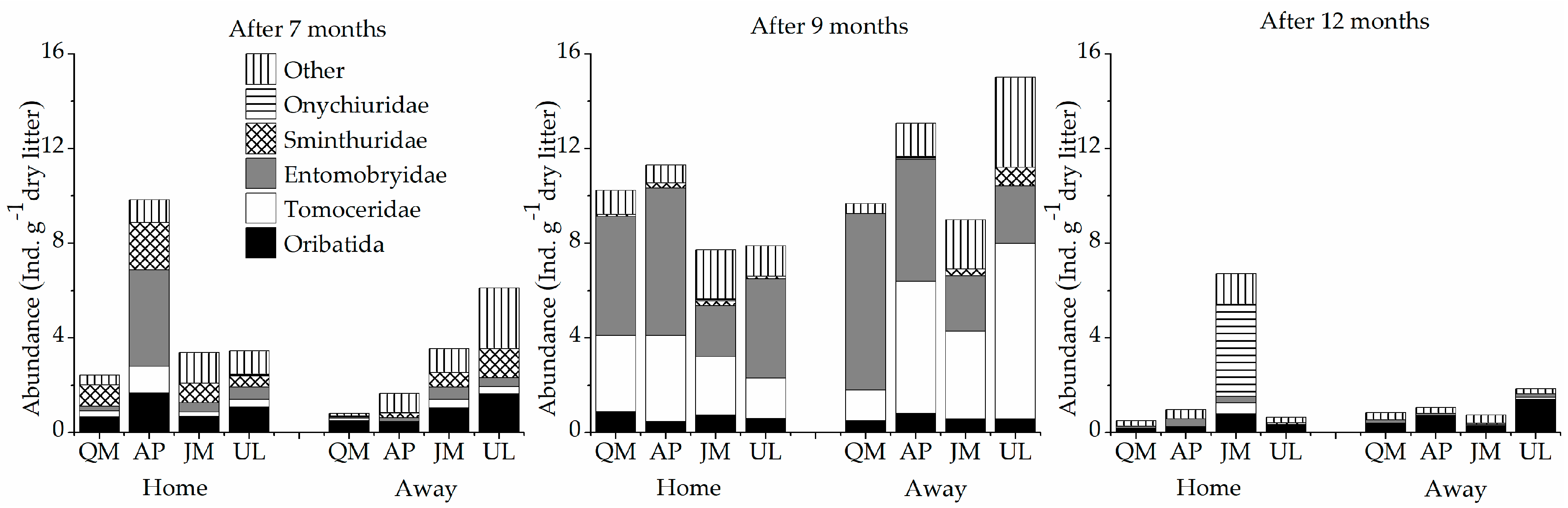
3.3. Soil Fauna Community Composition and Diversity
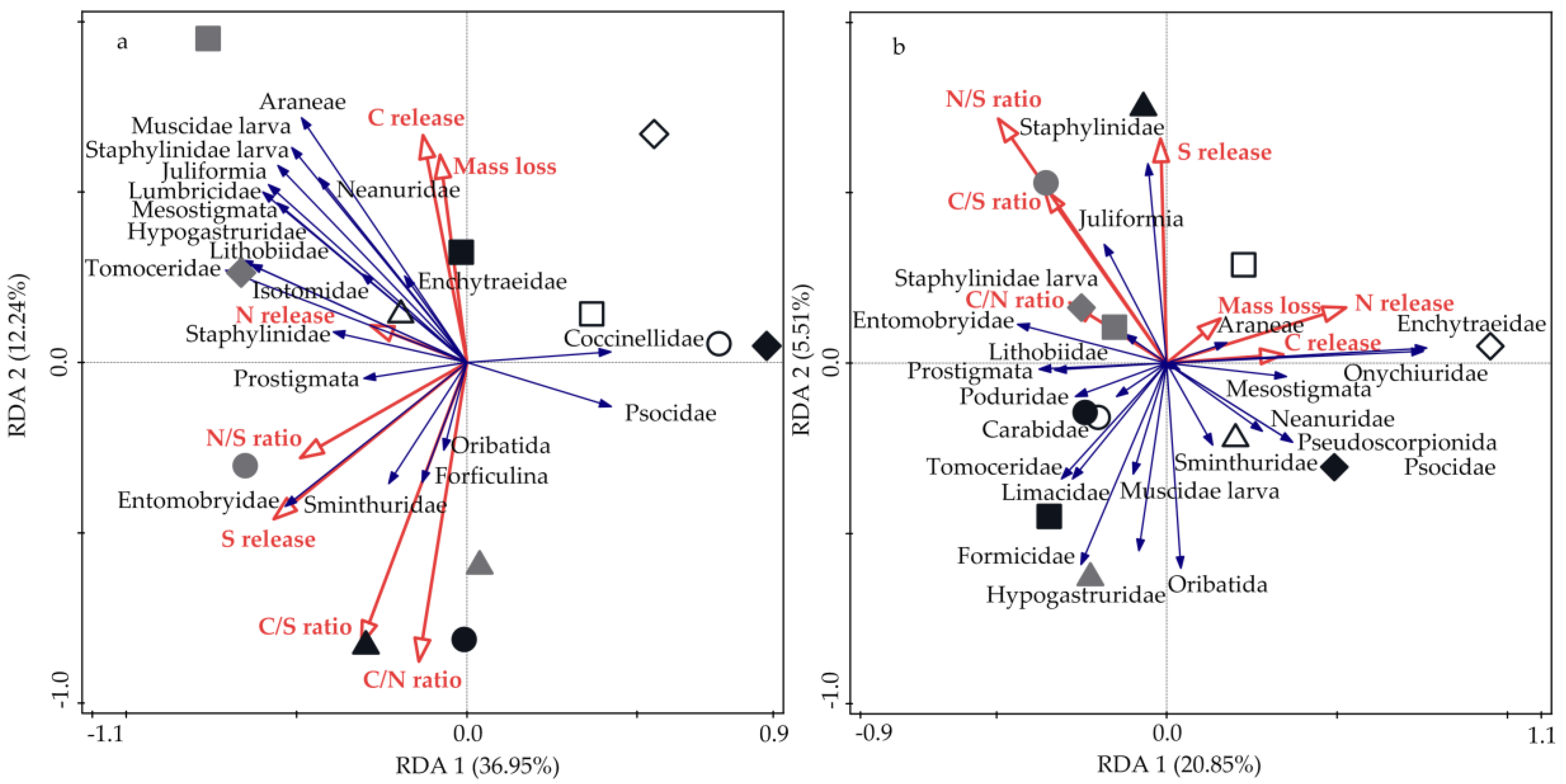
3.4. Relationships between Soil Fauna, Litter Mass Loss, and Nutrient Release
4. Discussion
4.1. Effects of Soil Fauna on the HFA of Litter Mass Losses
4.2. Effects of Soil Fauna on the HFA of Litter Nutrient Release
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Berg, B.; McClaugherty, C. Plant Litter: Decomposition, Humus Formation, Carbon Sequestration, 3rd ed.; Springer: Berlin, Germany, 2014. [Google Scholar]
- Ayres, E.; Steltzer, H.; Simmons, B.L.; Simpson, R.T.; Steinweg, J.M.; Wallenstein, M.D.; Mellor, N.; Parton, W.J.; Moore, J.C.; Wall, D.H. Home-field advantage accelerates leaf litter decomposition in forests. Soil Biol. Biochem. 2009, 41, 606–610. [Google Scholar] [CrossRef]
- Austin, A.T.; Vivanco, L.; González-Arzac, A.; Pérez, L.I. There’s no place like home? An exploration of the mechanisms behind plant litter–decomposer affinity in terrestrial ecosystems. New Phytol. 2014, 204, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Chomel, M.; Guittonny-Larchevêque, M.; Desrochers, A.; Baldy, V. Home field advantage of litter decomposition in pure and mixed plantations under boreal climate. Ecosystems 2015, 18, 1014–1028. [Google Scholar] [CrossRef]
- Palozzi, J.E.; Lindo, Z. Pure and mixed litters of Sphagnum and Carex exhibit a home-field advantage in Boreal peatlands. Soil Biol. Biochem. 2017, 115, 161–168. [Google Scholar] [CrossRef]
- Li, Y.B.; Li, Q.; Yang, J.J.; Lü, X.T.; Liang, W.J.; Han, X.G.; Bezemer, T.M. Home-field advantages of litter decomposition increase with increasing N deposition rates: A litter and soil perspective. Funct. Ecol. 2017, 31, 1792–1801. [Google Scholar] [CrossRef]
- Lin, H.; He, Z.H.; Hao, J.W.; Tian, K.; Jia, X.Q.; Kong, X.S.; Akbar, S.; Bei, Z.L.; Tian, X.J. Effect of N addition on home-field advantage of litter decomposition in subtropical forests. For. Ecol. Manag. 2017, 398, 216–225. [Google Scholar] [CrossRef]
- Yu, Z.P.; Huang, Z.Q.; Wang, M.H.; Liu, R.Q.; Zheng, L.J.; Wan, X.H.; Hu, Z.H.; Davis, M.R.; Lin, T.C. Nitrogen addition enhances home-field advantage during litter decomposition in subtropical forest plantations. Soil Biol. Biochem. 2015, 90, 188–196. [Google Scholar] [CrossRef]
- Gieβelmann, U.C.; Martins, K.G.; Brändlea, M.; Schädler, M.; Marques, R.; Brandl, R. Lack of home-field advantage in the decomposition of leaf litter in the Atlantic Rainforest of Brazil. Appl. Soil Ecol. 2011, 49, 5–10. [Google Scholar] [CrossRef]
- St. John, M.G.; Orwin, K.H.; Dickie, I.A. No ‘home’ versus ‘away’ effects of decomposition found in a grassland-forest reciprocal litter transplant study. Soil Biol. Biochem. 2011, 43, 1482–1489. [Google Scholar] [CrossRef]
- Yin, X.Q.; Song, B.; Dong, W.H.; Xin, W.D.; Wang, Y.Q. A review on the eco-geography of soil fauna in China. J. Geogr. Sci. 2010, 20, 333–346. [Google Scholar] [CrossRef]
- Meyer, I.I.I.W.M.; Ostertag, R.; Cowie, R.H. Macro-invertebrates accelerate litter decomposition and nutrient release in a Hawaiian rainforest. Soil Biol. Biochem. 2011, 43, 206–211. [Google Scholar] [CrossRef]
- Zhao, J.; Wan, S.Z.; Li, Z.A.; Shao, Y.H.; Xu, G.L.; Liu, Z.F.; Zhou, L.X.; Fu, S.L. Dicranopteris-dominated understory as major driver of intensive forest ecosystem in humid subtropical and tropical region. Soil Biol. Biochem. 2012, 4, 78–87. [Google Scholar] [CrossRef]
- Li, X.Q.; Yin, X.Q.; Wang, Z.H.; Fan, W.H. Interaction between decomposing litter and soil fauna of the Betula ermanii forest floor of the Changbai Mountains, China. Can. J. For. Res. 2014, 44, 1507–1514. [Google Scholar] [CrossRef]
- Li, X.Q.; Yin, X.Q.; Wang, Z.H.; Fan, W.H. Litter mass loss and nutrient release influenced by soil fauna of Betula ermanii forest floor of the Changbai Mountains, China. Appl. Soil Ecol. 2015, 95, 15–22. [Google Scholar] [CrossRef]
- Kampichler, C.; Bruckner, A. The role of microarthropods in terrestrial decomposition: A meta-analysis of 40 years of litterbag studies. Biol. Rev. 2009, 84, 375–389. [Google Scholar] [CrossRef]
- Perez, G.; Aubert, M.; Decaëns, T.; Trap, J.; Chauvat, M. Home-field advantage: A matter of interaction between litter biochemistry and decomposer biota. Soil Biol. Biochem. 2013, 67, 245–254. [Google Scholar] [CrossRef]
- Zha, T.G.; Zhang, Z.Q.; Sun, G.; Wang, G.M.; Yun, X.Q.; Wang, Y.K.; Liu, Y. Home-field advantage of litter decomposition and its soil biological driving mechanism: A review. Acta Ecol. Sin. 2012, 32, 7991–8000. [Google Scholar]
- Liao, S.; Ni, X.Y.; Yang, W.Q.; Li, H.; Wang, B.; Fu, C.K.; Xu, Z.F.; Tan, B.; Wu, F.Z. Water, rather than temperature, dominantly impacts how soil fauna affect dissolved carbon and nitrogen release from fresh litter during early litter decomposition. Forests 2016, 7, 249. [Google Scholar] [CrossRef]
- Swift, M.J.; Heal, O.W.; Anderson, J.M. Decompositi on in Terrestrial Ecosystems; University of California Press: Berkeley, CA, USA; Los Angeles, CA, USA, 1979. [Google Scholar]
- Xu, Y.; Fan, J.; Ding, W.; Gunina, A.; Chen, Z.; Bol, R.; Luo, J.; Bolan, N. Characterization of organic carbon in decomposing litter exposed to nitrogen and sulfur additions: Links to microbial community composition and activity. Geoderma 2017, 286, 116–124. [Google Scholar] [CrossRef]
- Wallwork, J.A. The Distribution and Diversity of Soil Fauna; Academic Press: New York, NY, USA, 1976. [Google Scholar]
- Yin, W.Y. Pictorial Keys to Soil Animals of China; Science Press: Beijing, China, 1998. [Google Scholar]
- Šmilauer, P.; Lepš, J. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Veen, G.F.; Sundqvist, M.K.; Wardle, D.A. Environmental factors and traits that drive plant litter decomposition do not determine home-field advantage effects. Funct. Ecol. 2015, 29, 981–991. [Google Scholar] [CrossRef]
- Strickland, M.S.; Osbern, E.; Lauber, C.; Fierer, N.; Bradford, M.A. Litter quality is in the eye of the beholder: Initial decomposition rates as a function of inoculum characteristics. Funct. Ecol. 2009, 23, 627–636. [Google Scholar] [CrossRef]
- González, G.; Seastedt, T.R. Comparison of the abundance and comparison of litter fauna in tropical and subalpine forests. Pedobiologia 2000, 44, 545–555. [Google Scholar] [CrossRef]
- Freschet, G.T.; Aerts, R.; Cornelissen, J.H.C. Multiple mechanisms for trait effects on litter decomposition: Moving beyond home field advantage with a new hypothesis. J. Ecol. 2012, 100, 619–630. [Google Scholar] [CrossRef]
- Schädler, M.; Brandl, R. Do invertebrate decomposers affect the disappearance rate of litter mixtures. Soil Biol. Biochem. 2005, 37, 329–337. [Google Scholar] [CrossRef]
- Wang, W.J.; Li, X.Q.; Nie, R.Y.; Gao, R.; Zhang, X.; Dai, Y.J. Effects of different forest types on meso-micro soil faunal diversity. Hans. J. Soil Sci. 2019, 7, 112–120. [Google Scholar] [CrossRef]
- Prescott, C.E. Litter decomposition: What controls it and how can we alter it to sequester more carbon in forest soils? Biogeochemistry 2010, 101, 133–149. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.F.; He, R.L.; Chen, Y.M.; Xu, Z.F.; Tan, B.; Zhang, L.; Xiao, J.J.; Zhu, P.; Chen, L.H.; et al. Higher soil fauna abundance accelerates litter carbon release across an alpine forest-tundra ecotone. Sci. Rep. 2019, 9, 10561. [Google Scholar] [CrossRef]
- Lin, D.M.; Pang, M.; Fanin, N.; Wang, H.J.; Qian, S.H.; Zhao, L.; Yang, Y.C.; Mi, X.C.; Ma, K.P. Fungi participate in driving home-field advantage of litter decomposition in a subtropical forest. Plant Soil 2019, 434, 467–480. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Chan, K.F. Experimental evidence that soil fauna enhance nutrient mineralization and plant nutrient uptake in montane grassland ecosystems. Soil Biol. Biochem. 1999, 31, 1007–1014. [Google Scholar] [CrossRef]
- Irmler, U. Changes in the fauna and its contribution to mass loss and N release during leaf litter decomposition in two deciduous forests. Pedobiologia 2000, 44, 105–118. [Google Scholar] [CrossRef]
- González, G.; Seastedt, T.R. Soil fauna and plant litter decomposition in tropical and subalpine forests. Ecology 2001, 82, 955–964. [Google Scholar] [CrossRef]
- Parton, W.J.; Silver, W.L.; Burke, I.C.; Grassens, L.; Harmon, M.E.; Currie, W.S.; King, J.Y.; Adair, E.C.; Brandt, L.A.; Hart, S.C.; et al. Global-scale similarities in nitrogen release patterns during long-term decomposition. Science 2007, 315, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Ayres, E.; Steltzer, H.; Berg, S.; Wall, D.H. Soil biota accelerate decomposition in high-elevation forests by specializing in the breakdown of litter produced by the plant species above them. J. Ecol. 2009, 97, 901–912. [Google Scholar] [CrossRef]
- Wang, C.Y.; Guo, P.; Han, G.M.; Feng, X.G.; Zhang, P.; Tian, X.J. Effect of simulated acid rain on the litter decomposition of Quercus acutissima and Pinus massoniana in forest soil microcosms and the relationship with soil enzyme activities. Sci. Total Environ. 2010, 408, 2706–2713. [Google Scholar] [CrossRef] [PubMed]
- Ayres, E.; Dromph, K.M.; Bardgett, R.D. Do plant species encourage soil biota that specialise in the rapid decomposition of their litter? Soil Biol. Biochem. 2006, 38, 183–186. [Google Scholar] [CrossRef]
| Source of Variance | df | Mass Loss | C Release | N Release | S Release | ||||
|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | ||
| Between subjects | |||||||||
| Litter types (LT) | 3 | 49.36 | <0.001 | 95.04 | <0.001 | 40.33 | <0.001 | 33.07 | <0.001 |
| Home vs. away (HA) | 1 | 0.20 | 0.655 | 0.07 | 0.801 | 23.35 | <0.001 | 0.04 | 0.853 |
| Mesh size (MS) | 1 | 35.84 | <0.001 | 86.53 | <0.001 | 0.57 | 0.455 | 5.38 | 0.027 |
| LT × HA | 3 | 1.21 | 0.321 | 1.74 | 0.178 | 3.06 | 0.042 | 4.60 | 0.009 |
| LT × MS | 3 | 7.80 | <0.001 | 8.60 | <0.001 | 5.56 | 0.003 | 19.44 | <0.001 |
| HA × MS | 1 | 0.60 | 0.808 | 0.46 | 0.501 | 6.12 | 0.019 | 8.69 | 0.006 |
| LT × HA × MS | 3 | 1.24 | 0.310 | 0.71 | 0.554 | 9.41 | <0.001 | 2.07 | 0.124 |
| Within subjects | |||||||||
| Sampling period (SP) | 2 | 217.44 | <0.001 | 308.61 | <0.001 | 24.85 | <0.001 | 28.47 | <0.001 |
| SP × LT | 6 | 8.19 | <0.001 | 8.72 | <0.001 | 19.83 | <0.001 | 45.26 | <0.001 |
| SP × HA | 2 | 1.18 | 0.304 | 4.50 | 0.026 | 14.87 | <0.001 | 3.46 | 0.037 |
| SP × MS | 2 | 2.50 | 0.107 | 10.52 | <0.001 | 2.65 | 0.096 | 6.47 | 0.003 |
| SP × LT × HA | 6 | 0.63 | 0.659 | 3.45 | 0.013 | 5.93 | <0.001 | 24.77 | <0.001 |
| SP × LT × MS | 6 | 1.67 | 0.166 | 1.57 | 0.193 | 9.62 | <0.001 | 6.28 | <0.001 |
| SP × HA × MS | 2 | 0.85 | 0.404 | 2.88 | 0.081 | 15.00 | <0.001 | 3.96 | 0.024 |
| SP × LT × HA × MS | 6 | 0.46 | 0.780 | 0.45 | 0.786 | 1798 | <0.001 | 17.52 | <0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Dong, W.; Song, Y.; Wang, W.; Zhan, W. Effect of Soil Fauna on Home-Field Advantages of Litter Mass Loss and Nutrient Release in Different Temperate Broad-Leaved Forests. Forests 2019, 10, 1033. https://doi.org/10.3390/f10111033
Li X, Dong W, Song Y, Wang W, Zhan W. Effect of Soil Fauna on Home-Field Advantages of Litter Mass Loss and Nutrient Release in Different Temperate Broad-Leaved Forests. Forests. 2019; 10(11):1033. https://doi.org/10.3390/f10111033
Chicago/Turabian StyleLi, Xiaoqiang, Weihua Dong, Yang Song, Weijie Wang, and Weiluan Zhan. 2019. "Effect of Soil Fauna on Home-Field Advantages of Litter Mass Loss and Nutrient Release in Different Temperate Broad-Leaved Forests" Forests 10, no. 11: 1033. https://doi.org/10.3390/f10111033
APA StyleLi, X., Dong, W., Song, Y., Wang, W., & Zhan, W. (2019). Effect of Soil Fauna on Home-Field Advantages of Litter Mass Loss and Nutrient Release in Different Temperate Broad-Leaved Forests. Forests, 10(11), 1033. https://doi.org/10.3390/f10111033





