The Effects of Trichoderma Fungi on the Tunneling, Aggregation, and Colony-Initiation Preferences of Black-Winged Subterranean Termites, Odontotermes formosanus (Blattodea: Termitidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Termites
2.2. Soil and Sand
2.3. Trichoderma Fungi
2.4. Tunneling-Choice Test
2.5. Aggregation-Choice Test
2.6. Colony-Initiation-Choice Test
2.7. Data Analyses
3. Results
3.1. Tunneling-Choice Tests
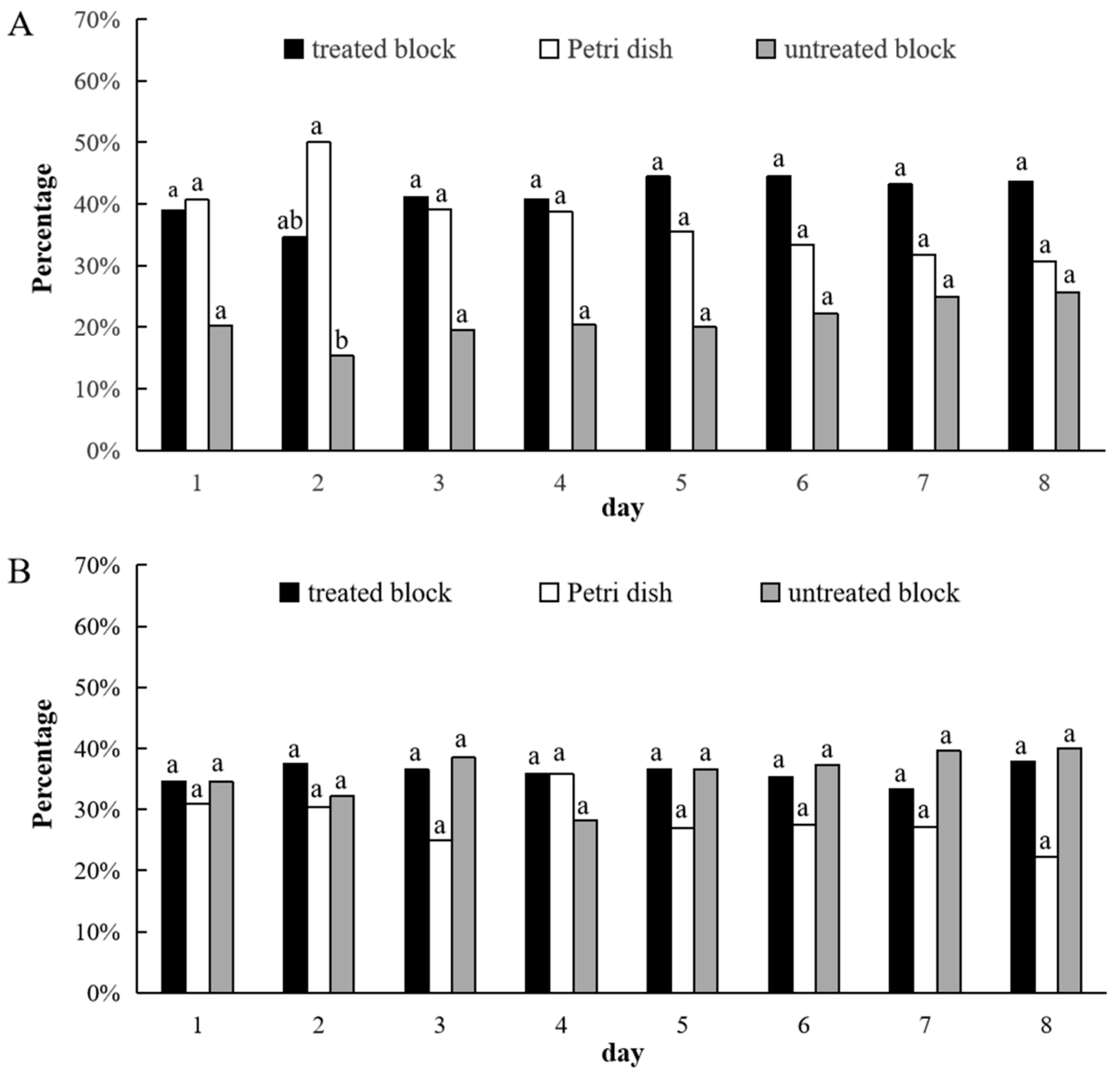
3.2. Aggregation-Choice Test
3.3. Colony-Initiation-Choice Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rust, M.K.; Su, N.Y. Managing social insects of urban importance. Annu. Rev. Entomol. 2012, 57, 355–375. [Google Scholar] [CrossRef] [PubMed]
- Denier, D.; Bulmer, M.S. Variation in subterranean termite susceptibility to fatal infections by local Metarhizium soil isolates. Insects Soc. 2015, 62, 219–226. [Google Scholar] [CrossRef]
- Hussain, A.; Li, Y.F.; Cheng, Y.; Liu, Y.; Chen, C.C.; Wen, S.Y. Immune-related transcriptome of Coptotermes formosanus Shiraki workers: The defense mechanism. PLoS ONE 2013, 8, e69543. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, C.; Lay, F.; Bulmer, M.S. Subterranean termite prophylactic secretions and external antifungal defenses. J. Insect Physiol. 2011, 57, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Peterson, B.F.; Scharf, M.E. Lower termite associations with microbes: Synergy, protection, and interplay. Front. Microbiol. 2016, 7, 422. [Google Scholar] [CrossRef]
- Chouvenc, T.; Elliott, M.L.; Šobotník, J.; Efstathion, C.A.; Su, N.Y. The termite fecal nest: A framework for the opportunistic acquisition of beneficial soil Streptomyces (Actinomycetales: Streptomycetaceae). Environ. Entomol. 2018, 47, 1431–1439. [Google Scholar] [CrossRef]
- Myles, T.G. Alarm, aggregation, and defense by Reticulitermes flavipes in response to a naturally occurring isolate of Metarhizium anisopliae. Sociobiology 2002, 40, 243–256. [Google Scholar]
- Bulmer, M.S.; Franco, B.A.; Fields, E.G. Subterranean termite social alarm and hygienic responses to fungal pathogens. Insects 2019, 10, 240. [Google Scholar] [CrossRef]
- Mankowski, M.E.; Kaya, H.K.; Grace, K.J.; Sipes, B. Differential susceptibility of subterranean termite castes to entomopathogenic nematodes. Biocontrol Sci. Technol. 2005, 15, 367–377. [Google Scholar] [CrossRef]
- Yanagawa, A.; Yokohari, F.; Shimizu, S. Influence of fungal odor on grooming behavior of the termite, Coptotermes formosanus. J. Insect Sci. 2010, 10, 141. [Google Scholar] [CrossRef]
- Yanagawa, A.; Fujiwara-Tsujii, N.; Akino, T.; Yoshimura, T.; Yanagawa, T.; Shimizu, S. Odor aversion and pathogen-removal efficiency in grooming behavior of the termite Coptotermes formosanus. PLoS ONE 2012, 7, e47412. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; Meconcelli, S.; Radek, R.; McMahon, D.P. Termites shape their collective behavioural response based on stage of infection. Sci. Rep. 2018, 8, 14433. [Google Scholar] [CrossRef] [PubMed]
- Chouvenc, T.; Su, N.Y. When subterranean termites challenge the rules of fungal epizootics. PLoS ONE 2012, 7, e34484. [Google Scholar] [CrossRef] [PubMed]
- Mburu, D.M.; Ochola, L.; Maniania, N.K.; Njagi, P.G.N.; Gitonga, L.M.; Ndung’u, M.W.; Hassanali, A. Relationship between virulence and repellency of entomopathogenic isolates of Metarhizium anisopliae and Beauveria bassiana to the termite Macrotermes michaelseni. J. Insect Physiol. 2009, 55, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Mburu, D.M.; Ndung’u, M.W.; Maniania, N.K.; Hassanali, A. Comparison of volatile blends and gene sequences of two isolates of Metarhizium anisopliae of different virulence and repellency toward the termite Macrotermes michaelseni. J. Exp. Biol. 2011, 214, 956–962. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, J.Z.; Fuxa, J.R.; Richter, A.; Ring, D. Interactions of Metarhizium anisoplae and tree-based mulches in repellence and mycoses against Coptotermes formosanus (Isoptera: Rhinotermitidae). Environ. Entomol. 2014, 37, 755–763. [Google Scholar] [CrossRef]
- Yanagawa, A.; Imai, T.; Akino, T.; Toh, Y.; Yoshimura, T. Olfactory cues from pathogenic fungus affect the direction of motion of termites, Coptotermes formosanus. J. Chem. Ecol. 2015, 41, 1118–1126. [Google Scholar] [CrossRef]
- De Roode, J.C.; Lefèvre, T. Behavioral immunity in insects. Insects 2012, 3, 789–820. [Google Scholar] [CrossRef]
- Shang, Y.; Feng, P.; Wang, C. Fungi that infect insects: Altering host behavior and beyond. PLoS Pathog. 2015, 11, e1005037. [Google Scholar] [CrossRef]
- Cremer, S.; Pull, C.D.; Fuerst, M.A. Social immunity: Emergence and evolution of colony-level disease protection. Annu. Rev. Entomol. 2018, 63, 105–123. [Google Scholar] [CrossRef]
- Meyel, S.V.; Koerner, M.; Meunier, J. Social immunity: Why we should study its nature, evolution and functions across all social systems. Curr. Opin. Insect Sci. 2018, 28, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zeng, Q.; Li, D.; Wu, J.; Wu, X.; Shen, J. GPR detection of several common subsurface voids inside dikes and dams. Eng. Geol. 2010, 111, 31–42. [Google Scholar] [CrossRef]
- Kasseney, B.D.; Deng, T.; Mo, J. Effect of wood hardness and secondary compounds on feeding preference of Odontotermes formosanus (Isoptera: Termitidae). J. Econ. Entomol. 2011, 104, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Mathew, G.M.; Ju, Y.M.; Lai, C.Y.; Mathew, D.C.; Huang, C.C. Microbial community analysis in the termite gut and fungus comb of Odontotermes formosanus: The implication of Bacillus as mutualists. FEMS. Microbiol. Ecol. 2012, 79, 504–517. [Google Scholar] [CrossRef]
- Benítez, T.; Rincón, A.M.; Limón, M.C.; Codon, A.C. Biocontrol mechanisms of Trichoderma strains. Intern. Microbiol. 2004, 7, 249–260. [Google Scholar]
- Um, S.; Fraimout, A.; Sapountzis, P.; Sapountzis, Oh.; Sapountzis, D.C.; Poulsen, M. The fungus-growing termite Macrotermes natalensis harbors bacillaene-producing Bacillus sp. that inhibit potentially antagonistic fungi. Sci. Rep. 2013, 3, 3250. [Google Scholar] [CrossRef]
- Mevers, E.J.; Saurí, A.; Moser, M.; Varlan, G.E.; Martin, J.; Clardy, C. Chemical warfare: The battle between termite-associated actinobacteria and Trichoderma harzianum, a fungal pathogen. Planta. Med. 2018, 82, S1–S381. [Google Scholar]
- Otani, S.; Challinor, V.L.; Kreuzenbeck, N.B.; Kildgaard, S.; Christensen, S.K.; Larsen, L.L.M.; Poulsen, M. Disease-free monoculture farming by fungus-growing termites. Sci. Rep. 2019, 9, 8819. [Google Scholar] [CrossRef]
- Xiong, H.; Xue, K.; Qin, W.; Chen, X.; Wang, H.; Shi, X.; Lin, W. Does soil treated with conidial formulations of Trichoderma spp. attract or repel subterranean termites? J. Econ. Entomol. 2018, 111, 808–816. [Google Scholar] [CrossRef]
- Huang, F.S.; Zhu, S.M.; Ping, Z.M.; He, X.S.; Li, G.X.; Gao, D.R. Isoptera; Science Press: Beijing, China, 2000; pp. 566–568. [Google Scholar]
- Ye, S.D.; Ying, S.H.; Chen, C.; Feng, M.G. New solid-state fermentation chamber for bulk production of aerial conidia of fungal biocontrol agents on rice. Biotechnol. Lett. 2006, 28, 799–804. [Google Scholar] [CrossRef]
- Locatelli, G.O.; dos Santos, G.F.; Botelho, P.S.; Finkler, C.L.L.; Buenod, L.A. Development of Trichoderma sp. Formulations in encapsulated granules (CG) and evaluation of conidia shelf-life. Biol. Control. 2018, 117, 21–29. [Google Scholar] [CrossRef]
- Gautam, B.K.; Henderson, G. Laboratory study of the influence of substrate type and temperature on the exploratory tunneling by Formosan subterranean termite. Insects 2012, 3, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Henderson, G.; Gautam, B.K. Behavioral response of Formosan subterranean termites (Isoptera: Rhinotermitidae) to soil with high clay content. J. Insect Behav. 2015, 28, 303–311. [Google Scholar] [CrossRef]
- Aitchison, J. The Statistical Analysis of Compositional Data; Chapman & Hall: London, UK; New York, NY, USA, 1986. [Google Scholar]
- Kucera, M.; Malmgren, B.A. Logratio transformation of compositional data: A resolution of the constant sum constraint. Mar. Micropaleontol. 1998, 34, 117–120. [Google Scholar] [CrossRef]
- MacDonald, P.L.; Gardner, R.C. Type I error rate comparisons of post hoc procedures for I×J chi-square tables. Educ. Psychol. Meas. 2000, 60, 735–754. [Google Scholar] [CrossRef]
- Wright, M.S.; Cornelius, M.L. Mortality and repellent effects of microbial pathogens on Coptotermes formosanus (Isoptera: Rhinotermitidae). BMC. Microbiol. 2012, 12, 291. [Google Scholar] [CrossRef]
- Bodawatta, K.H.; Poulsen, M.; Bos, N. Foraging Macrotermes natalensis fungus-growing termites avoid a mycopathogen but not an entomopathogen. Insects 2019, 10, 185. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Woo, S.L.; Lorito, M. Trichoderma-plant-pathogen interactions. Soil. Biol. Biochem. 2008, 40, 1–10. [Google Scholar] [CrossRef]
- Hermosa, R.; Viterbo, A.; Chet, I.; Monte, E. Plant-beneficial effects of Trichoderma and of its genes. Microbiology 2012, 158, 17–25. [Google Scholar] [CrossRef]
- Verma, M.; Brar, S.K.; Tyagi, R.D.; Surampalli, R.Y.; Valéro, J.R. Antagonistic fungi, Trichoderma spp.: Panoply of biological control. Biochem. Eng. J. 2007, 37, 1–20. [Google Scholar] [CrossRef]
- Pull, C.D.; Metzler, S.; Naderlinger, E.; Cremer, S. Protection against the lethal side effects of social immunity in ants. Curr. Biol. 2018, 28, R1139–R1140. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.M.; Madden, A.A.; Penick, C.A.; Epps, M.J.; Marting, P.R.; Stevens, J.L.; Fergus, D.J.; Dunn, R.R.; Meineke, E.K. Azteca ants maintain unique microbiomes across functionally distinct nest chambers. Proc. R. Soc. B 2019, 286, 20191026. [Google Scholar] [CrossRef] [PubMed]
- Bos, N.; Sundström, L.; Fuchs, S.; Freitak, D. Ants medicate to fight disease. Evolution 2015, 69, 2979–2984. [Google Scholar] [CrossRef]
- Rocha, S.L.; Evans, H.C.; Jorge, V.L.; Cardoso, L.A.; Pereira, F.S.; Rocha, F.B.; Barreto, R.W.; Hart, A.G.; Elliot, S.L. Recognition of endophytic Trichoderma species by leaf-cutting ants and their potential in a Trojan-horse management strategy. R. Soc. Open Sci. 2017, 4, 160628. [Google Scholar] [CrossRef]
- Pontieri, L.; Vojvodic, S.; Graham, R.; Pedersen, J.S.; Linksvayer, T.A. Ant colonies prefer infected over uninfected nest sites. PLoS ONE 2014, 9, e111961. [Google Scholar] [CrossRef]
- Bruetsch, T.; Felden, A.; Reber, A.; Chapuisat, M. Ant queens (Hymenoptera: Formicidae) are attracted to fungal pathogens during the initial stage of colony founding. Myrmecol. News 2014, 20, 71–76. [Google Scholar]
- Jayasimha, P.; Henderson, G. Fungi isolated from integument and guts of Coptotermes formosanus and their antagonistic effect on Gleophyllum trabeum. Ann. Entomol. Soc. Am. 2007, 100, 703–710. [Google Scholar] [CrossRef]
- Jayasimha, P.; Henderson, G. Suppression of growth of a brown rot fungus, Gloeophyllum trabeum, by Formosan subterranean termites (Isoptera: Rhinotermitidae). Ann. Entomol. Soc. Am. 2007, 100, 506–511. [Google Scholar] [CrossRef]
- Zoberi, M.H.; Grace, J.K. Fungi associated with the subterranean termite Reticulitermes flavipes in Ontario. Mycologia 1990, 82, 289–294. [Google Scholar] [CrossRef]
- Mankowski, M.E.; Schowalter, T.D.; Morrell, J.J.; Lyons, B. Feeding habits and gut fauna of Zootermopsis angusticollis (Isoptera: Termopsidae) in response to wood species and fungal associates. Environ. Entomol. 1998, 27, 1315–1322. [Google Scholar] [CrossRef]


| Trichoderma fungi | Strain No. | Source |
|---|---|---|
| Trichoderma longibrachiatum Rifai | Bio-68049 | CGMCC a |
| Trichoderma koningii Oud. | GIM-3.518 | GCCC b |
| Trichoderma harzianum Rifai | GIM-3.442 | GCCC |
| Trichoderma hamatum (Bon.) Bain | Bio-08848 | CGMCC |
| Trichoderma atroviride Karsten | Bio-08876 | CGMCC |
| Trichoderma viride Pers. ex Fries | GIM-3.432 | GCCC |
| Trichoderma spirale Indira and Kamala | Bio-088439 | CGMCC |
| Test | Measurement | Treated Sand | Untreated Sand | Two-Way ANOVA | Effect | ||
|---|---|---|---|---|---|---|---|
| F | d.f. | p | |||||
| Trichoderma longibrachiatum | Length (mm) | 505.0 ± 22.1 b | 583.5 ± 26.7 a | 6.60 | 1, 32 | 0.0151 | Repellent |
| Area (mm2) | 1322.6 ± 106.5 b | 1645.3 ± 90.0 a | 8.56 | 1, 32 | 0.0063 | Repellent | |
| Trichoderma koningii | Length (mm) | 523.2 ± 23.7 a | 563.8 ± 26.9 a | 1.86 | 1, 32 | 0.1826 | N.A. |
| Area (mm2) | 1364.4 ± 80.6 b | 1609.2 ± 78.2 a | 8.37 | 1, 32 | 0.0068 | Repellent | |
| Trichoderma harzianum | Length (mm) | 435.8 ± 25.6 a | 410.0 ± 14.2 a | 1.28 | 1, 32 | 0.2670 | N.A |
| Area (mm2) | 1497.0 ± 92.3 a | 1507.7 ± 75.1 a | 0.02 | 1, 32 | 0.9028 | N.A | |
| Trichoderma hamatum | Length (mm) | 388.9 ± 17.1 b | 471.4 ± 20.5 a | 10.89 | 1, 32 | 0.0024 | Repellent |
| Area (mm2) | 1228.6 ± 58.5 b | 1559.3 ± 67.0 a | 25.25 | 1, 32 | <0.0001 | Repellent | |
| Trichoderma atroviride | Length (mm) | 410.9 ± 18.3 b | 506.4 ± 23.8 a | 19.27 | 1, 32 | 0.0001 | Repellent |
| Area (mm2) | 1281.9 ± 51.9 b | 1702.6 ± 78.4 a | 35.51 | 1, 32 | <0.0001 | Repellent | |
| Trichoderma viride | Length (mm) | 428.9 ± 21.3 b | 504.9 ± 20.3 a | 9.67 | 1,32 | 0.0039 | Repellent |
| Area (mm2) | 1466.2 ± 77.4 b | 1710.9 ± 71.2 a | 6.12 | 1, 32 | 0.0189 | Repellent | |
| Trichoderma spirale | Length (mm) | 409.9 ± 26.7 b | 504.8 ± 20.9 a | 11.86 | 1, 32 | 0.0016 | Repellent |
| Area (mm2) | 1366.8 ± 103.0 b | 1739.0 ± 102.2 a | 17.11 | 1, 32 | 0.0002 | Repellent | |
| Test | Treated Block | Petri Dish | Untreated Block | Statistical Result | Effect | ||
|---|---|---|---|---|---|---|---|
| F | d.f. | p | |||||
| Trichoderma longibrachiatum | 66.9 ± 4.1 a | 18.0 ± 3.0 b | 15.2 ± 3.0 b | 97.87 | 2, 48 | <0.0001 | Attractive |
| Trichoderma koningii | 24.0 ± 3.5 b | 17.9 ± 3.4 b | 58.1 ± 4.6 a | 36.40 | 2, 48 | <0.0001 | Repellent |
| Trichoderma harzianum | 53.2 ± 5.9 a | 12.1 ± 2.7 b | 34.7 ± 5.4 a | 23.15 | 2, 48 | <0.0001 | N.A |
| Trichoderma hamatum | 71.6 ± 5.2 a | 3.5 ± 1.3 c | 24.9 ± 5.1 b | 90.17 | 2, 48 | <0.0001 | Attractive |
| Trichoderma atroviride | 37.1 ± 6.5 b | 5.8 ± 0.8 c | 57.1 ± 6.5 a | 32.28 | 2, 48 | <0.0001 | Repellent |
| Trichoderma viride | 55.7 ± 6.5 a | 4.8 ± 0.9 b | 39.5 ± 6.4 a | 37.59 | 2, 48 | <0.0001 | N.A |
| Trichoderma spirale | 34.2 ± 5.0 b | 10.0 ± 2.0 c | 55.8 ± 4.2 a | 68.49 | 2, 48 | <0.0001 | Repellent |
| Test | Day | χ2 | df | p |
|---|---|---|---|---|
| Trichodermalongibrachiatum | 1 | 5.1475 | 2 | 0.0762 |
| 2 | 6.1455 | 2 | 0.0463 | |
| 3 | 4.3529 | 2 | 0.1134 | |
| 4 | 3.7143 | 2 | 0.1561 | |
| 5 | 4.1333 | 2 | 0.1266 | |
| 6 | 3.3333 | 2 | 0.1889 | |
| 7 | 2.2273 | 2 | 0.3284 | |
| 8 | 2.0000 | 2 | 0.3679 | |
| Trichodermakoningii | 1 | 0.1455 | 2 | 0.9299 |
| 2 | 0.4643 | 2 | 0.7928 | |
| 3 | 1.6538 | 2 | 0.4374 | |
| 4 | 0.6038 | 2 | 0.7394 | |
| 5 | 0.9615 | 2 | 0.6183 | |
| 6 | 0.8235 | 2 | 0.6625 | |
| 7 | 1.1250 | 2 | 0.5698 | |
| 8 | 2.5333 | 2 | 0.2818 |
| Test | Treated Block | Petri Dish | Untreated Block | Statistical Results |
|---|---|---|---|---|
| Trichodermalongibrachiatum | 40.0 | 36.7 | 23.3 | χ2 = 1.4000; df = 2; p = 0.4966 |
| Trichoderma koningii | 37.8 | 22.2 | 40.0 | χ2 = 2.5333; df = 2; p = 0.2817 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, H.; Cai, J.; Chen, X.; Liang, S.; Wen, X.; Wang, C. The Effects of Trichoderma Fungi on the Tunneling, Aggregation, and Colony-Initiation Preferences of Black-Winged Subterranean Termites, Odontotermes formosanus (Blattodea: Termitidae). Forests 2019, 10, 1020. https://doi.org/10.3390/f10111020
Xiong H, Cai J, Chen X, Liang S, Wen X, Wang C. The Effects of Trichoderma Fungi on the Tunneling, Aggregation, and Colony-Initiation Preferences of Black-Winged Subterranean Termites, Odontotermes formosanus (Blattodea: Termitidae). Forests. 2019; 10(11):1020. https://doi.org/10.3390/f10111020
Chicago/Turabian StyleXiong, Hongpeng, Jiacheng Cai, Xuan Chen, Shiping Liang, Xiujun Wen, and Cai Wang. 2019. "The Effects of Trichoderma Fungi on the Tunneling, Aggregation, and Colony-Initiation Preferences of Black-Winged Subterranean Termites, Odontotermes formosanus (Blattodea: Termitidae)" Forests 10, no. 11: 1020. https://doi.org/10.3390/f10111020
APA StyleXiong, H., Cai, J., Chen, X., Liang, S., Wen, X., & Wang, C. (2019). The Effects of Trichoderma Fungi on the Tunneling, Aggregation, and Colony-Initiation Preferences of Black-Winged Subterranean Termites, Odontotermes formosanus (Blattodea: Termitidae). Forests, 10(11), 1020. https://doi.org/10.3390/f10111020





