Do Coarser Gap Mosaics in Conifer Plantations Induce More Seed Dispersal by Birds? Temporal Changes during 12 Years after Gap Creation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Gap-Mosaic Design
2.3. Seed Rain into Gaps
2.4. Factors Related to Seed Dispersal at 12 Years after Gap Creation
2.5. Data Analysis
2.5.1. Seed Dispersal by Frugivorous Birds
2.5.2. Effect of Gap Mosaic and Age
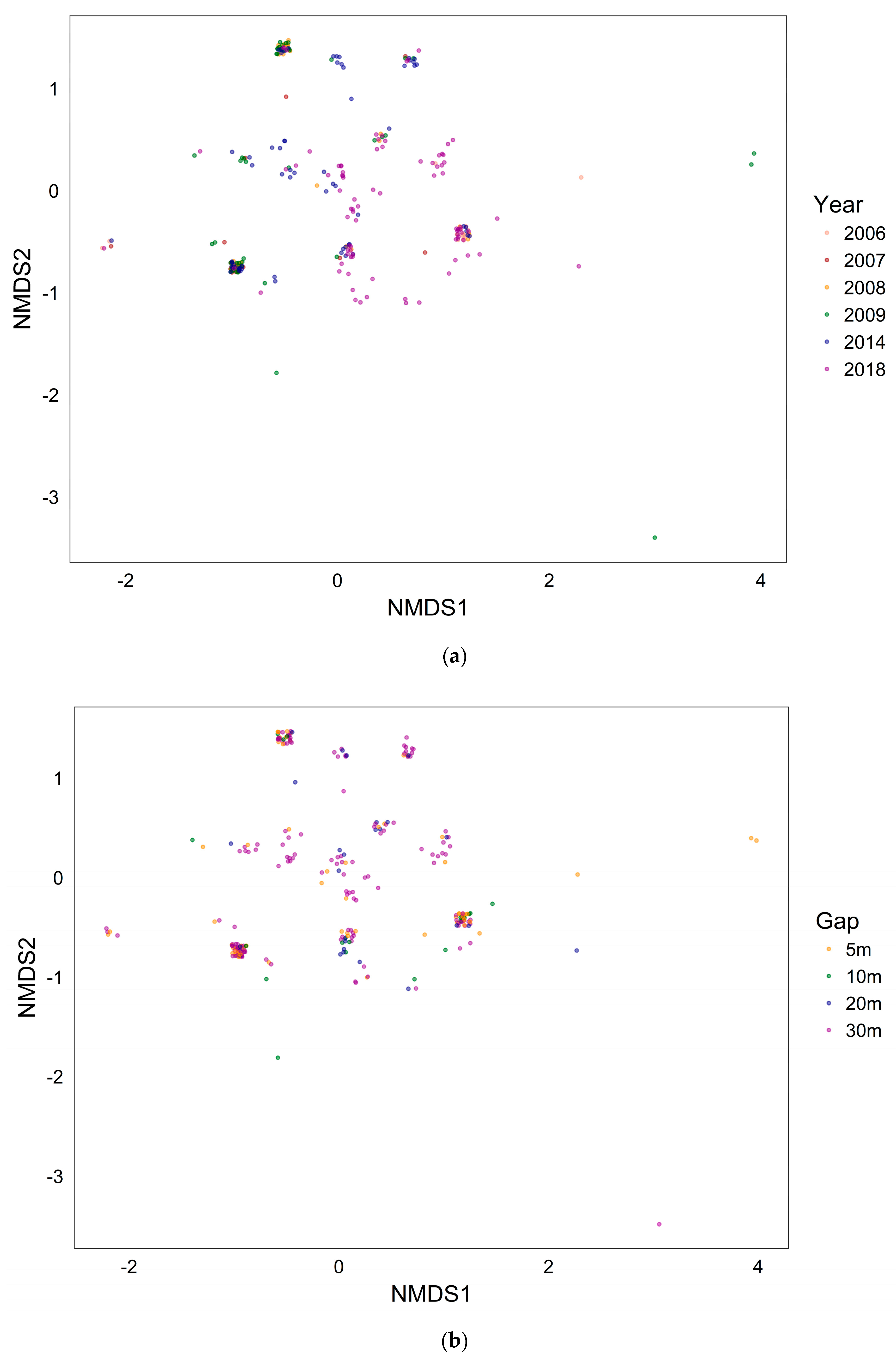
2.5.3. Ordination of Species Composition and Calculation of Species Diversity
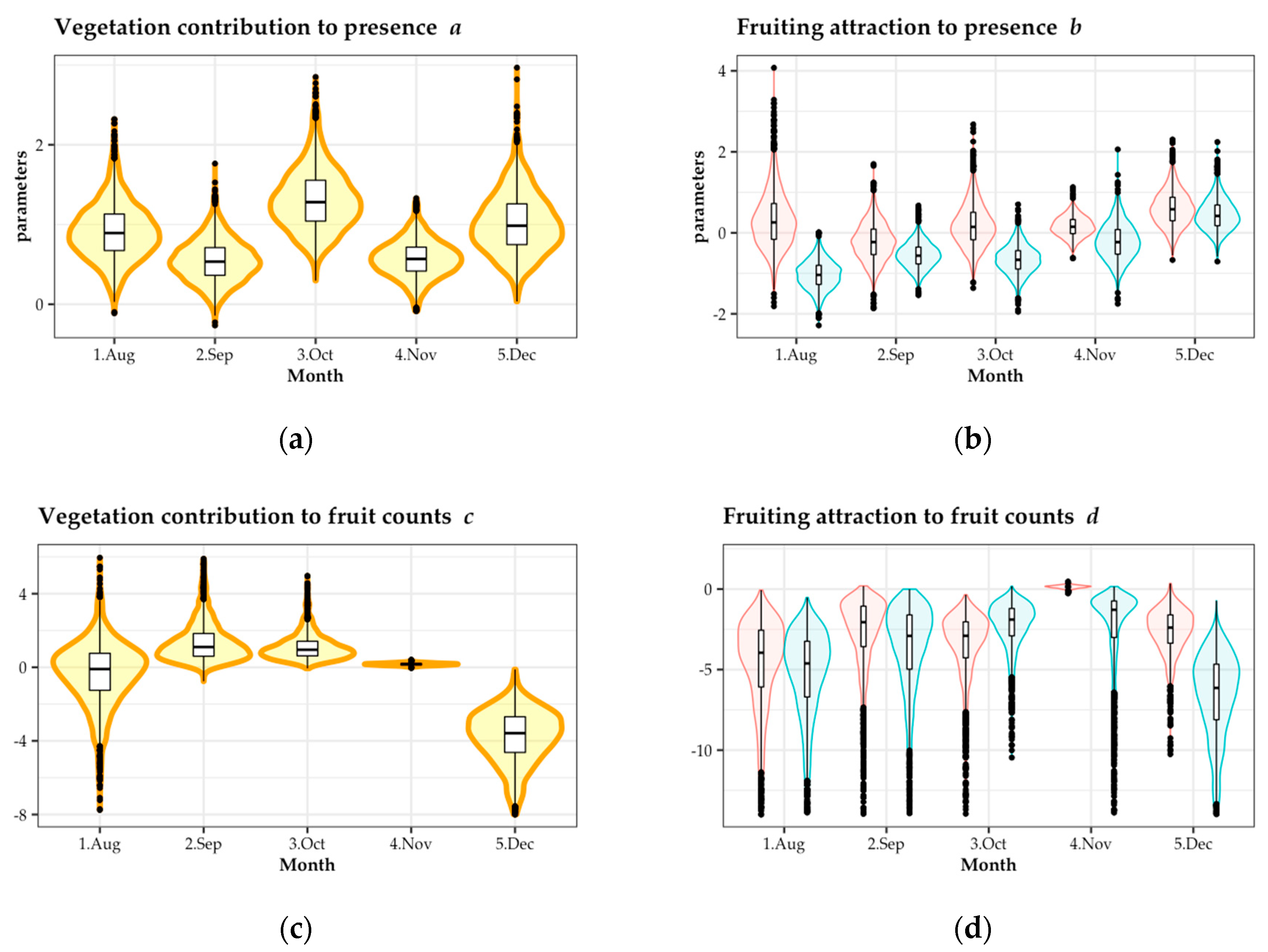
2.5.4. Bayesian Model for Factors Determining Dispersal Frequency
3. Results
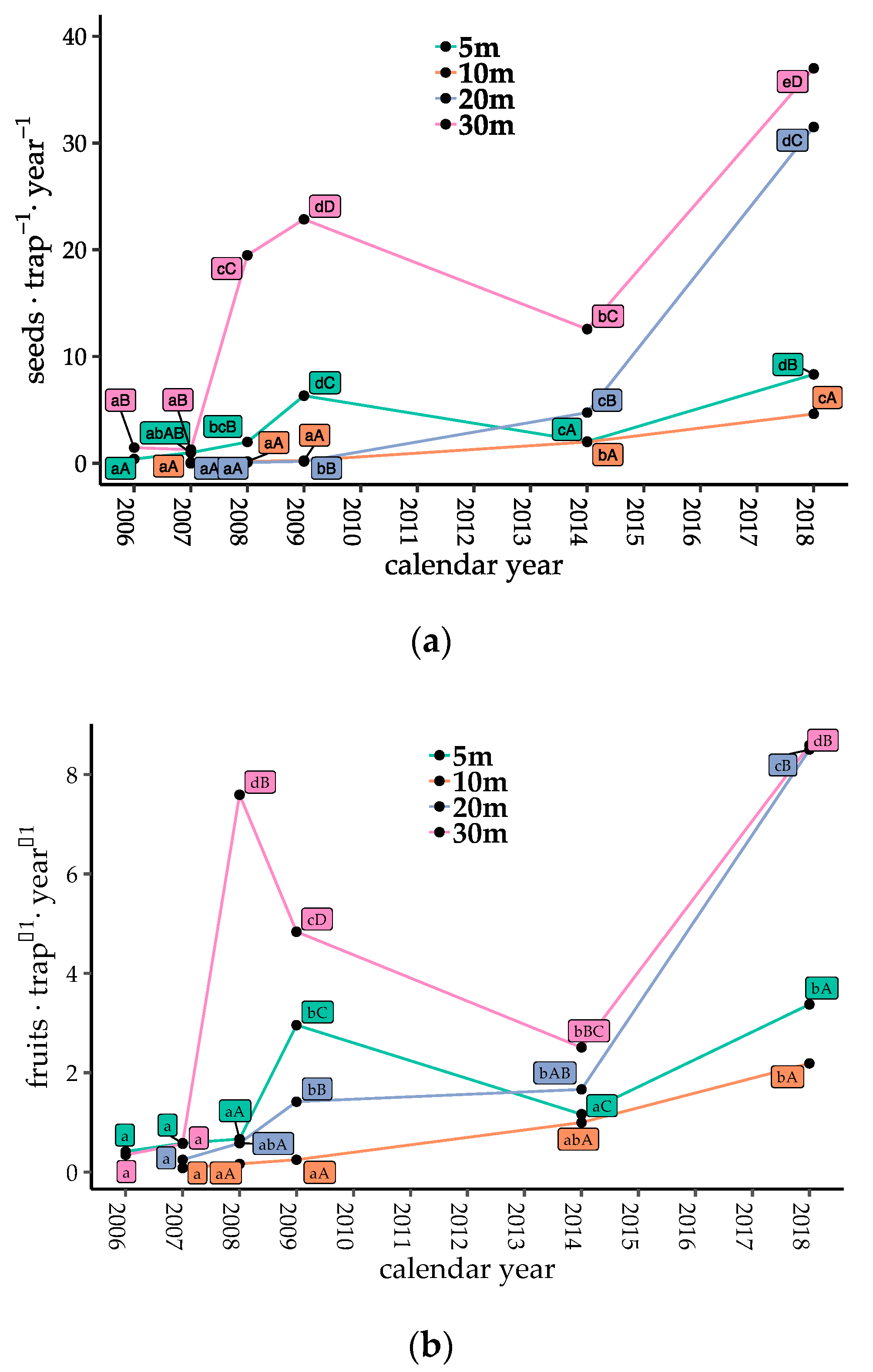
3.1. Temporal Changes in Seed Rain
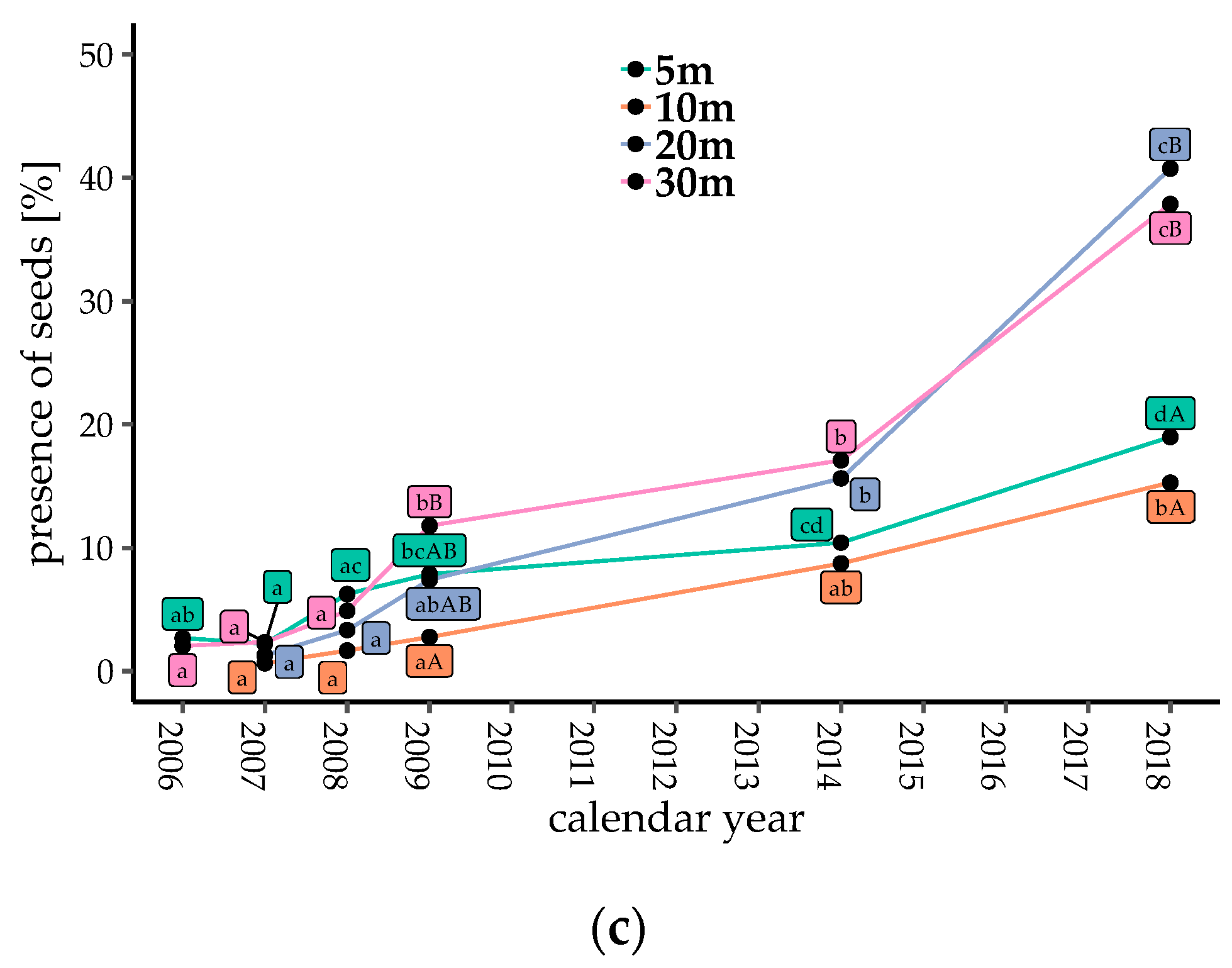
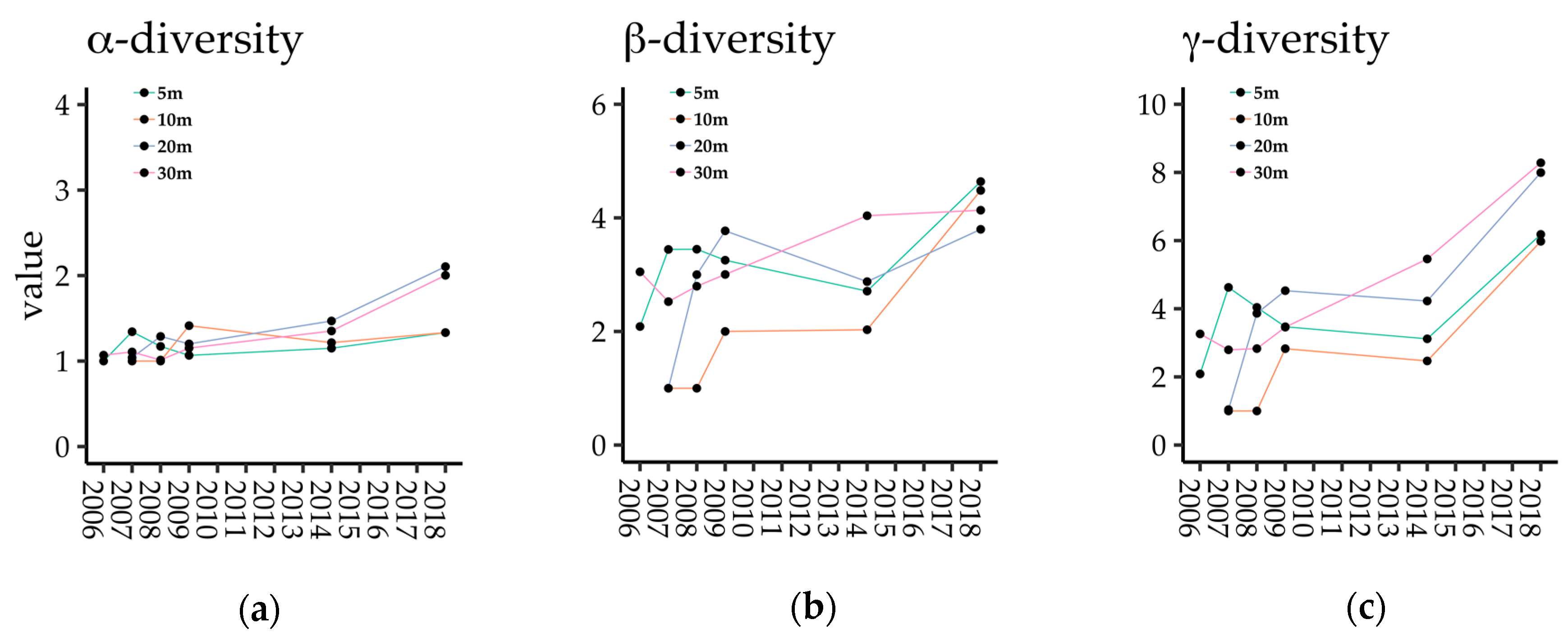
3.2. Transitions in Dispersed-Species Composition
3.3. Factors for Seed Dispersal by Frugivorous Birds
4. Discussion
4.1. Does Bird-Dispersed Seed Abundance Increase with Time after Gap Creation in a Plantation?
4.2. Do Finer or Coarser Gap Mosaics Induce More Bird-Dispersed Seeds?
4.3. How do Gap Age and Gap-Mosaic Arrangement Change Composition of Bird-Dispersed Woody Species?
4.4. Effect of Natural Forests
4.5. What Are Factors Affecting Seed Dispersal by Frugivorous Birds in Different Gap-Mosaic Arrangements?
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Seiwa, K. Steps in recovering biodiversity of conifer plantations: An effective combination of edge- and thinning effects. Jpn. J. Ecol. 2013, 63, 251–260. (In Japanese) [Google Scholar]
- Niiyama, K.; Ogawa, M.; Kushima, H.; Takahashi, K.; Sato, T.; Sakai, T.; Tanouchi, H. Collection and Evaluation of Literatures Related to Broadleaf Tree Regeneration for Leading Artificial Coniferous Forests to Broadleaf Forests. J. Jpn. For. Soc. 2010, 92, 292–296. [Google Scholar] [CrossRef]
- Tanouchi, H. Target Forest Type and Standard for Broadleaf Tree Regeneration. For. Sci. 2010, 59, 22–25. (In Japanese) [Google Scholar]
- Ito, S.; Ishigami, S.; Mizoue, N.; Buckley, G.P. Maintaining plant species composition and diversity of understory vegetation under strip-clearcutting forestry in conifer plantations in Kyushu, southern Japan. For. Ecol. Manag. 2006, 231, 234–241. [Google Scholar] [CrossRef]
- Utsugi, E.; Kanno, H.; Ueno, N.; Tomita, M.; Saitoh, T.; Kimura, M.; Kanou, K.; Seiwa, K. Hardwood recruitment into conifer plantations in Japan: Effects of thinning and distance from neighboring hardwood forests. For. Ecol. Manag. 2006, 237, 15–28. [Google Scholar] [CrossRef]
- Zhu, J.J.; Yang, K.; Yan, Q.L.; Liu, Z.G.; Yu, L.Z.; Wang, H.X. Feasibility of implementing thinning in even-aged Larix olgensis plantations to develop uneven-aged larch-broadleaved mixed forests. J. For. Res. 2010, 15, 71–80. [Google Scholar] [CrossRef]
- Mizunaga, H. Do finer gap mosaics provide a wider niche for Quercus gilva in young Japanese cedar plantations than coarser mosaics? Simulation of spatial heterogeneity of light availability and photosynthetic potential. Can. J. For. Res. 2007, 37, 1545–1553. [Google Scholar] [CrossRef]
- Fujishima, M.; Naramoto, M.; Mizunaga, H. Simulation of strip-gap arrangement in cedar plantations to regulate the light environment and competition between dwarf bamboo and beech seedlings. Forestry 2011, 84, 505–515. [Google Scholar] [CrossRef]
- Tanaka, H.; Tanouchi, H.; Sato, T.; Masaki, T.; Akashi, N.; Takahashi, K.; Kon, H.; Seiwa, K.; Shimada, H.; Nagaike, T.; et al. Handbook of Broadleaf Tree Regeneration 2010; Tanouchi, H., Tanaka, H., Hirata, A., Eds.; Forestry and Forest Products Research Institute: Kochi, Japan, 2010. (In Japanese) [Google Scholar]
- Tanaka, H.; Tanouchi, H.; Mizunaga, H.; Masaki, T.; Kominami, Y.; Koyama, Y.; Nagaike, T.; Sato, A.; Yamanaka, T.; Kayama, M.; et al. Handbook of Broadleaf Tree Regeneration 2012; Tanouchi, H., Tanaka, H., Hirata, A., Eds.; Forestry and Forest Products Research Institute: Kochi, Japan, 2012. (In Japanese) [Google Scholar]
- Masaki, T. Current and future studies on seed dispersal by vertebrates in Japan. Jpn. J. Ecol. 2009, 59, 13–24. [Google Scholar]
- Masaki, T.; Kominami, Y.; Nakashizuka, T. Spatial and seasonal patterns of seed dissemination of Cornus controversa in a temperate forest. Ecology 1994, 75, 1903–1910. [Google Scholar] [CrossRef]
- Nanami, S.; Kawaguchi, H.; Yamakura, T. Dioecy-induced spatial patterns of two codominant tree species, Podocarpus nagi and Neolitsea aciculata. J. Ecol. 1999, 87, 678–687. [Google Scholar] [CrossRef]
- Clark, C.J.; Poulsen, J.R.; Bolker, B.M.; Connor, E.F.; Parker, V.T. Comparative seed shadows of bird-, monkey-, and wind-dispersed trees. Ecology 2005, 86, 2684–2694. [Google Scholar] [CrossRef]
- Hoppes, W.G. Seedfall pattern of several species of bird-dispersed plants in an Illinois woodland. Ecology 1988, 69, 320–329. [Google Scholar] [CrossRef]
- Kon, H.; Akashi, N.; Minamino, K.; Kuramoto, S.; Iida, S. Seed dispersal of broad leaved species in Abies sachalinensis plantations on central Hokkaido, Japan. Jpn. J. Ecol. 2013, 63, 211–218. (In Japanese) [Google Scholar]
- Yamagawa, H.; Ito, S.; Nakao, T. Seed dispersal in clear-cut stands adjacent to lucidophyllous forest in 1 to 6 years after clear-cut. Jpn. J. Ecol. 2013, 63, 219–228. (In Japanese) [Google Scholar]
- Hoppes, W.G. Pre-and post-foraging movements of frugivorous birds in an eastern deciduous forest woodland, USA. Oikos 1987, 49, 281–290. [Google Scholar] [CrossRef]
- Gorchov, D.L.; Cornejo, F.; Ascorra, C.; Jaramillo, M. The role of seed dispersal in the natural regeneration of rain forest after strip-cutting in the Peruvian Amazon. Vegetatio 1993, 107, 339–349. [Google Scholar]
- Carlo, T.A.; Morales, J.M. Generalist birds promote tropical forest regeneration and increase plant diversity via rare-biased seed dispersal. Ecology 2016, 97, 1819–1831. [Google Scholar] [CrossRef]
- McDonnell, M.J.; Stiles, E.W. The structural complexity of old field vegetation and the recruitment of bird-dispersed plant species. Oecologia 1983, 56, 109–116. [Google Scholar] [CrossRef]
- Guevara, S.; Purata, S.E.; Van der Maarel, E. The role of remnant forest trees in tropical secondary succession. Vegetatio 1986, 66, 77–84. [Google Scholar]
- Herrera, J.M.; Garcia, D. The role of remnant trees in seed dispersal through the matrix: Being alone is not always so sad. Biol. Conserv. 2009, 142, 149–158. [Google Scholar] [CrossRef]
- Pizo, M.A.; dos Santos, B.T.P. Frugivory, Post-feeding Flights of Frugivorous Birds and the Movement of Seeds in a Brazilian Fragmented Landscape. Biotropica 2011, 43, 335–342. [Google Scholar] [CrossRef]
- Suhonen, J. Predation risk influences the use of foraging sites by tits. Ecology 1993, 74, 1197–1203. [Google Scholar] [CrossRef]
- Fink, R.D.; Lindell, C.A.; Morrison, E.B.; Zahawi, R.A.; Holl, K.D. Patch Size and Tree Species Influence the Number and Duration of Bird Visits in Forest Restoration Plots in Southern Costa Rica. Restor. Ecol. 2009, 17, 479–486. [Google Scholar] [CrossRef]
- Cole, R.J.; Holl, K.D.; Zahawi, R.A. Seed rain under tree islands planted to restore degraded lands in a tropical agricultural landscape. Ecol. Appl. 2010, 20, 1255–1269. [Google Scholar] [CrossRef]
- Morrison, E.B.; Lindell, C.A.; Holl, K.D.; Zahawi, R.A. Patch size effects on avian foraging behaviour: Implications for tropical forest restoration design. J. Appl. Ecol. 2010, 47, 130–138. [Google Scholar] [CrossRef]
- Lindell, C.A.; Reid, J.L.; Cole, R.J. Planting Design Effects on Avian Seed Dispersers in a Tropical Forest Restoration Experiment. Restor. Ecol. 2013, 21, 515–522. [Google Scholar] [CrossRef]
- Reid, J.L.; Holl, K.D.; Zahawi, R.A. Seed dispersal limitations shift over time in tropical forest restoration. Ecol. Appl. 2015, 25, 1072–1082. [Google Scholar] [CrossRef] [Green Version]
- Forest Ecosystem Section, Center for Education and Research in Field Sciences, Faculty of Agriculture, Shizuoka University. Available online: http://fc-forecol.agr.shizuoka.ac.jp/wordpress/ (accessed on 13 August 2019).
- Kominami, Y.; Sato, T.; Takeshita, K.; Manabe, T.; Endo, A.; Noma, N. Classification of bird-dispersed plants by fruiting phenology, fruit size, and growth form in a primary lucidophyllous forest: An analysis, with implications for the conservation of fruit-bird interactions. Ornithol. Sci. 2003, 2, 3–23. [Google Scholar] [CrossRef]
- Nakayama, S.; Inokuchi, M.; Minamitani, T. Seeds of Wild Plants in Japan; Tohoku University Press: Sendai, Japan, 2000. (In Japanese) [Google Scholar]
- Kitagawa, M.; Miyawaki, A.; Okuda, S.; Mochizuki, R. Handbook of Japanese Vegetation; Shibundo: Tokyo, Japan, 1978. (In Japanese) [Google Scholar]
- Satake, Y.; Hara, H.; Watari, S.; Tominari, T. Wild Flowers of Japan; Heibonsha: Tokyo, Japan, 1989. (In Japanese) [Google Scholar]
- Magurran, A.E. Biological Diversity; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Sato, S.; Sakai, A. Contribution of seed dispersal by birds on vegetation recoverty after clear-cutting of a coniferous plantation. Appl. For. Sci. 2003, 12, 23–28. [Google Scholar]
- Ichinose, T.; Katoh, K. A Relationship between Bird Communities and Vegetation Structure on Isolated Woodlots in Wintering Season in Tokorozawa City, Saitama Pref. J. Jpn. Inst. Landsc. Archit. 1995, 59, 73–76. [Google Scholar] [CrossRef] [Green Version]
- Toyoshima, Y.; Yamaura, Y.; Yabuhara, Y.; Nakamura, F. A preliminary study on the effects of line and selective thinning on bird communities in Hokkaido, northern Japan. J. For. Res. 2013, 24, 553–559. [Google Scholar] [CrossRef]
- Nakanishi, H. Fruit color and fruit size of bird-disseminated plants in Japan. Vegetatio 1996, 123, 207–218. [Google Scholar] [CrossRef]
- MacArthur, R.H.; Wilson, E.O. An equilibrium theory of insular zoogeography. Evolution 1963, 17, 373–387. [Google Scholar] [CrossRef]
- Baselga, A.; Orme, C.D.L. betapart: An R package for the study of beta diversity. Methods Ecol. Evol. 2012, 3, 808–812. [Google Scholar] [CrossRef]
- Hirata, R.; Hata, K.; Sone, K. Seed Dispersal by Frugivorous Birds in a Coniferous Plantation. J. Jpn. For. Soc. 2006, 88, 515–524. [Google Scholar] [CrossRef]
- Gonzales, R.S.; Nakashizuka, T. Broad-leaf species composition in Cryptomeria japonica plantations with respect to distance from natural forest. For. Ecol. Manag. 2010, 259, 2133–2140. [Google Scholar] [CrossRef]
- Hamao, S.; Miyashita, T.; Hagiwara, S.; Mori, Y. Seed dispersal by wintering birds in an urban space and relationship between gape width and fruit size. Jpn. J. Ornithol. 2010, 59, 139–147. [Google Scholar] [CrossRef]
- Fukui, A.W. The role of the brown-eared bulbul Hypsypetes amaurotis as a seed dispersal agent. Res. Popul. Ecol. 1995, 37, 211–218. [Google Scholar] [CrossRef]
- Noma, N.; Yumoto, T. Fruiting phenology of animal-dispersed plants in response to winter migration of frugivores in a warm temperate forest on Yakushima Island, Japan. Ecol. Res. 1997, 12, 119–129. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Isagi, Y. Dietary breadth of frugivorous birds in relation to their feeding strategies in the lowland forests of central Honshu, Japan. Oikos 2012, 121, 1041–1052. [Google Scholar] [CrossRef]
- Kiyosu, Y. The Birds of Japan I; Kodansha: Tokyo, Japan, 1978. (In Japanese) [Google Scholar]
- Lindenmayer, D.B.; Likens, G.E. Adaptive monitoring: A new paradigm for long-term research and monitoring. Trends Ecol. Evol. 2009, 24, 482–486. [Google Scholar] [CrossRef]
- Karasti, H.; Baker, K.S. Digital data practices and the long term ecological research program growing global. Int. J. Digit. Curation 2008, 3. [Google Scholar] [CrossRef]
- Johnson, J.C.; Christian, R.R.; Brunt, J.W.; Hickman, C.R.; Waide, R.B. Evolution of Collaboration within the US Long Term Ecological Research Network. Bioscience 2010, 60, 931–940. [Google Scholar] [CrossRef]
- Michener, W.K.; Porter, J.; Servilla, M.; Vanderbilt, K. Long term ecological research and information management. Ecol. Inform. 2011, 6, 13–24. [Google Scholar] [CrossRef]
- Hobbie, J.E. Scientific accomplishments of the long term ecological research program: An introduction. Bioscience 2003, 53, 17–20. [Google Scholar] [CrossRef]
- Alcantara, J.M.; Rey, P.J.; Valera, F.; Sanchez-Lafuente, A.M. Factors shaping the seedfall pattern of a bird-dispersed plant. Ecology 2000, 81, 1937–1950. [Google Scholar] [CrossRef]
- Gonzales, R.S.; Ingle, N.R.; Lagunzad, D.A.; Nakashizuka, T. Seed Dispersal by Birds and Bats in Lowland Philippine Forest Successional Area. Biotropica 2009, 41, 452–458. [Google Scholar] [CrossRef]
- McCay, T.S.; McCay, D.H.; Czajka, J.L. Deposition of exotic bird-dispersed seeds into three habitats of a fragmented landscape in the northeastern United States. Plant Ecol. 2009, 203, 59–67. [Google Scholar] [CrossRef]
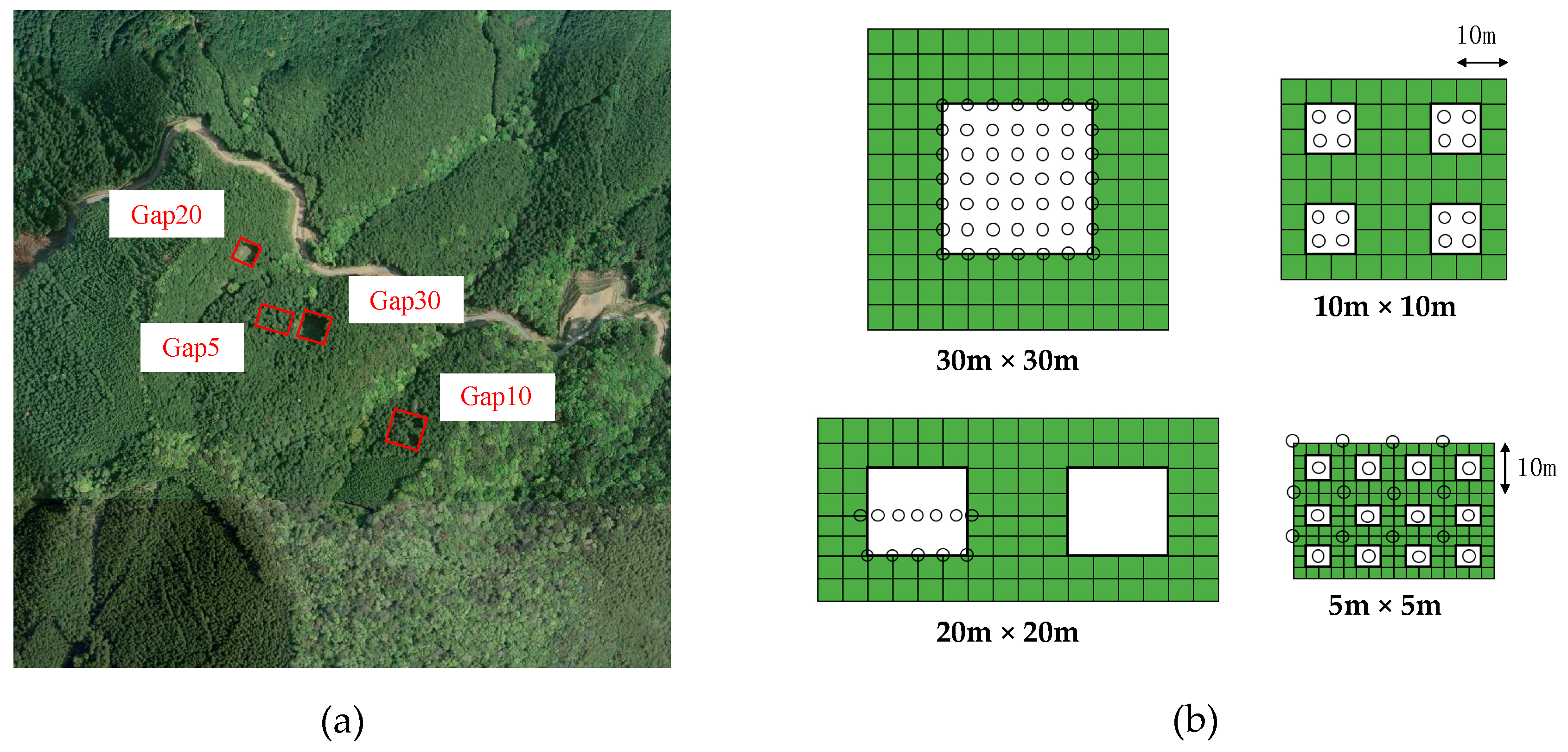
| Plot | Gap Size (m) | Plot Area (m2) | Year of Gap Establishment | Slope Direction | Gap Number | Seed-Trap Number | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2006 | 2007 | 2008 | 2009 | 2014 | 2018 | ||||||
| Gap5 | 5 × 5 | 1200 | 2006 | NW | 12 | 24 | 24 | 24 | 24 | 24 | 24 |
| Gap10 | 10 × 10 | 1800 | 2007 | NW | 4 | - | 12 | 12 | 12 | 10 | 16 |
| Gap20 | 20 × 20 | 3200 | 2007 | E | 2 | - | 12 | 12 | 12 | 12 | 12 |
| Gap30 | 30 × 30 | 3600 | 2006 | NW | 1 | 49 | 49 | 49 | 49 | 49 | 49 |
| Type | Variables | Description |
|---|---|---|
| Data | S | Fruit counts |
| Vg | Vegetation index | |
| Ft | Fruiting index | |
| M | Month | |
| Parameters | a (M) | Effect of vegetation on dispersal presence |
| b (M, Ft) | Effect of fruiting on dispersal presence | |
| c (M) | Effect of vegetation on fruit counts | |
| d (M, Ft) | Effect of fruiting on fruit counts | |
| pf (M) | Effect of vegetation on fruiting | |
| pic (M) | Intercept on fruiting | |
| a_base–pic_base | Hyperparameters of parameters a, b, c, d, pf, and pic for average and standard deviation | |
| std_a–std_pic | ||
| Models | logit(q) ~ a (M) × Vg + b (M, Ft) | |
| lambda = exp (c (M) × Vg + d (M, Ft)) | ||
| S ~ ZIP (logit(q), lambda) | ||
| Ft ~ pf (M) × Vg + pic (M) | ||
| a (M) ~ normal (a_base, std_a) | ||
| b (M) ~ normal (b_base, std_b) | ||
| c (M) ~ normal (c_base, std_c) | ||
| d (M) ~ normal (d_base, std_d) | ||
| pf (M) ~ normal (pf_base, std_pf) | ||
| pic (M) ~ normal (pic_base, std_pic) | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takano, T.; Kominami, Y.; Mizunaga, H. Do Coarser Gap Mosaics in Conifer Plantations Induce More Seed Dispersal by Birds? Temporal Changes during 12 Years after Gap Creation. Forests 2019, 10, 918. https://doi.org/10.3390/f10100918
Takano T, Kominami Y, Mizunaga H. Do Coarser Gap Mosaics in Conifer Plantations Induce More Seed Dispersal by Birds? Temporal Changes during 12 Years after Gap Creation. Forests. 2019; 10(10):918. https://doi.org/10.3390/f10100918
Chicago/Turabian StyleTakano, Tsubasa, Yohsuke Kominami, and Hiromi Mizunaga. 2019. "Do Coarser Gap Mosaics in Conifer Plantations Induce More Seed Dispersal by Birds? Temporal Changes during 12 Years after Gap Creation" Forests 10, no. 10: 918. https://doi.org/10.3390/f10100918




