Conversion of Pinus nigra Plantations with Natural Regeneration in the Slovenian Karst: The Importance of Intermediate, Gradually Formed Canopy Gaps
Abstract
1. Introduction
2. Materials and Methods
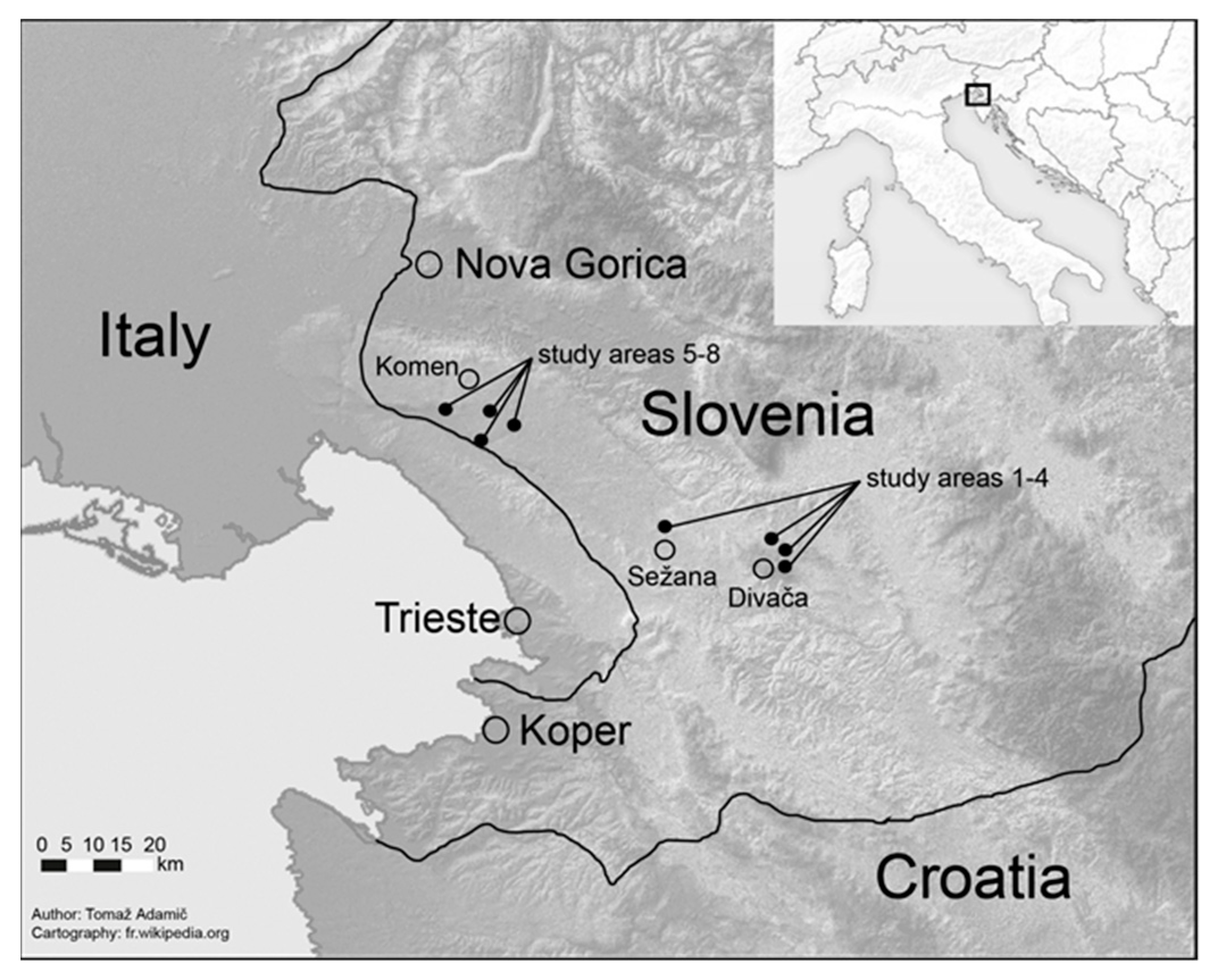
2.1. Stand and Site Characteristics
2.2. Sampling Design and Recordings
2.3. Data Analysis
3. Results
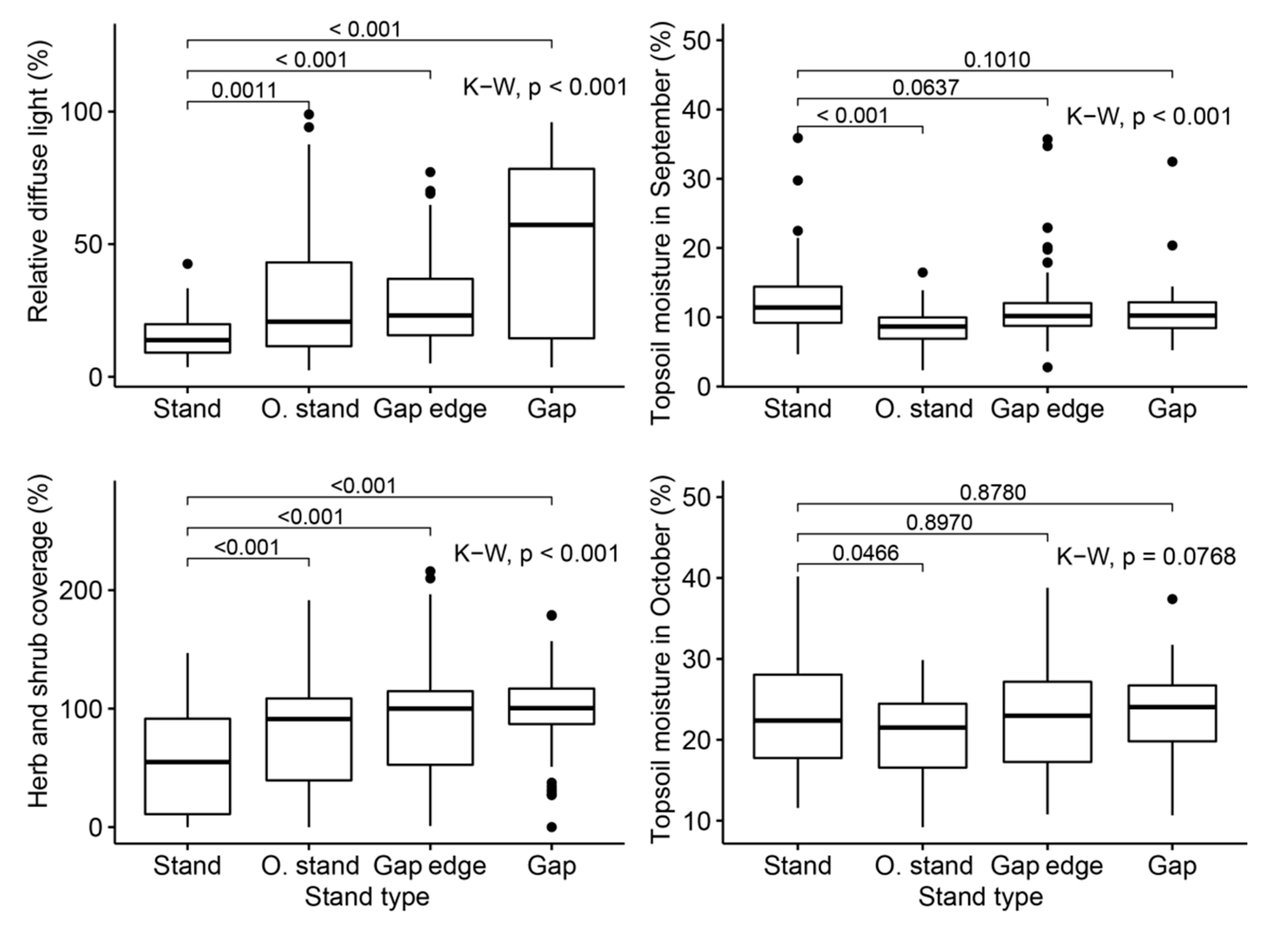
3.1. Variability of Ecological Factors within and between Stand Types
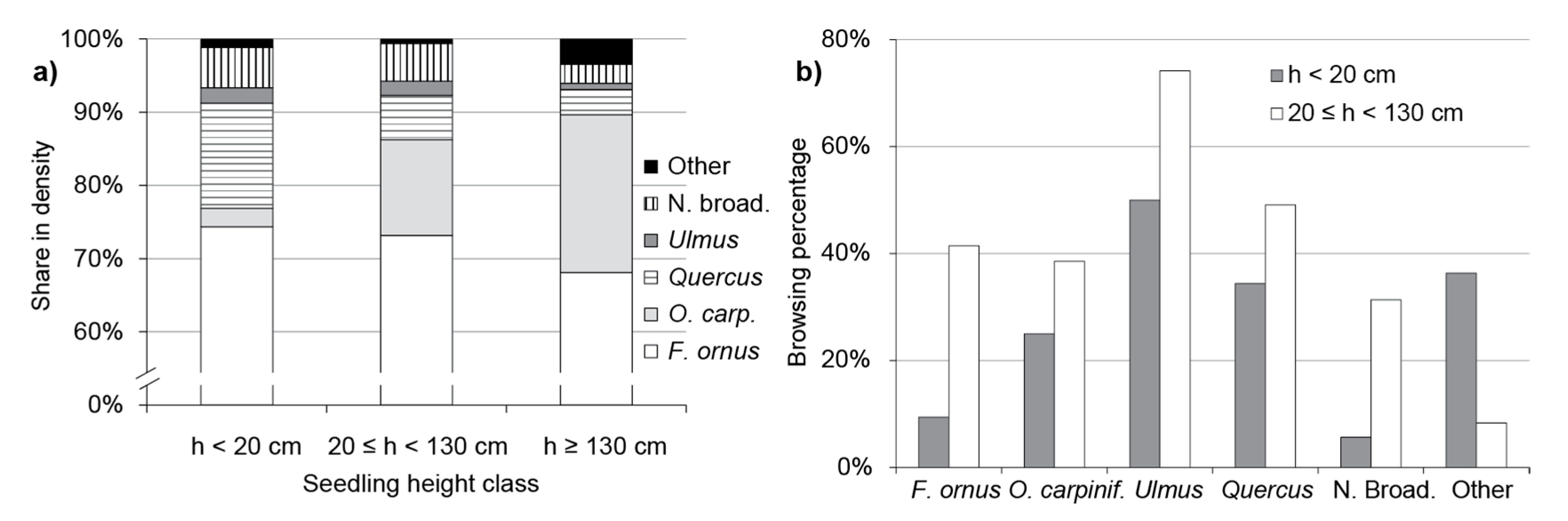
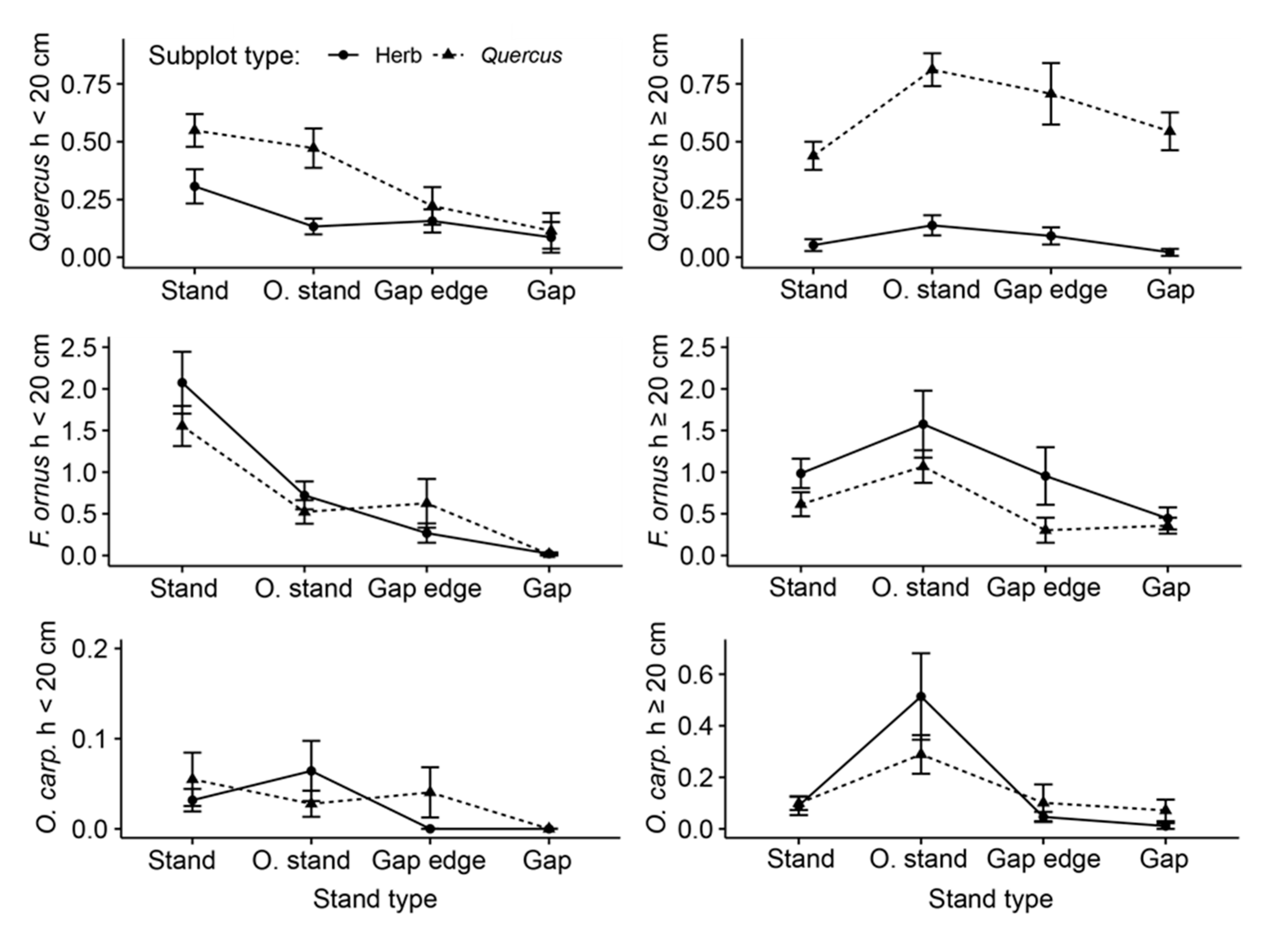
3.2. Natural Regeneration
3.3. Herb Vegetation and Indication of Ecological Factors
3.4. Drivers of Regeneration Dynamics
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Trees | Frequency % | Regeneration % | Shrubs | Frequency % |
| Fraxinus ornus | 70 | 73 | Hedera helix | 39 |
| Quercus sp. | 10 | Clematis vitalba | 35 | |
| Quercus pubescens | 20 | Ligustrum vulgare | 30 | |
| Quercus cerris | 11 | Rubus hirtus | 25 | |
| Quercus petraea | 3 | Cornus mas | 21 | |
| Quercus rubra | 1 | Prunus spinosa | 19 | |
| Ostrya carpinifolia | 37 | 9 | Crataegus monogyna | 15 |
| Ulmus glabra aggr. | 2 | Prunus mahaleb | 14 | |
| Ulmus glabra | 2 | Frangula rupestris | 11 | |
| Ulmus minor | 5 | Rubus ulmifolius | 10 | |
| Corylus avellana | 6 | |||
| Noble Broadleaves | Cotinus coggygria | 6 | ||
| Acer pseudoplatanus | 5 | 3 | Lonicera xylosteum | 6 |
| Fraxinus excelsior | 4 | 2 | Lonicera etrusca | 5 |
| Tilia sp. | <1 | Rosa sp. | 4 | |
| Tilia platyphyllos | 2 | Berberis vulgaris | 2 | |
| Tilia cordata | 1 | Rhamnus cathartica | 2 | |
| Prunus avium | 1 | <1 | Euonymus verrucosus | 2 |
| Sorbus torminalis | 2 | <1 | Rosa canina | 2 |
| Cornus sanguinea | 2 | |||
| Other tree species | Euonymus europaeus | 2 | ||
| Robinia pseudoacacia | 3 | <1 | Rubus sp. | 2 |
| Sorbus aria | 2 | 1 | Viburnum lantana | 2 |
| Abies alba | 2 | <1 | Rubus fruticosus aggr. | 1 |
| Acer campestre | 2 | <1 | Frangula alnus | 1 |
| Castanea sativa | <1 | <1 | Rosa arvensis | <1 |
| Juglans regia | <1 | <1 | Rosa gallica | <1 |
| Malus sylvestris | <1 | <1 | Rubus bungeana | <1 |
| Pyrus pyraster | <1 | <1 | Rhamnus saxatilis | <1 |
| Sorbus aucuparia | <1 | <1 | Rosa guleana | <1 |
| Acer monspessulanum | <1 | <1 | Rubus caesius | <1 |
| Celtis australis | <1 | <1 | Rubus idaeus | <1 |
| Pinus nigra | <1 | <1 |
References
- Bastin, J.F.; Finegold, Y.; Garcia, C.; Mollicone, D.; Rezende, M.; Routh, D.; Zohner, C.M.; Crowther, T.W. The global tree restoration potential. Science 2019, 365, 76. [Google Scholar] [CrossRef] [PubMed]
- Löf, M.; Madsen, P.; Metslaid, M.; Witzell, J.; Jacobs, D.F. Restoring forests: Regeneration and ecosystem function for the future. New For. 2019, 50, 139–151. [Google Scholar] [CrossRef]
- Krajnc, A. History of deforestation and reforestation in the Dinaric Karst. Geogr. Res. 2009, 47, 15–23. [Google Scholar]
- Šercelj, A. The Origins and Development of Forests in Slovenia; Slovene Academy of Arts and Sciences: Ljubljana, Slovenia, 1996. (In Slovenian) [Google Scholar]
- Portoghesi, L.; Consalvo, M.; Angelini, A.; Ferrari, B.; Barbati, A.; Castaldi, C.; Corona, P. Multifunctional management of mountain reforestations: Thoughts and perspectives from a case study in central Italy. Ital. J. For. Mt. Environ. 2013, 68, 305–315. [Google Scholar] [CrossRef][Green Version]
- Sánchez-Salguero, R.; Camarero, J.J.; Dobbertin, M.; Fernández-Cancio, Á.; Vilà-Cabrera, A.; Manzanedo, R.D.; Zavala, M.A.; Navarro-Cerrillo, R.M. Contrasting vulnerability and resilience to drought-induced decline of densely planted vs. Natural rear-edge Pinus nigra forests. For. Ecol. Manag. 2013, 310, 956–967. [Google Scholar] [CrossRef]
- Gajsek, D.; Jarni, K.; Brus, R. Conversion of old black pine stands using broadleaf tree species in the Slovenian karst. Dendrobiology 2015, 74, 77–84. [Google Scholar] [CrossRef][Green Version]
- Nocentini, S. La rinaturalizzazione dei rimboschimenti. Una prova su pino nero e laricio nel complesso di Monte Morello (Firenze). L’Italia Forestale e Montana 1995, 50, 425–435. [Google Scholar]
- Mercurio, R.; Spinelli, R. Exploring the silvicultural and economic viability of gap cutting in Mediterranean softwood plantations. For. Stud. China 2012, 14, 63–69. [Google Scholar] [CrossRef]
- Zerbe, S. Restoration of natural broad-leaved woodland in central Europe on sites with coniferous forest plantations. For. Ecol. Manag. 2002, 167, 27–42. [Google Scholar] [CrossRef]
- Zlatanov, T.; Velichkov, I.; Lexer, M.J.; Dubravac, T. Regeneration dynamics in aging black pine (Pinus nigra Arn.) plantations on the south slopes of the middle Balkan range in Bulgaria. New For. 2010, 40, 289–303. [Google Scholar] [CrossRef]
- Anić, I. Promjena sastojinskog oblika prirodnim pomlađivanjem na primjeru šumske kulture crnoga bora (Pinus nigra Arn.) u Senjskoj Dragi. Šumarski List 2003, 77, 41–49. [Google Scholar]
- Mosandl, R.; Kleinert, A. Development of oaks (Quercus petraea (Matt.) Liebl.) emerged from bird-dispersed seeds under old-growth pine (Pinus silvestris L.) stands. For. Ecol. Manag. 1998, 106, 35–44. [Google Scholar] [CrossRef]
- Dobrowolska, D. Oak natural regeneration and conversion processes in mixed Scots pine stands. Forestry 2006, 79, 503–513. [Google Scholar] [CrossRef]
- Vallauri, D.R.; Aronson, J.; Barbero, M. An analysis of forest restoration 120 years after reforestation on badlands in the southwestern Alps. Restor. Ecol. 2002, 10, 16–26. [Google Scholar] [CrossRef]
- Gams, I. Origin of the term “karst” and the transformation of the Classical Karst (kras). Environ. Geol. 1993, 21, 110–114. [Google Scholar] [CrossRef]
- ARSO. Eionet (Environmental Information and Observation Network); ARSO Environmental Agency of the Republic of Slovenia: Ljubljana, Slovenia, 2019. Available online: http://eionet-en.Arso.Gov.Si/ (accessed on 22 November 2019).
- Parent, S.; Messier, C. A simple and efficient method to estimate microsite light availability under a forest canopy. Can. J. For. Res. 1996, 26, 151–154. [Google Scholar] [CrossRef]
- IMKO. Trime-ez/-ezc/-It/-Itc User Manual. Available online: http://95.110.175.13/rid=1Q22RVC3H-QC1NPP-BKZ/Imko_Humedad_suelo.pdf (accessed on 22 November 2019).
- ARSO. Pregled Hidroloških Razmer Površinskih Voda v Sloveniji; Poročilo o Monitoringu za leto 2012; Ministrstvo za Okolje in Prostor, Agencija RS za Okolje: Ljubljana, Slovenia, 2012.
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 24 November 2019).
- Bolker, B.; Skaug, H.; Magnusson, A.; Nielsen, A. Getting Started with the GlmmADMB Package. 2012. Available online: http://glmmadmb.r-forge.r-project.org/glmmADMB.pdf (accessed on 18 March 2015).
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with r; Springer: New York, NY, USA, 2009; p. 574. [Google Scholar]
- Robinson, A.P.; Hamann, J.D. Forest Analytics with R: An Introduction; Springer: New York, NY, USA, 2011; p. 354. [Google Scholar]
- Landolt, E.; Bäumler, B.; Erhardt, A.; Hegg, O.; Klötzli, F.; Lämmler, W.; Nobis, M.; Rudmann-Maurer, K.; Schweingruber, F.; Theurillat, J. Flora Indicativa: Ökologische Zeigerwerte und Biologische Kennzeichen zur Flora der Schweiz und der Alpen; Haupt: Bern, Switzerland, 2010; p. 378. [Google Scholar]
- Lepš, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003; p. 362. [Google Scholar]
- Oksanen, J.; Guillaume, B.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Community Ecology Package. Available online: https//CRAN.R-project.org/package=vegan (accessed on 22 November 2019).
- Legendre, P.; Anderson, M.J. Distance-based redundancy analysis: Testing multispecies responses in multifactorial ecological experiments. Ecol. Monogr. 1999, 69, 1–24. [Google Scholar] [CrossRef]
- Cáceres, M.D.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef]
- Nussbaumer, A.; Waldner, P.; Etzold, S.; Gessler, A.; Benham, S.; Thomsen, I.M.; Jørgensen, B.B.; Timmermann, V.; Verstraeten, A.; Sioen, G. Patterns of mast fruiting of common beech, sessile and common oak, Norway spruce and Scots pine in Central and Northern Europe. For. Ecol. Manag. 2016, 363, 237–251. [Google Scholar] [CrossRef]
- Caldeira, M.C.; Ibáñez, I.; Nogueira, C.; Bugalho, M.N.; Lecomte, X.; Moreira, A.; Pereira, J.S. Direct and indirect effects of tree canopy facilitation in the recruitment of Mediterranean oaks. J. Appl. Ecol. 2014, 51, 349–358. [Google Scholar] [CrossRef]
- Pulido, F.J.; Díaz, M. Regeneration of a Mediterranean oak: A whole-cycle approach. Ecoscience 2005, 12, 92–102. [Google Scholar] [CrossRef]
- Oberlechner, V.; Vacik, H. Regeneration and growth success of downy oak (Quercus pubescens) in secondary Austrian black pine forests (Pinus nigra) in Vinschgau, Southtyrol. Allgemeine Forst und Jagdzeitung 2003, 174, 219–226. [Google Scholar]
- Gray, A.N.; Spies, T.A.; Easter, M.J. Microclimatic and soil moisture responses to gap formation in coastal Douglas-fir forests. Can. J. For. Res. 2002, 32, 332–343. [Google Scholar] [CrossRef]
- Vilhar, U.; Roženbergar, D.; Simončič, P.; Diaci, J. Variation in irradiance, soil features and regeneration patterns in experimental forest canopy gaps. Ann. For. Sci. 2015, 72, 253–266. [Google Scholar] [CrossRef]
- Prevosto, B.; Monnier, Y.; Ripert, C.; Fernandez, C. Can we use shelterwoods in Mediterranean pine forests to promote oak seedling development? For. Ecol. Manag. 2011, 262, 1426–1433. [Google Scholar] [CrossRef]
- Eiberle, K.; Nigg, H. Grundlagen zur Beurteilung des Wildverbisses im Gebirgswald. Schweiz. Z. Forstwes. 1987, 138, 747–780. [Google Scholar]
- Hester, A.; Bergman, M.; Iason, G.; Moen, J. Impacts of large herbivores on plant community structure and dynamics. In Large Herbivore Ecology, Ecosystem Dynamics and Conservation; Danell, K., Bergstrom, R., Duncan, P., Pastor, J., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 97–141. [Google Scholar]
- Barbalat, S.; Junod, P.; Plachta, M. Silviculture of two heliophilic tree species and insect fauna diversity. Schweiz. Z. Forstwes. 1997, 148, 789–807. [Google Scholar]
- Mercurio, R.; Mallamaci, C.; Muscolo, A.; Sidari, M. Gap size effects on tree regeneration in afforestations of black pine (Pinus nigra arn.). Forest 2009, 6, 312–319. [Google Scholar] [CrossRef]
- Schütz, J.-P.; Saniga, M.; Diaci, J.; Vrska, T. Comparing close-to-nature silviculture with processes in pristine forests: Lessons from central europe. Ann. For. Sci. 2016, 73, 911–921. [Google Scholar] [CrossRef]


| Herb Subplots | |||||||
| Height Class | F. ornus | O. carpinif. | Quercus | Ulmus | N. Brd. | Other | Sum |
| h < 20 cm | 9616 | 321 | 1859 | 270 | 710 | 152 | 12,928 |
| 20 ≤ h < 130 cm | 9632 | 1724 | 794 | 253 | 744 | 51 | 13,164 |
| h ≥ 130 cm | 1335 | 422 | 68 | 17 | 51 | 68 | 1960 |
| Total | 20,583 | 2467 | 2721 | 541 | 1504 | 270 | 28,086 |
| Quercus Subplots | |||||||
| h < 20 cm | 8494 | 353 | 4237 | 166 | 228 | 42 | 13,520 |
| 20 ≤ h < 130 cm | 6397 | 1059 | 6044 | 332 | 187 | 145 | 14,164 |
| h ≥ 130 cm | 748 | 602 | 166 | 0 | 0 | 21 | 1537 |
| Total | 15,639 | 2015 | 10,447 | 498 | 415 | 208 | 29,221 |
| Variable | Quercus < 20 cm | Quercus ≥ 20 cm | F. ornus < 20 cm | F. ornus ≥ 20 cm | O. carpinif. ≥ 20 cm |
|---|---|---|---|---|---|
| Intercept | −0.78 (1.55) | −5.65 (0.90) *** | 12.16 (2.64) *** | 9.72 (2.25) *** | 15.33 (3.60) *** |
| Stand type: closed vs. open | −0.26 (0.20) | 0.94 (0.16) *** | −0.72 (0.21) *** | 0.44 (0.19) * | −0.61 (0.40) |
| Stand type: closed vs. gap edge | −0.62 (0.28) * | 0.57 (0.22) ** | −1.30 (0.30) *** | −0.06 (0.27) | −0.89 (0.39) * |
| Stand type: closed vs. gap centre | −0.17 (0.35) *** | 0.13 (0.22) | −3.61 (0.67) *** | −0.004 (0.28) | −1.28 (0.36) *** |
| Transect: Quercus vs. Herb | 0.92 (0.17) *** | 1.95 (0.16) *** | ns | −0.40 (0.17) * | ns |
| Soil depth | 0.34 (0.12) ** | ns | ns | ns | ns |
| LIV nutrients | −0.83 (0.37) * | ns | −2.32 (0.48) *** | ns | ns |
| LIV continentality | 0.77 (0.35) * | ns | −2.21 (0.42) *** | −2.79 (0.39) *** | −2.23 (0.57) *** |
| LIV light | ns | 0.48 (0.18) ** | −0.92 (0.33) ** | ns | −1.74 (0.49) *** |
| LIV moisture | ns | 0.74 (0.27) ** | ns | −2.09 (0.43) *** | −1.70 (0.69) * |
| LIV soil reaction | ns | ns | 1.39 (0.41) *** | 1.30 (0.38) *** | ns |
| Coverage S. autumnalis | −0.29 (0.11) ** | ns | ns | 0.42 (0.09) *** | ns |
| Distance to Quercus seed trees | −0.12 (0.05) * | 0.08 (0.04) * | nt | nt | nt |
| Rockiness | ns | −0.48 (0.22) * | ns | ns | −1.73 (0.44) *** |
| LFH | ns | ns | ns | 0.09 (0.03) ** | ns |
| Relief: flat vs other | ns | ns | ns | −0.39 (0.20) * | ns |
| Random effects: a Plot | <0.01 (<0.01) | <0.01 (<0.01) | <0.01 (<0.01) | <0.01 (0.09) | <0.01 (<0.01) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaci, J.; Adamič, T.; Rozman, A.; Fidej, G.; Roženbergar, D. Conversion of Pinus nigra Plantations with Natural Regeneration in the Slovenian Karst: The Importance of Intermediate, Gradually Formed Canopy Gaps. Forests 2019, 10, 1136. https://doi.org/10.3390/f10121136
Diaci J, Adamič T, Rozman A, Fidej G, Roženbergar D. Conversion of Pinus nigra Plantations with Natural Regeneration in the Slovenian Karst: The Importance of Intermediate, Gradually Formed Canopy Gaps. Forests. 2019; 10(12):1136. https://doi.org/10.3390/f10121136
Chicago/Turabian StyleDiaci, Jurij, Tomaž Adamič, Andrej Rozman, Gal Fidej, and Dušan Roženbergar. 2019. "Conversion of Pinus nigra Plantations with Natural Regeneration in the Slovenian Karst: The Importance of Intermediate, Gradually Formed Canopy Gaps" Forests 10, no. 12: 1136. https://doi.org/10.3390/f10121136
APA StyleDiaci, J., Adamič, T., Rozman, A., Fidej, G., & Roženbergar, D. (2019). Conversion of Pinus nigra Plantations with Natural Regeneration in the Slovenian Karst: The Importance of Intermediate, Gradually Formed Canopy Gaps. Forests, 10(12), 1136. https://doi.org/10.3390/f10121136






