Autotoxicity Hinders the Natural Regeneration of Cinnamomum migao H. W. Li in Southwest China
Abstract
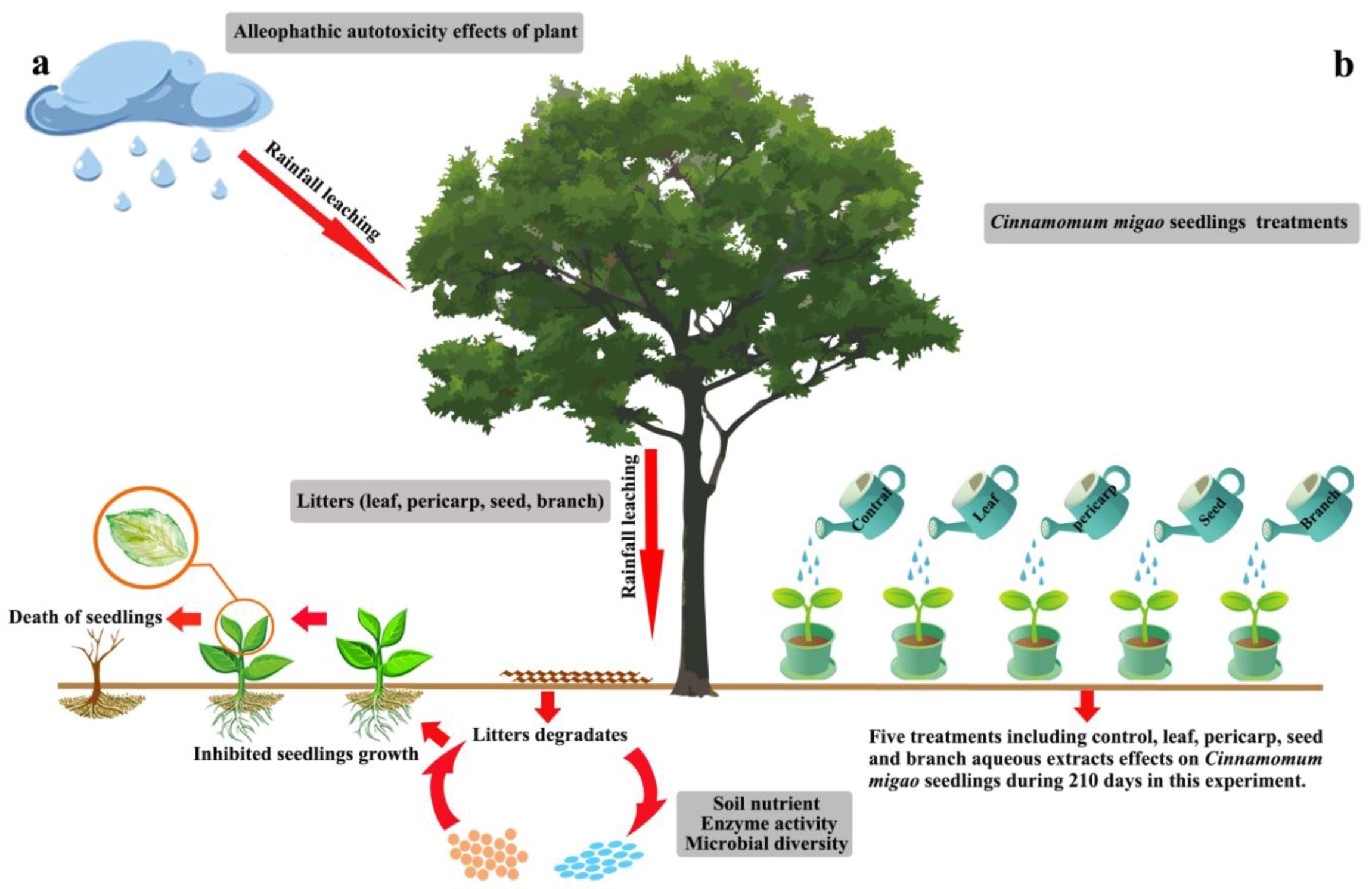
:1. Introduction
2. Material and Methods
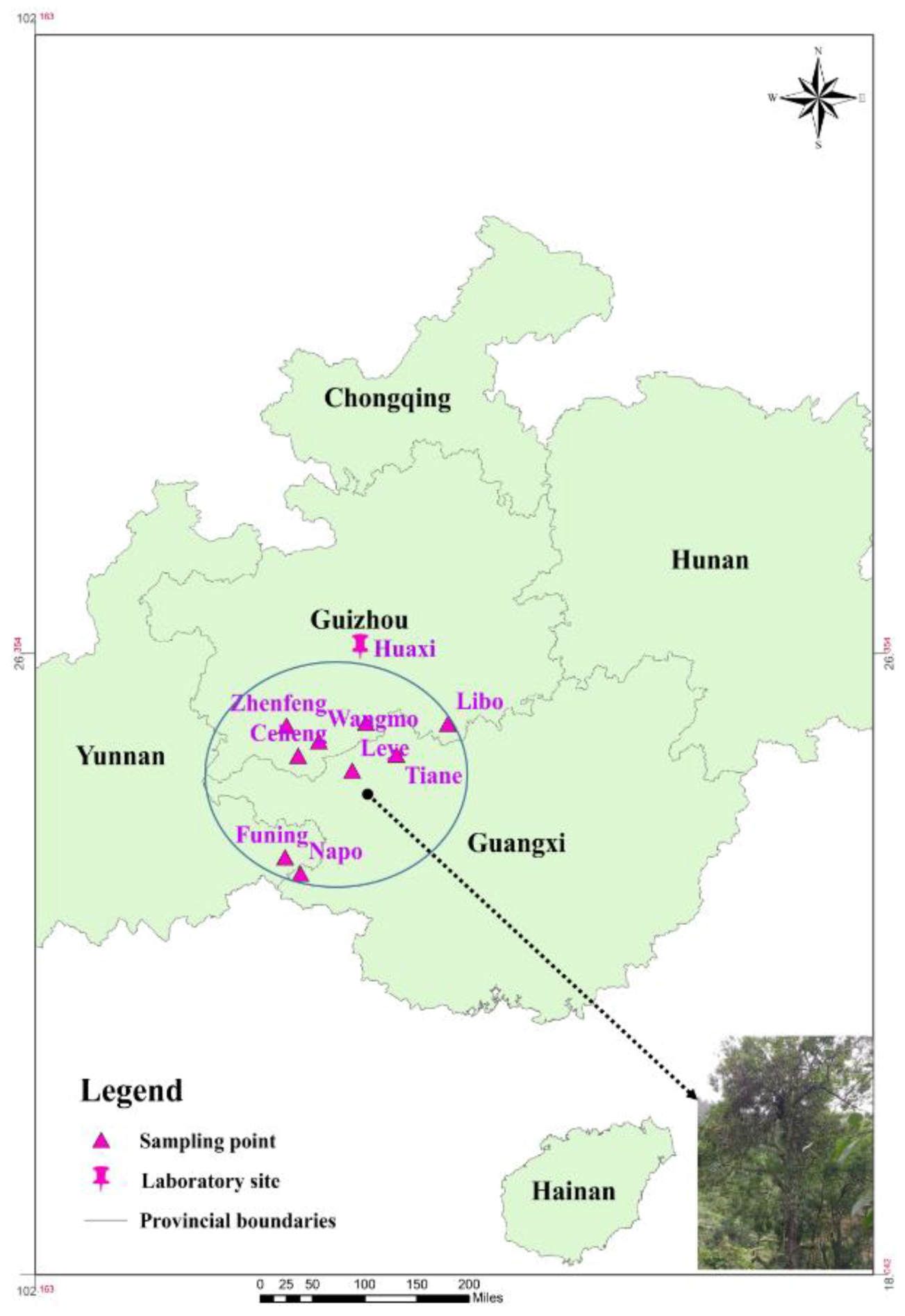
2.1. Experimental Site
2.2. Plant Materials
2.3. Experimental Design
2.4. Chemical Analyses
2.4.1. Analysis of Seedling Growth and Physiology
2.4.2. Analysis of Soil Physicochemical Properties and Soil Enzyme Activity
2.4.3. Analysis of Soil Fungi
2.5. Statistical Analysis
3. Results
3.1. Effects of C. migao Litter Aqueous Extract on Seedling Growth
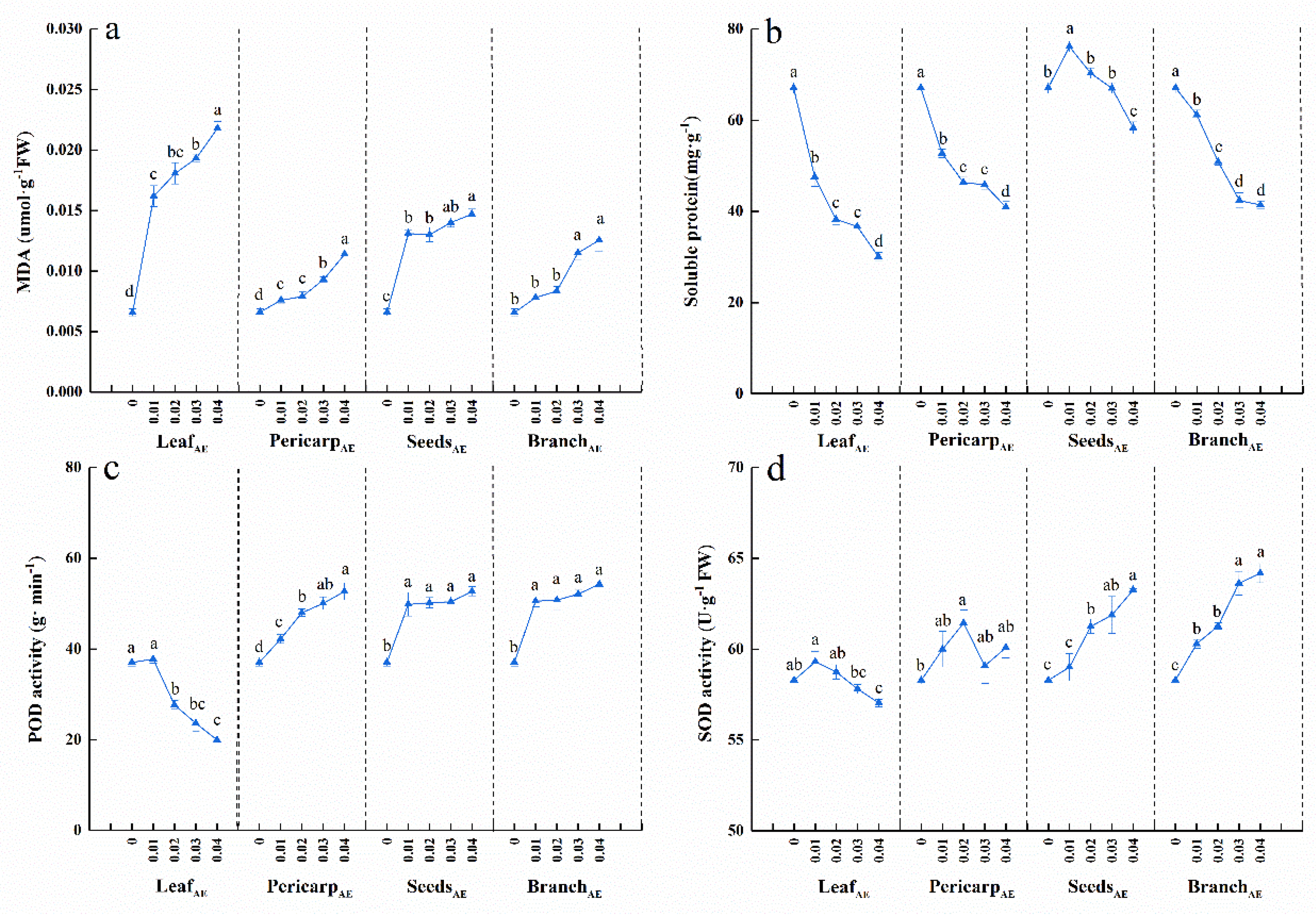
3.2. Effects of C. migao Litter Aqueous Extract on the Antioxidant Systems of Seedlings
3.3. Effects of C. migao Litter Aqueous Extracts on N, P, and K in Soils of Potted Seedlings
3.4. Effects of C. Migao Litter Aqueous Extract on Soil Enzyme Activities of Potted Seedlings
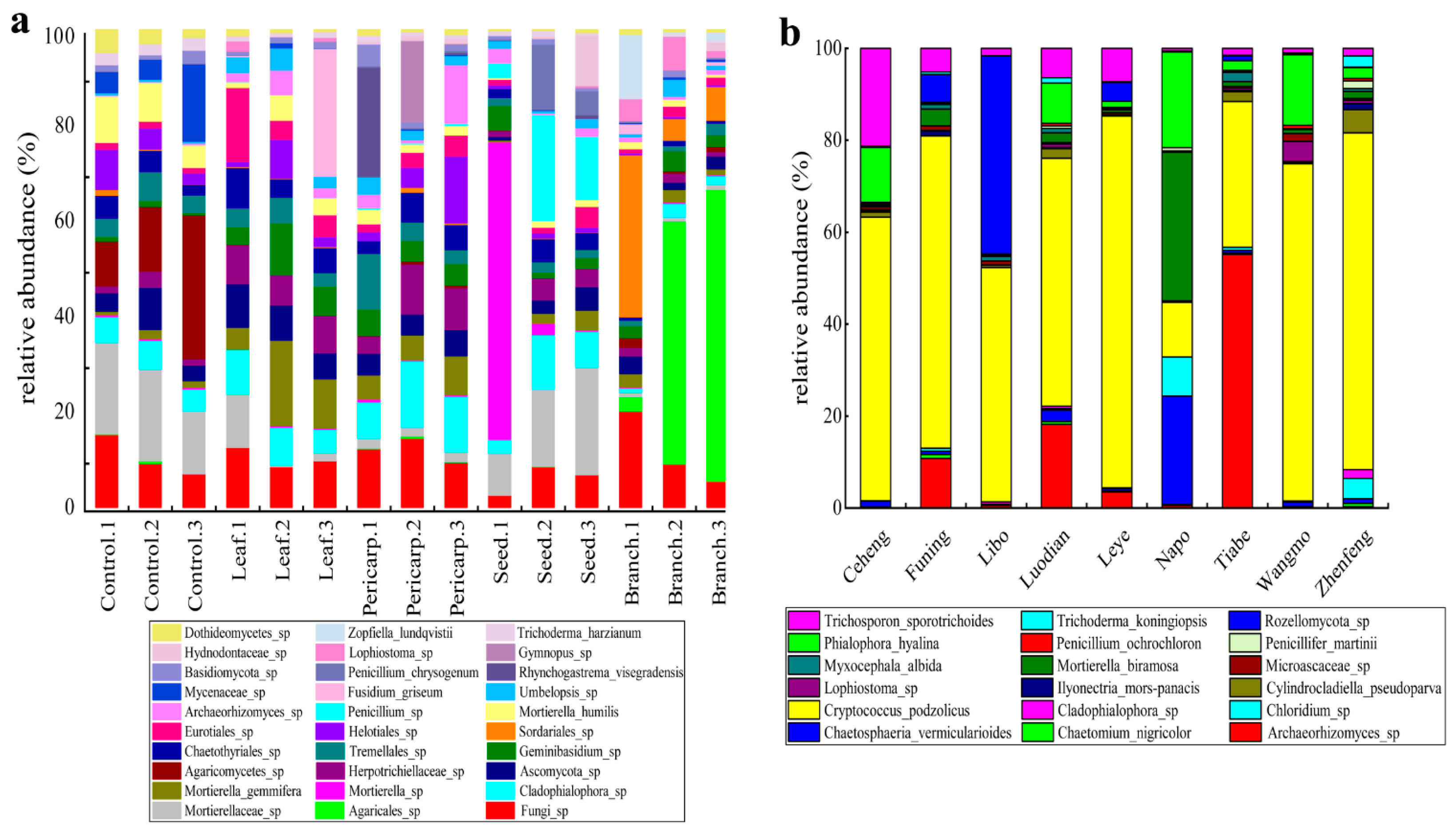
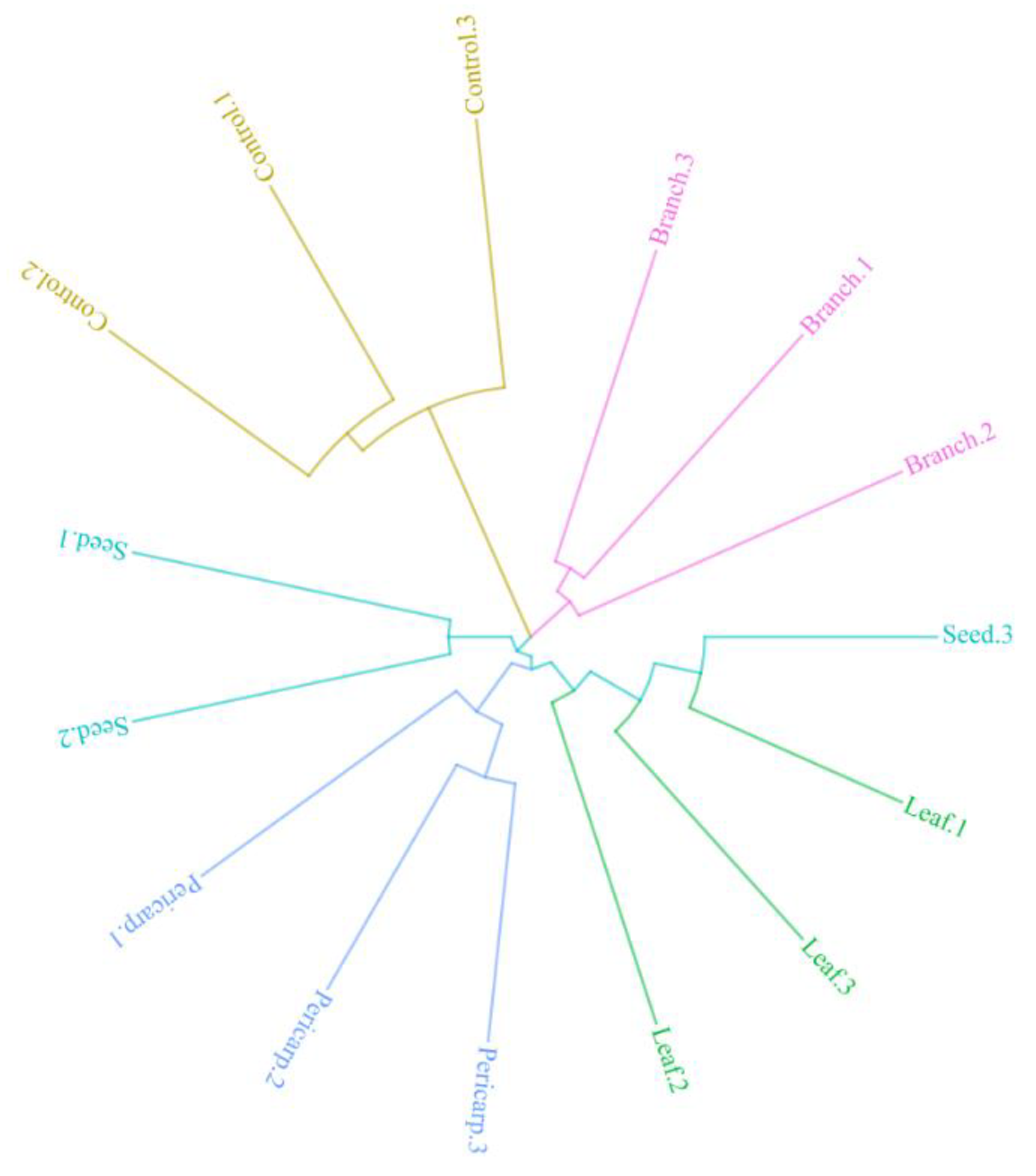
3.5. Composition of Fungi in Different Plots and Experimental Treatments
4. Discussion
4.1. Seedling Growth and Antioxidant System Responses to Aqueous Extracts from Different Anatomical Parts of C. migao
4.2. Soil Nutrition and Enzyme Activity Responses to Aqueous Extracts of Different Anatomical Parts of C. migao
4.3. Soil Fungal Component with Different Treatments and in Contrast to that in the Field
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lorenzo, P.; Rodríguezecheverría, S. Influence of soil microorganisms, allelopathy and soil origin on the establishment of the invasive Acacia dealbata. Plant Ecol. Divers. 2012, 5, 67–73. [Google Scholar] [CrossRef]
- Xia, R.; Xiaofeng, H.; Zhongfeng, Z.; Zhiqiang, Y.; Hui, J.; Xiuzhuang, L.; Bo, Q. Isolation, Identification, and Autotoxicity Effect of Allelochemicals from Rhizosphere Soils of Flue-Cured Tobacco. J. Agri. Food Chem. 2015, 63, 8975. [Google Scholar]
- Fuentes-Ramírez, A.; Pauchard, A.; Cavieres, L.A.; García, R.A. Survival and growth of Acacia dealbata vs. native trees across an invasion front in south-central Chile. Forest Ecol. Manag. 2011, 261, 1003–1009. [Google Scholar] [CrossRef]
- Blum, U. Plant—Plant Allelopathic Interactions; Springer Netherlands: Dordrecht, The Netherlands, 2011; p. 17. [Google Scholar]
- Fernandez, C.; Monnier, Y.; Santonja, M.; Gallet, C.; Weston, L.A.; Prévosto, B.; Saunier, A.; Baldy, V.; Bousquet-Mélou, A. The Impact of Competition and Allelopathy on the Trade-Off between Plant Defense and Growth in Two Contrasting Tree Species. Front Plant Sci. 2016, 7, 594. [Google Scholar] [CrossRef] [PubMed]
- Xia, R.; Yan, Z.Q.; He, X.F.; Li, X.Z.; Bo, Q. Allelochemicals from rhizosphere soils of Glycyrrhiza uralensis Fisch: Discovery of the autotoxic compounds of a traditional herbal medicine. Ind. Crops Prod. 2017, 97, 302–307. [Google Scholar]
- Ahmed, R. Allelopathic effects of leaf litters of Eucalyptus camaldulensis on some forest and agricultural crops. J. For. Res. 2008, 19, 19–24. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Heikki, S.L.; Putten, W.H.V.D.; Wall, D.H. Ecological linkages between aboveground and belowground biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef]
- Mitić, N.; Stanišić, M.; Savić, J.; Ćosić, T.; Stanisavljević, N.; Miljuš-Đukić, J.; Marin, M.; Radović, S.; Ninković, S. Physiological and cell ultrastructure disturbances in wheat seedlings generated by Chenopodium murale hairy root exudate. Protoplasma 2018, 255, 1683–1692. [Google Scholar] [CrossRef]
- Yang, L.; Peng, W.; Kong, C. Effect of larch (Larix gmelini Rupr.) root exudates on Manchurian walnut (Juglans mandshurica Maxim.) growth and soil juglone in a mixed-species plantation. Plant Soil 2010, 329, 249–258. [Google Scholar] [CrossRef]
- Feng, Y.; Hu, Y.; Wu, J.; Chen, J.; Yrjala, K.; Yu, W. Change in microbial communities, soil enzyme and metabolic activity in a Torreya grandis plantation in response to root rot disease. Forest Ecol. Manag. 2019, 432, 932–941. [Google Scholar] [CrossRef]
- Chomel, M.; Guittonny-Larchevêque, M.; Fernandez, C.; Gallet, C.; Baldy, V. Plant secondary metabolites: A key driver of litter decomposition and soil nutrient cycling. J. Ecol. 2016, 104, 1527–1541. [Google Scholar] [CrossRef]
- Gavinet, J.; Santonja, M.; Baldy, V.; Hashoum, H.; Bousquet-Mélou, A. Phenolics of the understory shrub Cotinus coggygria influence Mediterranean oak forests diversity and dynamics. Forest Ecol. Manag. 2019, 441, 262–270. [Google Scholar] [CrossRef]
- Lobón, N.C.; Cruz, I.F.D.L.; Gallego, J.C.A. Autotoxicity of Diterpenes Present in Leaves of Cistus ladanifer L. Plants 2019, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Margherita, G.; Osborne, B.A. Resource competition in plant invasions: Emerging patterns and research needs. Front Plant Sci. 2014, 5, 501. [Google Scholar] [CrossRef]
- Santonja, M.; Foucault, Q.; Rancon, A.; Gauquelin, T.; Mirleau, P. Contrasting responses of bacterial and fungal communities to plant litter diversity in a Mediterranean oak forest. Soil Biol. Biochem. 2018, 125, 27–36. [Google Scholar] [CrossRef]
- Fernandez, C.; Santonja, M.; Gros, R.; Monnier, Y.; Chomel, M.; Baldy, V.; Bousquet-Mélou, A. Allelochemicals of Pinus halepensis as drivers of biodiversity in;mediterranean open mosaic habitats during the colonization stage of secondary Succession. J. Chem. Ecol. 2013, 39, 298–311. [Google Scholar] [CrossRef]
- Rose, S.L.; Perry, D.A.; Pilz, D.; Schoeneberger, M.M. Allelopathic effects of litter on the growth and colonization of mycorrhizal fungi. J. Chem. Ecol. 1983, 9, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Chahartaghi, M.; Langel, R.; Scheu, S.; Ruess, L. Feeding guilds in Collembola based on nitrogen stable isotope ratios. Soil Biol. Biochem. 2005, 37, 1718–1725. [Google Scholar] [CrossRef]
- Santonja, M.; Fernandez, C.; Proffit, M.; Gers, C.; Gauquelin, T.; Reiter, I.M.; Cramer, W.; Baldy, V. Plant litter mixture partly mitigates the negative effects of extended drought on soil biota and litter decomposition in a Mediterranean oak forest. J. Ecol. 2016, 105, 801–815. [Google Scholar] [CrossRef]
- Yeasmin, R.; Motoki, S.; Yamamoto, S.; Nishihara, E. Allelochemicals inhibit the growth of subsequently replanted asparagus (Asparagus officinalis L.). Biol. Agric. Hortic. 2013, 29, 165–172. [Google Scholar] [CrossRef]
- Nilsson, M.C.A.Z. Characterisation of the differential interference effects of two boreal dwarf shrub species. Oecologia 2000, 123, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Alías, J.C.; Sosa, T.; Escudero, J.C.; Chaves, N. Autotoxicity Against Germination and Seedling Emergence in Cistus ladanifer L. Plant Soil 2006, 282, 327–332. [Google Scholar] [CrossRef]
- Li, T.X.; Wang, J.K. Supercritical carbon dioxide extraction of essential oil from Cinnamomum migao HW Li. J. Chin. Med. Mater. 2003, 26, 178–180. [Google Scholar]
- Hu, L.; Xue, R.; Xu, C.; Zhang, Z.; Zhang, G.; Zeng, R.; Song, Y. Autotoxicity in the cultivated medicinal herb Andrographis paniculata. Allelopath. J. 2018, 45, 141–151. [Google Scholar] [CrossRef]
- Zhou, T.; Yang, Z.N.; Jiang, W.K.; Ai, K.; Guo, W.K. Variation and regularity of volatile oil constituents in fruits of national medicine Cinnamomum migao. China J. Chin. Mater. Med. 2010, 35, 852–856. [Google Scholar]
- Zhao, S.; Li, H.Y.; Qiu, D.W.; Liu, Z.R.; Liu, N. Cinnamomum migao resources and ecological investigation: Guizhou, Northern Guangxi and the Contiguous Areas of Hunan, Guizhou and Guangxi. J. Guiyang Univ. Chin. Med. 1991, 59–61. [Google Scholar] [CrossRef]
- Xie, M.; Yan, Z.Q.; Ren, X.; Li, X.Z.; Qin, B. Autotoxin in cultivated soil of codonopsis pilosula (franch.) nannf. J. Agri. Food Chem. 2017, 65, 2032–2038. [Google Scholar] [CrossRef]
- Chen, J.Z.; Liu, J.M.; Xiong, X.; Huang, X.L.; Tong, B.L.; Wen, A.L. Assessing the feasibility of tree-medicinal plant intercropping system of Cinnamomum migao and Aomum villosumt based on allelopathy. Chinese J. Ecol. 2019, 38, 1322–1330. [Google Scholar]
- Muhl, R.M.W.; Roelke, D.L.; Zohary, T.; Moustaka-Gouni, M.; Sommer, U.; Borics, G.; Gaedke, U.; Withrow, F.G.; Bhattacharyya, J. Resisting annihilation: Relationships between functional trait dissimilarity, assemblage competitive power and allelopathy. Ecol. Lett. 2018, 21, 1390–1400. [Google Scholar] [CrossRef]
- Hodges, D.; Forney, C.; Prange, R.J. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Lowry, O.H.R.N. Determination of protein by Folin-Phenol reagent. Food Drug 2011, 13, 147–151. [Google Scholar]
- Venisse, J.S.; Malnoy, M.M.; Paulin, J.P.; Brisset, M.N. Modulation of defense responses of Malus spp. during compatible and incompatible interactions with Erwinia amylovora. Mol. Plant Microbe Interact 2002, 15, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Lacan, D.; Baccou, J.C. High levels of antioxidant enzymes correlate with delayed senescence in nonnetted muskmelon fruits. Planta 1998, 204, 377–382. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, S.; Huang, Y. Comparisons of litterfall, litter decomposition and nutrient return in a monoculture Cunninghamia lanceolata and a mixed stand in southern China. Forest Ecol. Manag. 2008, 255, 1210–1218. [Google Scholar] [CrossRef]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis: Part 2, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Agronomy Society of America and Soil Science Society of America: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Dorich, R.A.; Nelson, D.W. Evaluation of manual cadmium reduction methods for determination of nitrate in potassium chloride extracts of soils. Soil Sci. Soc. Am. J. 1984, 48, 72–75. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agricultural Chemistry Analysis, 3rd ed.; China Agricultural Press: Beijing, China, 2013; pp. 56–58. [Google Scholar]
- Bughio, F.; Mangrio, S.; Abro, S.; Jahangir, D.T.M.; Bux, H. Physio-morphological responses of native Acacia nilotica to Eucalyptus allelopathy. Pak. J. Bot. 2013, 45, 97–105. [Google Scholar]
- Santos, F.M.; Balieiro, F.D.C.; Diniz, A.R.; Chaer, G.M. Dynamics of aboveground biomass accumulation in monospecific and mixed-species plantations of Eucalyptus and Acacia on a Brazilian sandy soil. Forest Ecol. Manag. 2016, 363, 86–97. [Google Scholar] [CrossRef]
- Alrababah, M.A.; Tadros, M.J.; Samarah, N.H.; Ghosheh, H. Allelopathic effects of Pinus halepensis and Quercus coccifera on the germination of Mediterranean crop seeds. New For. 2009, 38, 261–272. [Google Scholar] [CrossRef]
- An, M.; Pratley, J.E.; Haig, T. Phytotoxicity of vulpia residues: III. Biological activity of identified allelochemicals from Vulpia myuros. J. Chem. Ecol. 2001, 27, 383–394. [Google Scholar] [CrossRef]
- Lin, W.X.; Kim, K.U.; Shin, D.H. Rice allelopathic potential and its modes of action on Barnyardgrass (Echinochloa crus-galli). Allelopath. J. 2000, 7, 215–224. [Google Scholar]
- Strom, S.L. Microbial ecology of ocean biogeochemistry: A community perspective. Science 2008, 320, 1043–1045. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Sun, Y.; Xiao, C.L.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Physiological basis of different allelopathic reactions of cucumber and figleaf gourd plants to cinnamic acid. J. Exp. Bot. 2007, 58, 3765–3773. [Google Scholar] [CrossRef] [PubMed]
- Amit, D.; Portis, A.R.; Henry, D. Enhanced translation of a chloroplast-expressed RbcS gene restores small subunit levels and photosynthesis in nuclear RbcS antisense plants. Proc. Natl. Acad. Sci. USA 2004, 101, 6315–6320. [Google Scholar]
- Haichar, F.E.Z.; Santaella, C.; Heulin, T.; Achouak, W. Root exudates mediated interactions belowground. Soil Biol. Biochem. 2014, 77, 69–80. [Google Scholar] [CrossRef]
- Stefano, M.; Giuliano, B.; Guido, I.; Maria Luisa, C.; Pasquale, T.; Antonio, M.; Mauro, S.; Francesco, G.; Fabrizio, C.; Max, R. Inhibitory and toxic effects of extracellular self-DNA in litter: A mechanism for negative plant-soil feedbacks? New Phytol. 2015, 205, 1195–1210. [Google Scholar]
- Zornoza, R.; Guerrero, C.; Matax-Solera, J.; Arcenegui, V.; García-Orenes, F.M.J. Assessing air-drying and rewetting pre-treatment effect on some soil enzyme activities under Mediterranean conditions. Soil Biol. Biochem. 2006, 38, 2125–2134. [Google Scholar] [CrossRef]
- Freschet, G.T.; Violle, C.; Bourget, M.Y.; Scherer-Lorenzen, M.; Fort, F. Allocation, morphology, physiology, architecture: The multiple facets of plant above- and below-ground responses to resource stress. New Phytol. 2018, 219. [Google Scholar] [CrossRef]
- Chen, C.; Chen, D.; Shu, K.L. Simulation of Nitrous Oxide Emission and Mineralized Nitrogen under Different Straw Retention Conditions Using a Denitrification–Decomposition Model. CLEAN—Soil Air Water 2015, 43, 577–583. [Google Scholar] [CrossRef]
- Mao, J.; Yang, L.; Shi, Y.; Jian, H.U.; Zhe, P.; Mei, L.; Yin, S. Crude extract of Astragalus mongholicus root inhibits crop seed germination and soil nitrifying activity. Soil Biol. Biochem. 2006, 38, 201–208. [Google Scholar] [CrossRef]
- Corneo, P.E.; Alberto, P.; Luca, C.; Marco, R.; Marco, C.; Cesare, G.; Ilaria, P. Microbial community structure in vineyard soils across altitudinal gradients and in different seasons. Fems Microbiol. Ecol. 2013, 84, 588–602. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, J.; Wu, F. Soil microbial communities in cucumber monoculture and rotation systems and their feedback effects on cucumber seedling growth. Plant Soil 2017, 415, 507–520. [Google Scholar] [CrossRef]
- van Dam, N.M.; Bouwmeester, H.J. Metabolomics in the Rhizosphere: Tapping into Belowground Chemical Communication. Trends Plant Sci. 2016, 21, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Jackrel, S.L.; Wootton, J.T. Cascading effects of induced terrestrial plant defences on aquatic and terrestrial ecosystem function. Proc. Biol. Sci. 2015, 282, 264–266. [Google Scholar] [CrossRef] [PubMed]
- Frey, S.D.; Ollinger, S.; Nadelhoffer, K.; Bowden, R.; Brzostek, E.; Burton, A.; Caldwell, B.A.; Crow, S.; Goodale, C.L.; Grandy, A.S. Chronic nitrogen additions suppress decomposition and sequester soil carbon in temperate forests. Biogeochemistry 2014, 121, 305–316. [Google Scholar] [CrossRef]
- Saiya-Cork, K.R.; Sinsabaugh, R.L.; Zak, D.R. The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol. Biochem. 2002, 34, 1309–1315. [Google Scholar] [CrossRef]
- Hause, B.; Schaarschmidt, S. The role of jasmonates in mutualistic symbioses between plants and soil-born microorganisms. Phytochemistry 2009, 69, 1589–1599. [Google Scholar] [CrossRef]


| Soil Properties | pH | Total N (g·kg−1) | Total P (g·kg−1) | Total K (g·kg−1) | Available N (g·kg−1) | Available P (mg·kg−1) | Available K (mg·kg−1) | Catalase mL·g−1·20 min | Urease mgNH3−N/g | Phopatase umol·g−1·d−1 | Invertase mg·g−1·d−1 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 6.06 | 1.2 | 0.2 | 0.29 | 52.5 | 3.5 | 13.03 | 1.43 | 0.015 | 23.53 | 1.5 |
| Group | Concentration (g·mL−1) | Height (cm) | Biomass (g) | Leaf Area (cm2) |
|---|---|---|---|---|
| LeafAE | 0 | 162.53 ± 3.75 a | 148.30 ± 7.10 a | 17.69 ± 4.30 a |
| 0.01 | 143.07 ± 7.30 b | 110.80 ± 8.70 b | 15.57 ± 2.88 bc | |
| 0.02 | 131.23 ± 8.60 bc | 99.80 ± 9.10 bc | 14.35 ± 1.24 bc | |
| 0.03 | 129.30 ± 6.80 c | 95.30 ± 4.40 c | 12.41 ± 2.65 bc | |
| 0.04 | 110.40 ± 4.96 d | 75.60 ± 5.70 d | 10.26 ± 3.68 c | |
| PericarpAE | 0 | 162.53 ± 3.75 d | 148.30 ± 7.10 c | 17.69 ± 4.30 b |
| 0.01 | 220.60 ± 6.10 c | 176.30 ± 6.40 b | 23.46 ± 3.41 ab | |
| 0.02 | 225.50 ± 7.50 bc | 182.40 ± 6.30 b | 22.33 ± 3.62 ab | |
| 0.03 | 235.80 ± 5.20 b | 183.80 ± 5.50 b | 22.89 ± 3.05 ab | |
| 0.04 | 255.60 ± 5.70 a | 203.70 ± 7.70 a | 26.69 ± 3.67 a | |
| SeedAE | 0 | 162.53 ± 3.75 d | 148.30 ± 7.10 b | 17.69 ± 4.30 |
| 0.01 | 168.80 ± 5.30 cd | 152.50 ± 7.30 b | 19.43 ± 2.78 | |
| 0.02 | 178.50 ± 6.00 c | 157.00 ± 6.80 b | 19.59 ± 3.88 | |
| 0.03 | 225.50 ± 6.70 b | 176.60 ± 8.20 a | 21.47 ± 4.26 | |
| 0.04 | 235.50 ± 4.80 a | 186.30 ± 5.80 a | 24.76 ± 3.86 | |
| BranchAE | 0 | 162.53 ± 3.75 c | 148.30 ± 7.10 c | 17.69 ± 4.30 c |
| 0.01 | 170.60 ± 5.90 c | 150.30 ± 4.80 c | 19.82 ± 4.18 ab | |
| 0.02 | 192.50 ± 6.70 b | 164.50 ± 6.20 b | 23.52 ± 4.11 ab | |
| 0.03 | 232.80 ± 7.50 a | 187.50 ± 6.60 a | 24.38 ± 3.65 ab | |
| 0.04 | 235.50 ± 5.10 a | 193.10 ± 5.70 a | 26.06 ± 3.72 a |
| Title | Df | Sum of Squares | Mean Squares | F. Model | R2 | P (>F) |
|---|---|---|---|---|---|---|
| Group_factor | 4 | 1.7057 | 0.42642 | 3.4234 | 0.57795 | 0.001 |
| Residuals | 10 | 1.2456 | 0.12456 | 0.42205 | ||
| Total | 14 | 2.9513 | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Chen, J.; Liu, J.; Li, J.; Wu, M.; Tong, B. Autotoxicity Hinders the Natural Regeneration of Cinnamomum migao H. W. Li in Southwest China. Forests 2019, 10, 919. https://doi.org/10.3390/f10100919
Huang X, Chen J, Liu J, Li J, Wu M, Tong B. Autotoxicity Hinders the Natural Regeneration of Cinnamomum migao H. W. Li in Southwest China. Forests. 2019; 10(10):919. https://doi.org/10.3390/f10100919
Chicago/Turabian StyleHuang, Xiaolong, Jingzhong Chen, Jiming Liu, Jia Li, Mengyao Wu, and Bingli Tong. 2019. "Autotoxicity Hinders the Natural Regeneration of Cinnamomum migao H. W. Li in Southwest China" Forests 10, no. 10: 919. https://doi.org/10.3390/f10100919
APA StyleHuang, X., Chen, J., Liu, J., Li, J., Wu, M., & Tong, B. (2019). Autotoxicity Hinders the Natural Regeneration of Cinnamomum migao H. W. Li in Southwest China. Forests, 10(10), 919. https://doi.org/10.3390/f10100919




