Dehydration Sensitivity at the Early Seedling Establishment Stages of the European Beech (Fagus sylvatica L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Seed Viability after Dehydration
2.3. Electrolyte Leakage
2.4. Protein Extraction
2.5. Electrophoresis
2.6. Western Blot Analysis
2.7. Lipid Extraction
2.8. Ultrastructural Analyses
2.9. Lipid Storage Material Visualization
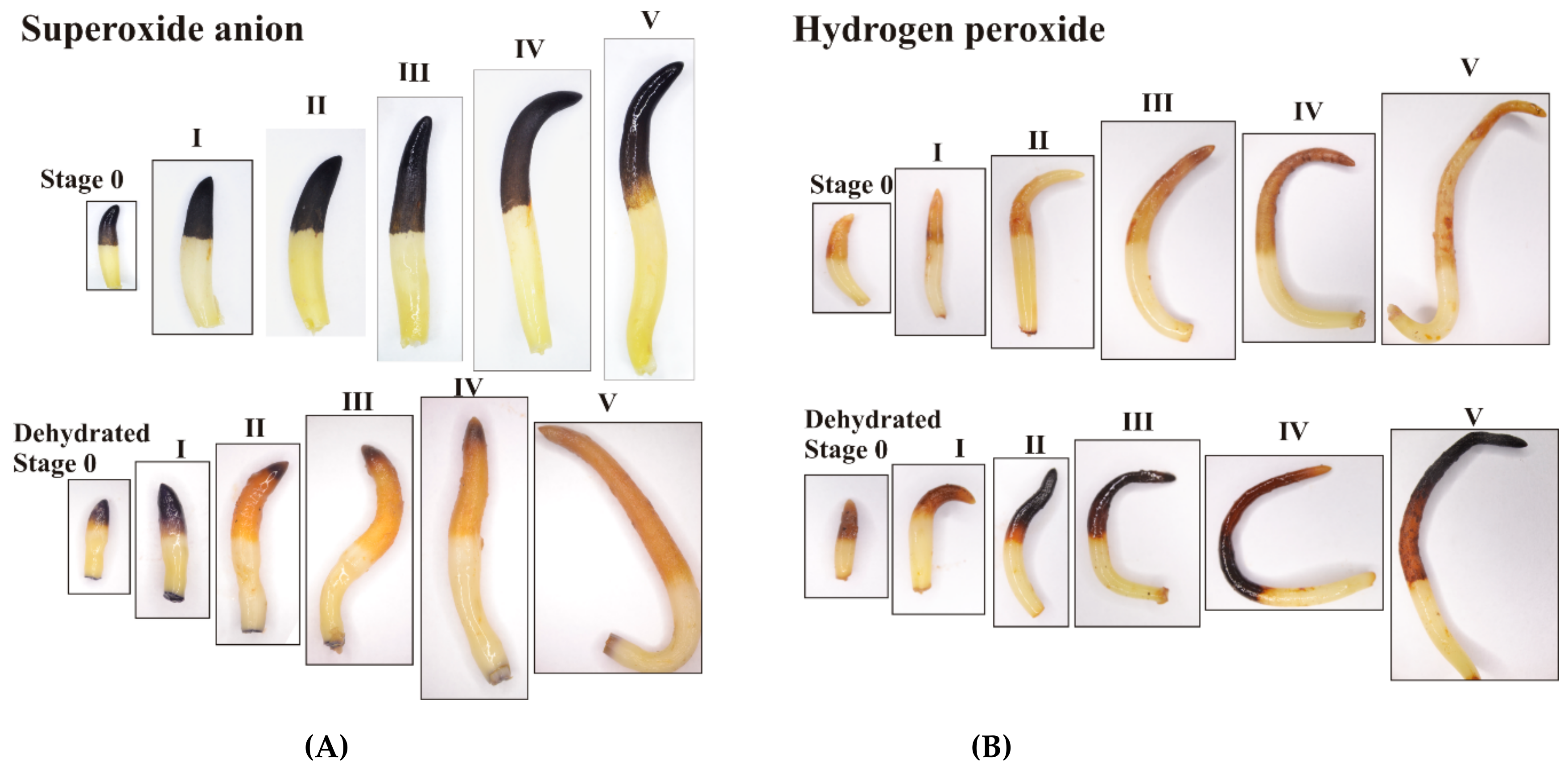
2.10. Histochemical Detection of ROS
2.11. Statistical Analysis
3. Results
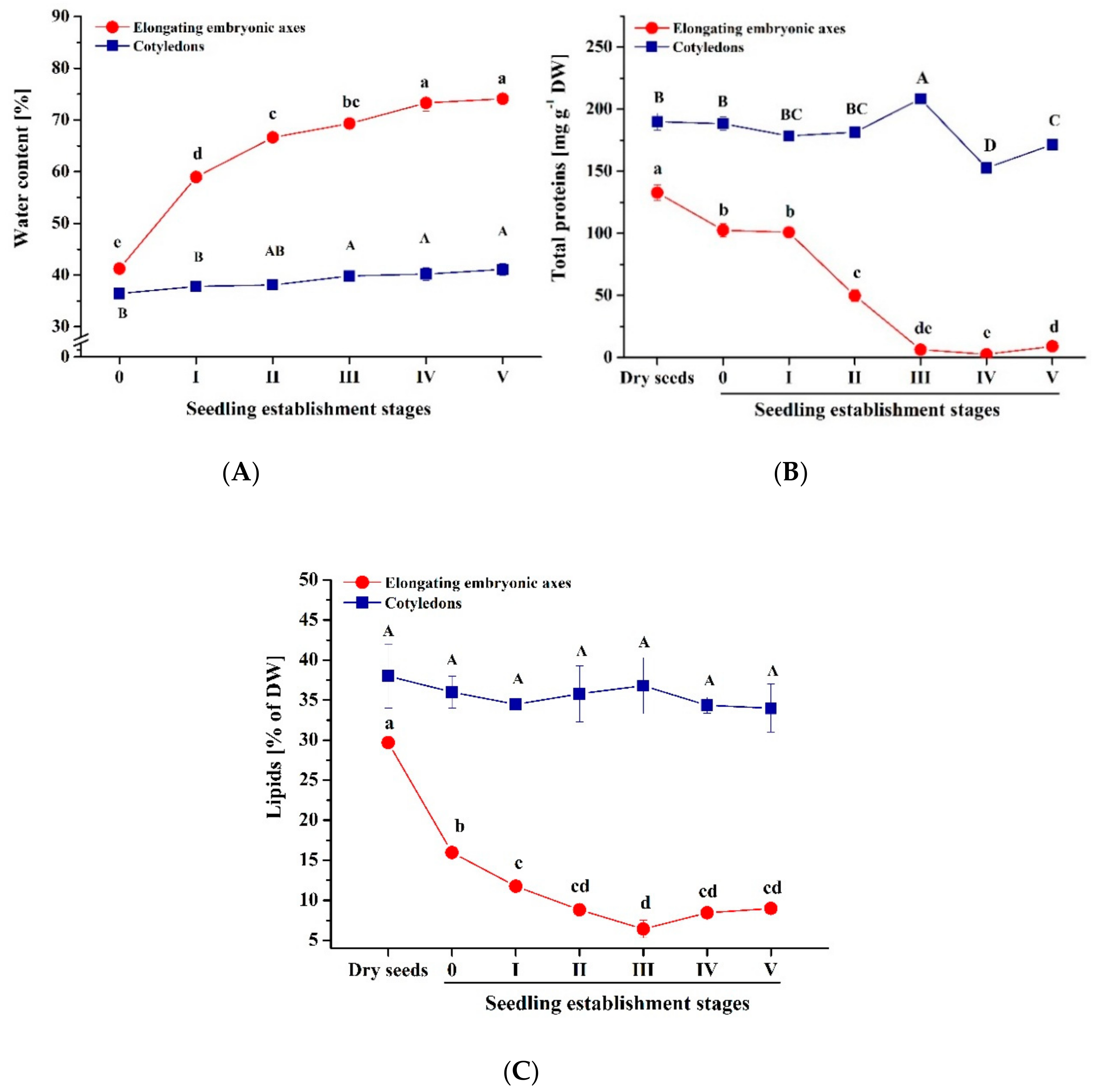
3.1. Water Status and Storage Materials under Normal Conditions
3.2. Localization of Storage Materials
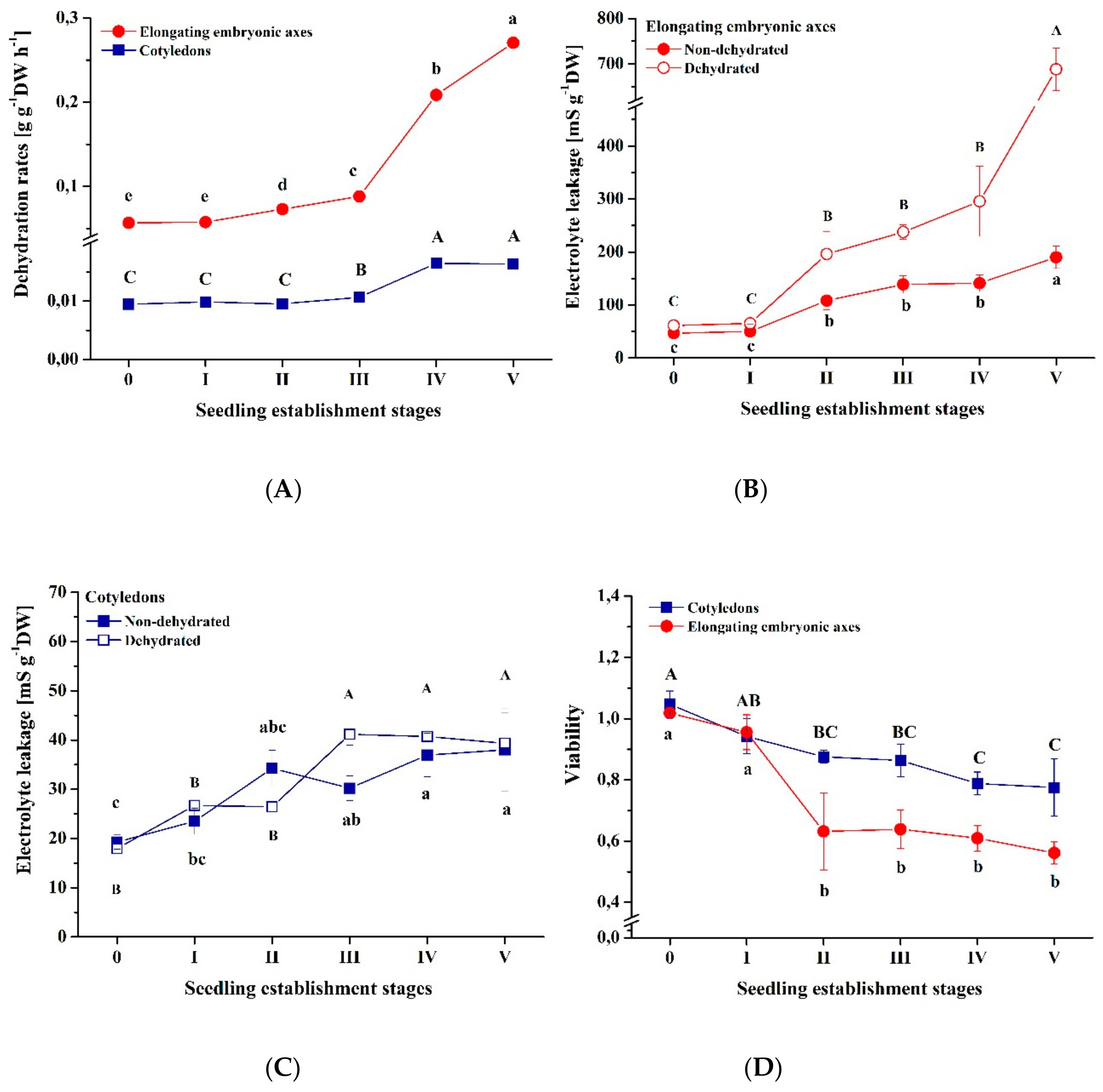
3.3. Response to Dehydration
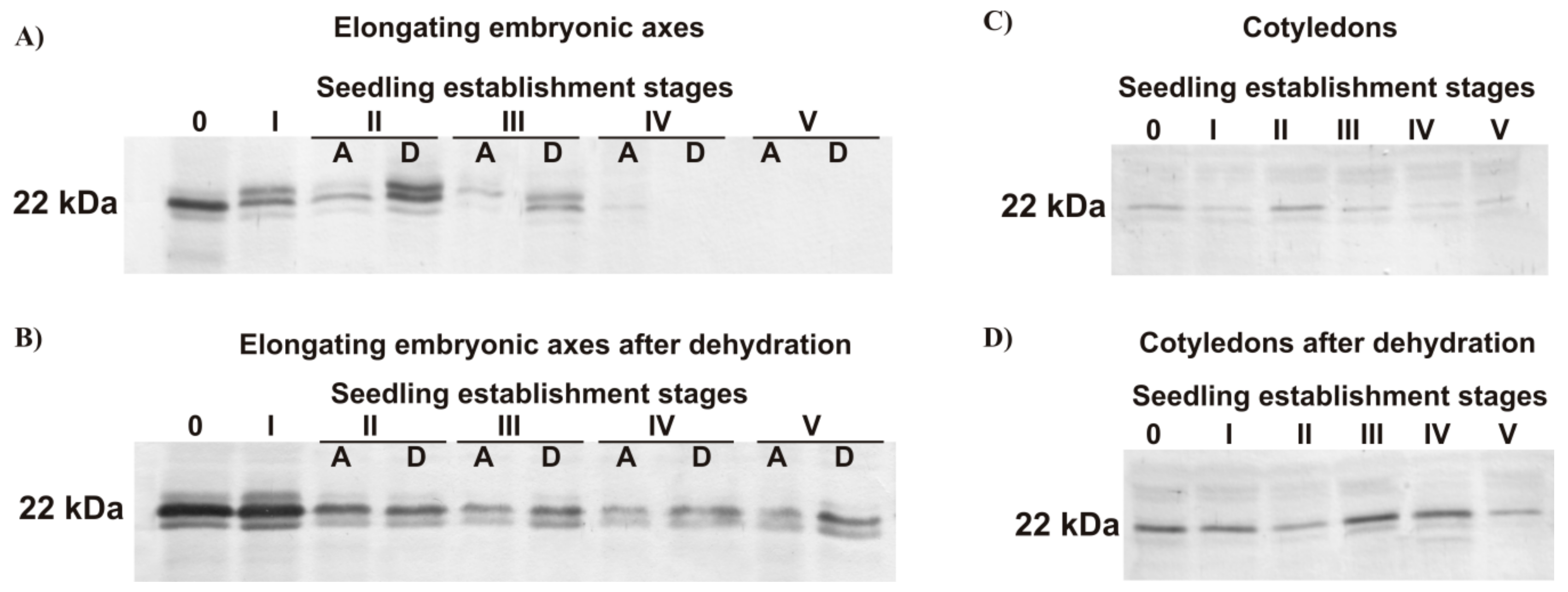
3.4. Chaperones
3.4.1. Dehydrin-like Proteins
3.4.2. Small Heat Shock Protein
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Walters, C. Orthodoxy, recalcitrance and in-between: Describing variation in seed storage characteristics using threshold responses to water loss. Planta 2015, 242, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Buitink, J.; Vu, B.L.; Satour, P.; Leprince, O. The re-establishment of desiccation tolerance in germinated radicles of Medicago truncatula Gaertn. seeds. Seed Sci. Res. 2003, 13, 273–286. [Google Scholar] [CrossRef]
- Kalemba, E.M.; Janowiak, F.; Pukacka, S. Desiccation tolerance acquisition in developing beech (Fagus sylvatica L.) seeds: The contribution of dehydrin-like protein. Trees 2009, 23, 305–315. [Google Scholar] [CrossRef]
- León-Lobos, P.; Ellis, R.H. Seed storage behaviour of Fagus sylvatica and Fagus crenata. Seed Sci. Res. 2002, 12, 31–37. [Google Scholar] [CrossRef]
- Bogdziewicz, M.; Szymkowiak, J.; Kasprzyk, I.; Grewling, L.; Borowski, Z.; Borycka, K.; Kantorowicz, W.; Myszkowska, D.; Piotrowicz, K.; Ziemianin, M.; et al. Masting in wind-pollinated trees: The role of weather and pollination dynamics in driving seed production. Ecology 2017, 98, 2615–2625. [Google Scholar] [CrossRef] [PubMed]
- Augustaitis, A.; Jasineviciene, D.; Girgzdiene, R.; Kliucius, A.; Marozas, V. Sensitivity of beech trees to global environmental changes at most north-eastern latitude of their occurrence in Europe. Sci. World J. 2012, 2012, 743926. [Google Scholar] [CrossRef] [PubMed]
- Pukacka, S.; Hoffmann, S.K.; Goslar, J.; Pukacki, P.M.; Wójkiewicz, E. Water and lipid relations in beech (Fagus sylvatica L.) seeds and its effect on storage behaviour. Biochim. Biophys. Acta 2003, 1621, 48–56. [Google Scholar] [CrossRef]
- Övergaard, R.; Agestam, E.; Ekö, P.M. Seed production and natural regeneration of beech (Fagus sylvatica L.) in southern Sweden. Stud. For. Suec. 2009, 218, 1–30. [Google Scholar]
- Tang, J.; Busso, C.A.; Jiang, D.; Wang, Y.; Wu, D.; Musa, A.; Miao, R.; Miao, C. Seed burial depth and soil water content affect seedling emergence and growth of Ulmus pumila var. sabulosa in the Horqin Sandy Land. Sustainability 2016, 8, 68. [Google Scholar]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Seeds: Physiology of Development; Germination and Dormancy, 3rd ed.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Penfield, S.; Graham, S.; Graham, I.A. Storage reserve mobilization in germinating oilseeds: Arabidopsis as a model system. Biochem. Soc. Trans. 2005, 33, 380–383. [Google Scholar] [CrossRef]
- Farrant, J.; Pammenter, N.; Berjak, P.; Walters, C. Subcellular organization and metabolic activity during the development of seeds that attain different levels of desiccation tolerance. Seed Sci. Res. 1997, 7, 135–144. [Google Scholar] [CrossRef] [Green Version]
- Ratajczak, E.; Pukacka, S. Decrease in beech (Fagus sylvatica) seed viability caused by temperature and humidity conditions as related to membrane damage and lipid composition. Acta Physiol. Plant. 2005, 27, 3–11. [Google Scholar] [CrossRef]
- Ratajczak, E.; Kalemba, E.M.; Pukacka, S. Age-related changes in protein metabolism of beech (Fagus sylvatica L.) seeds during alleviation of dormancy and in the early stage of germination. Plant. Physiol. Biochem. 2015, 94, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, B.J.; Costa, M.C.; Maia, J.; Bentsink, L.; Ligterink, W.; Hilhorst, H.W. Acquisition and loss of desiccation tolerance in seeds: From experimental model to biological relevance. Planta 2015, 241, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.C.D.; Righetti, K.; Nijveen, H.; Yazdanpanah, F.; Ligterink, W.; Buitink, J.; Hilhorst, H.W.M. A gene co-expression network predicts functional genes controlling the re-establishment of desiccation tolerance in germinated Arabidopsis thaliana seeds. Planta 2015, 242, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Maia, J.; Dekkers, B.J.; Provart, N.J.; Ligterink, W.; Hilhorst, H.W. The re-establishment of desiccation tolerance in germinated Arabidopsis thaliana seeds and its associated transcriptome. PLoS ONE 2011, 6, e29123. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Vinocur, B.; Shoseyov, O.; Altman, A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant. Sci. 2004, 9, 244–252. [Google Scholar] [CrossRef]
- Radwan, A.; Hara, M.; Kleinwächter, M.; Selmar, D. Dehydrin expression in seeds and maturation drying: A paradigm change. Plant. Biol. (Stuttg) 2014, 16, 853–855. [Google Scholar] [CrossRef]
- Close, T.J.; Fenton, R.D.; Moonan, F. A view of plant dehydrins using antibodies specific to the carboxy terminal peptide. Plant. Mol. Biol. 1993, 23, 279–286. [Google Scholar] [CrossRef]
- Kalemba, E.M.; Bagniewska-Zadworna, A.; Ratajczak, E. Multiple subcellular localizations of dehydrin-like proteins in the embryonic axes of common beech (Fagus sylvatica L.) seeds during maturation and dry storage. J. Plant. Growth Regul. 2015, 34, 137–149. [Google Scholar] [CrossRef]
- Kalemba, E.M.; Pukacka, S. Changes in late embryogenesis abundant proteins and a small heat shock protein during storage of beech (Fagus sylvatica L.) seeds. Environ. Exp. Bot. 2008, 63, 274–280. [Google Scholar] [CrossRef]
- Kalemba, E.M.; Litkowiec, M. Functional characterization of a dehydrin protein from Fagus sylvatica seeds using experimental and in silico approaches. Plant. Physiol. Biochem. 2015, 97, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Vaseva, I.I.; Anders, I.; Feller, U. Identification and expression of different dehydrin subclasses involved in the drought response of Trifolium repens. J. Plant. Physiol. 2014, 171, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Wehmeyer, N.; Hernandez, L.D.; Finkelstein, R.R.; Vierling, E. Synthesis of small heat-shock proteins is part of the developmental program of late seed maturation. Plant. Physiol. 1996, 112, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Petla, B.P.; Kamble, N.U.; Singh, A.; Rao, V.; Salvi, P.; Ghosh, S.; Majee, M. Differentially expressed seed aging responsive heat shock protein OsHSP18.2 implicates in seed vigor.; longevity and improves germination and seedling establishment under abiotic stress. Front. Plant. Sci. 2015, 6, 713. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Chen, S.; Wang, T.; Dai, S. Proteomic insights into seed germination in response to environmental factors. Proteomics 2013, 13, 1850–1870. [Google Scholar] [CrossRef] [PubMed]
- Kalemba, E.M.; Pukacka, S. Carbonylated proteins accumulated as vitality decreases during long-term storage of beech (Fagus sylvatica L.) seeds. Trees 2014, 28, 503–515. [Google Scholar] [CrossRef]
- Jeevan Kumar, S.P.; Rajendra Prasad, S.; Banerjee, R.; Thammineni, C. Seed birth to death: Dual functions of reactive oxygen species in seed physiology. Ann. Bot. 2015, 116, 663–668. [Google Scholar] [CrossRef]
- Bailly, C.; El-Maarouf-Bouteau, H.; Corbineau, F. From intracellular signaling networks to cell death, the dual role of reactive oxygen species in seed physiology. C. R. Biol. 2008, 331, 806–814. [Google Scholar] [CrossRef]
- Kwak, J.M.; Mori, I.C.; Pei, Z.M.; Leonhardt, N.; Torres, M.A.; Dang, J.L.; Bloom, R.E.; Bodde, S.; Jones, J.D.G.; Schroeder, J.I. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 2003, 22, 2623–2633. [Google Scholar] [CrossRef]
- Liszkay, A.; van der Zalm, E.; Schopfer, P. Production of reactive oxygen intermediates (O2-.; H2O2.; and ⋅OH) by maize roots and their role in wall loosening and elongation growth. Plant. Physiol. 2004, 136, 3114–3123. [Google Scholar] [CrossRef] [PubMed]
- Tsukagoshi, H.; Busch, W.; Benfey, P.N. Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell 2010, 143, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Schopfer, P.; Plachy, C.; Frahry, G. Release of reactive oxygen intermediates (superoxide radicals, hydrogen peroxide, and hydroxyl radicals) and peroxidase in germinating radish seeds controlled by light, gibberellin, and abscisic acid. Plant. Physiol. 2001, 125, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Pukacka, S.; Ratajczak, E. Ascorbate and glutathione metabolism during development and desiccation of orthodox and recalcitrant seeds of the genus Acer. Funct. Plant. Biol. 2007, 34, 601–613. [Google Scholar] [CrossRef]
- Pukacka, S.; Ratajczak, E. Age-related biochemical changes during storage of beech (Fagus sylvatica L.) seeds. Seed Sci. Res. 2007, 17, 45–53. [Google Scholar] [CrossRef]
- Chandra, J.; Keshavkant, S. Desiccation-induced ROS accumulation and lipid catabolism in recalcitrant Madhuca latifolia seeds. Physiol. Mol. Biol. Plants 2018, 24, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Sershen; Varghese, B.; Naidoo, C.; Pammenter, N.W. The use of plant stress biomarkers in assessing the effects of desiccation in zygotic embryos from recalcitrant seeds: Challenges and considerations. Plant. Biol. (Stuttg) 2016, 18, 433–444. [Google Scholar] [CrossRef]
- Li, C.; Sun, W.Q. Desiccation sensitivity and activities of free radical scavenging enzymes in recalcitrant Theobroma cacao seeds. Seed Sci. Res. 1999, 9, 209–217. [Google Scholar] [CrossRef]
- Kalemba, E.M.; Ratajczak, E. The effect of a doubled glutathione level on parameters affecting the germinability of recalcitrant Acer saccharinum seeds during drying. J. Plant. Physiol. 2018, 223, 72–83. [Google Scholar] [CrossRef]
- Topham, A.T.; Taylor, R.E.; Yan, D.; Nambara, E.; Johnston, I.G.; Bassel, G.W. Temperature variability is integrated by a spatially embedded decision-making center to break dormancy in Arabidopsis seeds. Proc. Natl. Acad. Sci. USA 2017, 114, 6629–6634. [Google Scholar] [CrossRef]
- Sliwinska, E.; Bassel, G.W.; Bewley, J.D. Germination of Arabidopsis thaliana seeds is not completed as a result of elongation of the radicle but of the adjacent transition zone and lower hypocotyl. J. Exp. Bot. 2009, 60, 3587–3594. [Google Scholar] [CrossRef] [PubMed]
- International Rules for Seed Testing; The Topographical Tetrazolium Test; The International Seed Testing Association (ISTA): Bassersdorf, Switzerland, 2016; p. i-6-26.
- Chen, W.P.; Li, P.H.; Chen, T.H.H. Glycinebetaine increases chilling tolerance and reduces chilling-induced lipid peroxidation in Zea mays L. Plant. Cell Environ. 2000, 23, 609–618. [Google Scholar] [CrossRef]
- Bagniewska-Zadworna, A.; Wojciechowicz, M.K.; Zenkteler, M.; Jeżowski, S.; Zenkteler, E. Cytological analysis of hybrid embryos of Intergeneric crosses between Salix viminalis and Populus species. Austr. J. Bot. 2010, 58, 42–48. [Google Scholar] [CrossRef]
- Krishnamurthy, K.V. Methods in Cell Wall Cytochemistry; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Daudi, A.; O’Brien, J.A. Detection of hydrogen peroxide by DAB staining in Arabidopsis Leaves. Bio. Protoc. 2012, 2, e263. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Yusuf, M.A.; Singh, P.; Sardar, M.; Sarin, N.B. Histochemical detection of superoxide and H2O2 accumulation in Brassica juncea seedlings. Bio. Protoc. 2014, 4, e1108. [Google Scholar] [CrossRef]
- Robson, T.M.; Rodríguez-Calcerrada, J.; Sánchez-Gómez, D.; Aranda, I. Summer drought impedes beech seedling performance more in a sub-Mediterranean forest understory than in small gaps. Tree Physiol. 2009, 29, 249–259. [Google Scholar] [CrossRef]
- Norberg, P.; Liljenberg, C. Lipids of plasma membranes prepared from oat root cells: effects of induced water-deficit tolerance. Plant. Physiol. 1991, 96, 1136–1141. [Google Scholar] [CrossRef]
- Sun, W.Q.; Leopold, A.C. Acquisition of desiccation tolerance in soybeans. Physiol. Plant. 1993, 87, 403–409. [Google Scholar] [CrossRef]
- Da Costa, C.T.; de Almeida, M.R.; Ruedell, C.M.; Schwambach, J.; Maraschin, F.S.; Fett-Neto, A.G. When stress and development go hand in hand: Main hormonal controls of adventitious rooting in cuttings. Front. Plant. Sci. 2013, 4, 133. [Google Scholar] [CrossRef]
- Prasad, R.B.N.; Guelz, P.G. Composition of lipids of beech Fagus sylvatica L. seed oil. Zeitschrift fuer Naturforschung C 1989, 44, 735–738. [Google Scholar] [CrossRef]
- Collada, C.; Aragoncillo, P.; Aragoncillo, C. Development of protein bodies in cotyledons of Fagus sylvatica. Physiol. Plant. 1993, 89, 354–359. [Google Scholar] [CrossRef]
- Eliasova, K.; Pesek, B.; Vondrakova, Z. Storage compounds, ABA and fumarase in Fagus sylvatica embryos during stratification. Dendrobiology 2015, 74, 25–33. [Google Scholar] [CrossRef]
- Murphy, D.J. Plant Lipids, Biology, Utilisation and Manipulation; Blackwells: Oxford, UK, 2004. [Google Scholar]
- Feenstra, A.D.; Alexander, L.E.; Song, Z.; Korte, A.R.; Yandeau-Nelson, M.D.; Nikolau, B.J.; Lee, Y.J. Spatial mapping and profiling of metabolite distributions during germination. Plant. Physiol. 2017, 174, 2532–2548. [Google Scholar] [CrossRef] [PubMed]
- Van der Schoot, C.; Paul, L.K.; Paul, S.B.; Rinne, P.L. Plant lipid bodies and cell-cell signaling: A new role for an old organelle? Plant. Signal. Behav. 2011, 6, 1732–1738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.Q.; Liu, S.J.; Song, S.Q.; Møller, I.M. Proteomics of seed development, desiccation tolerance, germination and vigor. Plant. Physiol. Biochem. 2015, 86, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Personat, J.M.; Tejedor-Cano, J.; Prieto-Dapena, P.; Almoguera, C.; Jordano, J. Co-overexpression of two Heat Shock Factors results in enhanced seed longevity and in synergistic effects on seedling tolerance to severe dehydration and oxidative stress. BMC Plant. Biol. 2014, 14, 56. [Google Scholar] [CrossRef] [PubMed]
- Roach, T.; Ivanova, M.; Beckett, R.P.; Minibayeva, F.V.; Green, I.; Pritchard, H.W.; Kranner, I. An oxidative burst of superoxide in embryonic axes of recalcitrant sweet chestnut seeds as induced by excision and desiccation. Physiol. Plant. 2008, 133, 131–139. [Google Scholar] [CrossRef]
- Singh, K.L.; Chaudhuri, A.; Kar, R.K. Superoxide and its metabolism during germination and axis growth of Vigna radiata (L.) Wilczek seeds. Plant. Signal. Behav. 2014, 9, e29278. [Google Scholar] [CrossRef]
- Kranner, I.; Roach, T.; Beckett, R.P.; Whitaker, C.; Minibayeva, F.V. Extracellular production of reactive oxygen species during seed germination and early seedling growth in Pisum sativum. J. Plant. Physiol. 2010, 167, 805–811. [Google Scholar] [CrossRef]
- El-Maarouf-Bouteau, H.; Bailly, C. Oxidative signaling in seed germination and dormancy. Plant. Signal. Behav. 2008, 3, 175–182. [Google Scholar] [CrossRef] [Green Version]
- Kagenishi, T.; Yokawa, K.; Baluška, F. MES buffer affects Arabidopsis root apex zonation and root growth by suppressing superoxide generation in root apex. Front. Plant. Sci. 2016, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Tamma, G.; Valenti, G.; Grossini, E.; Donnini, S.; Marino, A.; Marinelli, R.A.; Calamita, G. Aquaporin membrane channels in oxidative stress, cell signaling, and aging: recent advances and research trends. Oxidative Med. Cell Longev. 2018, 1501847. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Umemoto, Y.; Kawano, T. Hydrogen peroxide-independent generation of superoxide by plant peroxidase: Hypotheses and supportive data employing ferrous ion as a model stimulus. Front. Plant. Sci. 2014, 5, 285. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Meng, Y.L.; Feldman, L.J. Quiescent center formation in maize roots is associated with an auxin-regulated oxidizing environment. Development 2003, 130, 1429–1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boddington, K.F.; Graether, S.P. Binding of a Vitis riparia dehydrin to DNA. Plant. Sci. 2019, 287, 110172. [Google Scholar] [CrossRef] [PubMed]
- Halder, T.; Upadhyaya, G.; Basak, C.; Das, A.; Chakraborty, C.; Ray, S. Dehydrins impart protection against oxidative stress in transgenic tobacco plants. Front. Plant. Sci. 2018, 9, 136. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Hou, X.; Zhang, Y.; Meng, Y.; Zhang, H.; Liu, S.; Wang, X.; Chen, R. CaDHN5, a dehydrin gene from pepper, plays an important role in salt and osmotic stress responses. Int. J. Mol. Sci. 2019, 20, 1989. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalemba, E.M.; Bagniewska-Zadworna, A.; Suszka, J.; Pukacka, S. Dehydration Sensitivity at the Early Seedling Establishment Stages of the European Beech (Fagus sylvatica L.). Forests 2019, 10, 900. https://doi.org/10.3390/f10100900
Kalemba EM, Bagniewska-Zadworna A, Suszka J, Pukacka S. Dehydration Sensitivity at the Early Seedling Establishment Stages of the European Beech (Fagus sylvatica L.). Forests. 2019; 10(10):900. https://doi.org/10.3390/f10100900
Chicago/Turabian StyleKalemba, Ewa M., Agnieszka Bagniewska-Zadworna, Jan Suszka, and Stanisława Pukacka. 2019. "Dehydration Sensitivity at the Early Seedling Establishment Stages of the European Beech (Fagus sylvatica L.)" Forests 10, no. 10: 900. https://doi.org/10.3390/f10100900
APA StyleKalemba, E. M., Bagniewska-Zadworna, A., Suszka, J., & Pukacka, S. (2019). Dehydration Sensitivity at the Early Seedling Establishment Stages of the European Beech (Fagus sylvatica L.). Forests, 10(10), 900. https://doi.org/10.3390/f10100900





