Modeling Migratory Flight in the Spruce Budworm: Circadian Rhythm
Abstract
:1. Introduction
2. Materials and Methods
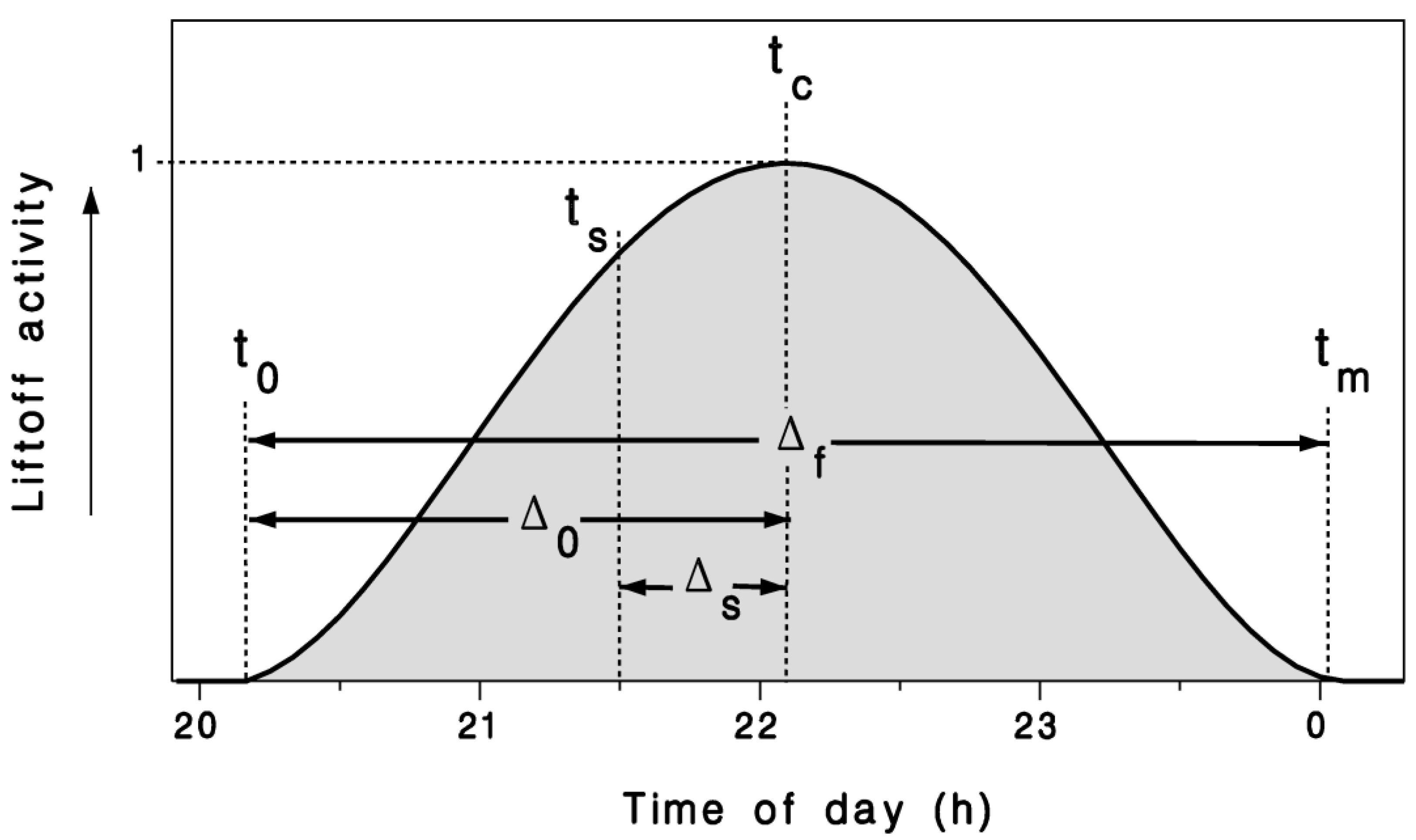
2.1. Mathematical Model
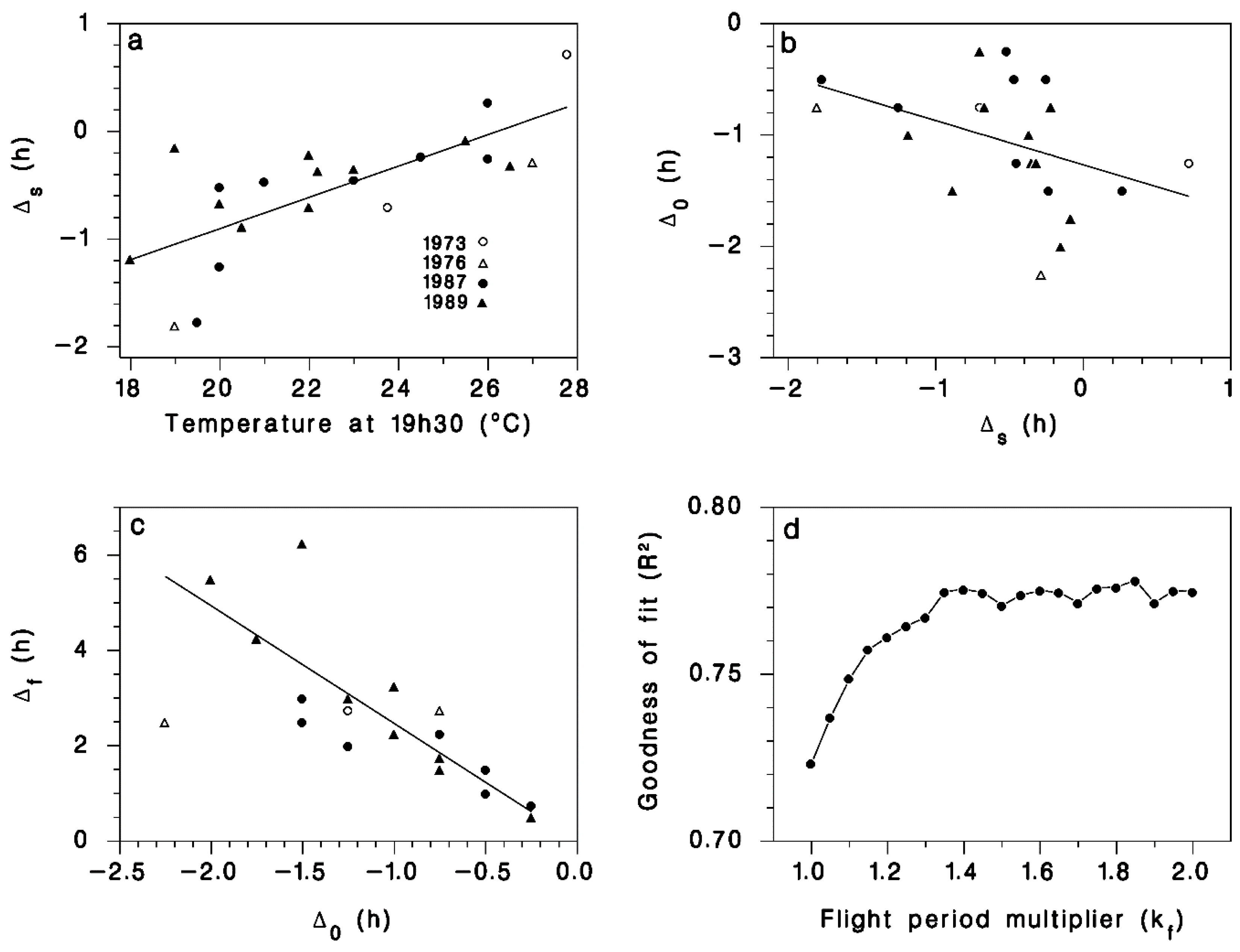
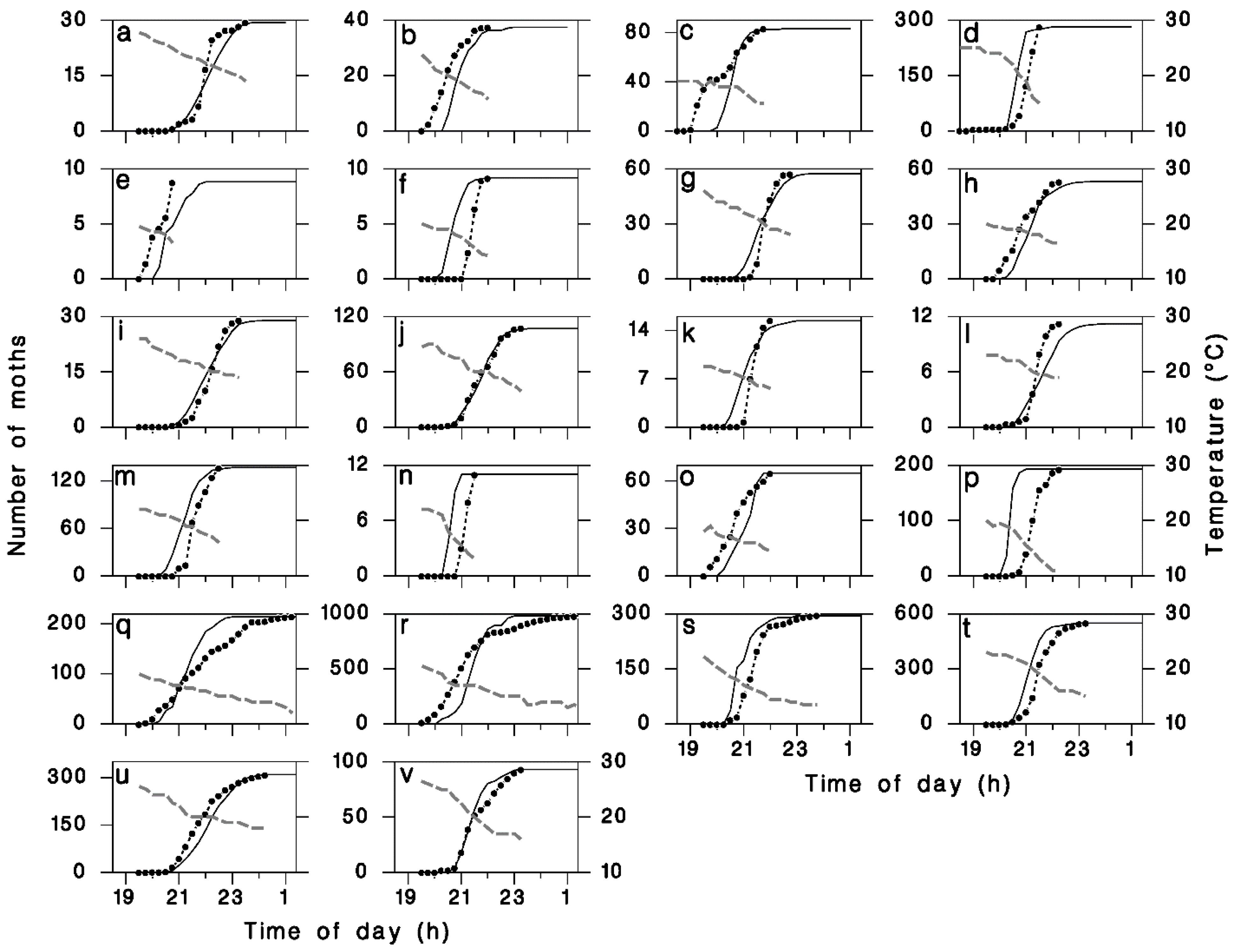
2.2. Observations and Model Calibration
2.3. Simulations
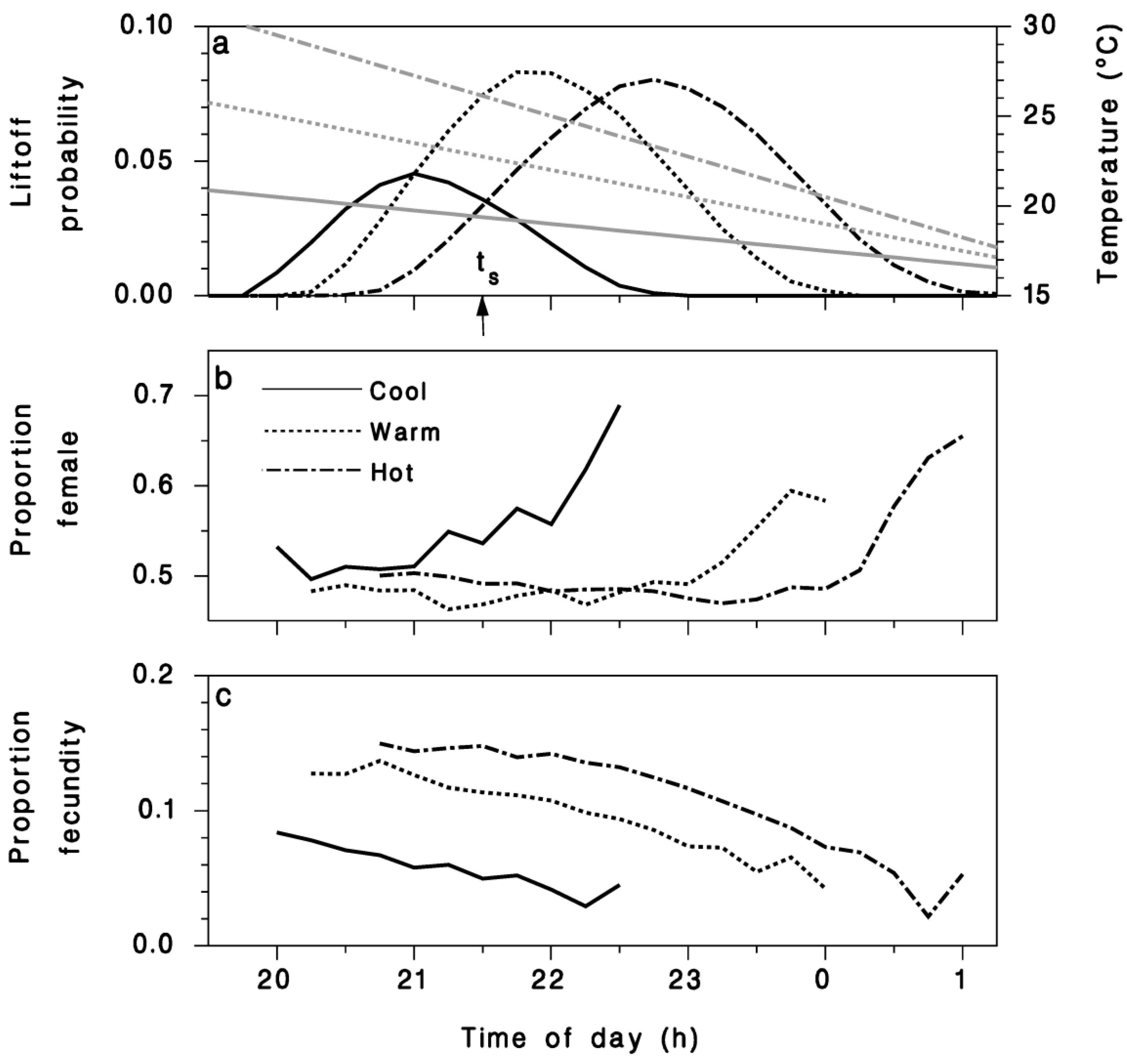
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dingle, H. Migration: The Biology of Life on the Move, 2nd ed.; Oxford University Press: New York, NY, USA, 2014; 326p. [Google Scholar] [CrossRef]
- Régnière, J.; Delisle, J.; Sturtevant, B.R.; Garcia, M.; St-Amant, R. Modeling migratory flight in the spruce budworm: Temperature constraints. Forests 2019, 10, 802. [Google Scholar] [CrossRef]
- Hu, G.; Lim, K.S.; Reynolds, D.R.; Reynolds, A.M.; Chapman, J.W. Wind-related orientation patterns in diurnal, crepuscular and nocturnal high-altitude insect migrants. Front. Behav. Neurosci. 2016, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, A.Y.; Plotkin, D.; Hamilton, C.A.; Gough, H.; St Laurent, R.; Owens, H.L.; Homziak, N.T.; Barber, J.R. Diel behavior in moths and butterflies: A synthesis of data illuminates the evolution of temporal activity. Org. Divers. Evol. 2018, 18, 13–27. [Google Scholar] [CrossRef]
- Rund, S.; O’Donnell, A.; Gentile, J.; Reece, S. Daily rhythms in mosquitoes and their consequences for malaria transmission. Insects 2016, 7, 14. [Google Scholar] [CrossRef]
- Broadhead, G.T.; Basu, T.; von Arx, M.; Raguso, R.A. Diel rhythms and sex differences in the locomotor activity of hawkmoths. J. Exp. Biol. 2017, 220, 1472–1480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wellington, W.G. The light reactions of the spruce budworm, Choristoneura fumiferana Clemens (Lepidoptera: Tortricidae). Can. Entomol. 1948, 80, 56–82. [Google Scholar] [CrossRef]
- Henson, W.R. Mass flights of the spruce budworm. Can. Entomol. 1951, 83, 240. [Google Scholar] [CrossRef]
- Schaefer, G.W. Radar observations of insect flight. Symp. R. Entomol. Soc. Lond. 1976, 7, 157–197. [Google Scholar]
- Greenbank, D.O.; Schaefer, G.W.; Rainey, R.C. Spruce budworm (Lepidoptera: Tortricidae) moth flight and dispersal: New understanding from canopy observations, radar and aircraft. Mem. Entomol. Soc. Can. 1980, 112, 1–49. [Google Scholar] [CrossRef]
- Kipp, L.R.; Lonergan, G.C.; Bell, W.J. Male periodicity and the timing of mating in the spruce budworm (Lepidoptera: Tortricidae): Influence of population density and temperature. Environ. Entomol. 1995, 24, 1150–1159. [Google Scholar] [CrossRef]
- Cardé, R.T.; Roelofs, W.L. Temperature modification of male sex pheromone response and factors affecting female calling in Holomelina immaculata (Lepidoptera: Arctiidae). Can. Entomol. 1973, 105, 1505–1512. [Google Scholar] [CrossRef]
- Cardé, R.T.; Comeau, A.; Baker, T.C.; Roelofs, W.L. Moth mating periodicity: Temperature regulates the circadian gate. Experientia 1975, 31, 46–48. [Google Scholar] [CrossRef] [PubMed]
- Comeau, A.; Cardé, R.T.; Roelofs, W.L. Relationship of ambient temperatures to diel periodicities of sex attraction in six species of Lepidoptera. Can. Entomol. 1976, 108, 415–418. [Google Scholar] [CrossRef]
- Alerstam, T.; Chapman, J.W.; Bäckman, J.; Smith, A.D.; Karlsson, H.; Nilsson, C.; Reynolds, D.R.; Klaassen, H.G.; Hill, J.K. Convergent patterns of long-distance nocturnal migration in noctuid moths and passerine birds. Proc. R. Soc. B Biol. Sci. 2011, 278, 3074–3080. [Google Scholar] [CrossRef]
- Krauel, J.J.; Westbrook, J.K.; McCracken, G.F. Weather-driven dynamics in a dual-migrant system: Moths and bats. J. Anim. Ecol. 2015, 84, 604–614. [Google Scholar] [CrossRef]
- Krauel, J.J.; Brown, V.A.; Westbrook, J.K.; McCracken, G.F. Predator–prey interaction reveals local effects of high-altitude insect migration. Oecologia 2018, 186, 49–58. [Google Scholar] [CrossRef]
- Sanders, C.J. Daily activity patterns and sex pheromone specificity as sexual isolating mechanisms in two species of Choristoneura (Lepidoptera: Tortricidae). Can. Entomol. 1971, 103, 498–502. [Google Scholar] [CrossRef]
- Sanders, C.J.; Lucuik, G.S. Effects of photoperiod and size on flight activity and oviposition in the eastern spruce budworm (Lepidoptera: Tortricidae). Can. Entomol. 1975, 107, 1289–1299. [Google Scholar] [CrossRef]
- Simmons, G.A.; Chen, C.W. Application of harmonic analysis and polynomial regression to study flight activity of Choristoneura fumiferana (Clem.) (Lepidoptera: Tortricidae) in the field. Abstracts, Forty-Seventh Annual Meeting, Eastern Branch Entomological Society of America. J. N. Y. Entomol. Soc. 1975, 83, 266. [Google Scholar]
- Régnière, J.; Powell, J.; Bentz, B.; Nealis, V. Effects of temperature on development, survival and reproduction of insects: Experimental design, data analysis and modeling. J. Insect Physiol. 2012, 58, 634–647. [Google Scholar] [CrossRef]
- Wellington, W.G.; Henson, W.R. Notes on the effects of physical factors on the spruce budworm, Choristoneura fumiferana (Clem.). Can. Entomol. 1947, 79, 168–170. [Google Scholar] [CrossRef]
- Dickison, R.B.B.; Haggis, M.J.; Rainey, R.C. Spruce budworm moth flight and storms: Case study of a cold front system. J. Clim. Appl. Meteorol. 1983, 22, 278–286. [Google Scholar] [CrossRef]
- Dickison, R.B.B.; Haggis, M.J.; Rainey, R.C.; Burns, L.M.D. Spruce budworm moth flight and storms: Further studies using aircraft and radar. J. Clim. Appl. Meteorol. 1986, 25, 1600–1608. [Google Scholar] [CrossRef]
- Pedgley, D.E.; Reynolds, D.R.; Riley, J.R.; Tucker, M.R. Flying insects reveal small-scale wind systems. Weather 1982, 37, 295–306. [Google Scholar] [CrossRef]
- Drake, V.A. The vertical distribution of macro-insects migrating in the nocturnal boundary layer: A radar study. Bound. Layer Meteorol. 1984, 28, 353–374. [Google Scholar] [CrossRef]
- Drake, V.A. Radar observations of moths migrating in a nocturnal low-level jet. Ecol. Entomol. 1985, 10, 259–265. [Google Scholar] [CrossRef]
- Reynolds, D.R.; Chapman, J.W.; Edwards, A.S.; Smith, A.D.; Wood, C.R.; Barlow, J.F.; Woiwod, I.P. Radar studies of the vertical distribution of insects migrating over southern Britain: The influence of temperature inversions on nocturnal layer concentrations. Bull. Entomol. Res. 2005, 95, 259–274. [Google Scholar] [CrossRef]
- Reynolds, D.R.; Smith, A.D.; Chapman, J.W. A radar study of emigratory flight and layer formation by insects at dawn over southern Britain. Bull. Entomol. Res. 2008, 98, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.W.; Drake, V.A.; Reynolds, D.R. Recent insights from radar studies of insect flight. Ann. Rev. Entomol. 2011, 56, 337–356. [Google Scholar] [CrossRef]
- Westbrook, J.K.; Eyster, R.S.; Wolf, W.W. WSR-88D doppler radar detection of corn earworm moth migration. Int. J. Biometeorol. 2014, 58, 931–940. [Google Scholar] [CrossRef]
- Boulanger, Y.; Fabry, F.; Kilambi, A.; Pureswaran, D.S.; Sturtevant, B.R.; Saint-Amant, R. The use of weather surveillance radar and high-resolution three-dimensional weather data to monitor a spruce budworm mass exodus flight. Agric. For. Meteorol. 2017, 234, 127–135. [Google Scholar] [CrossRef]
- Westbrook, J.K.; Eyster, R.S. Doppler weather radar detects emigratory flights of noctuids during a major pest outbreak. Remote Sens. Appl. Soc. Environ. 2017, 8, 64–70. [Google Scholar] [CrossRef]
- Sanders, J.C.; Wallace, D.R.; Luicuik, G.S. Flight activity of female eastern spruce budworm (Lepidoptera: Tortricidae) at constant temperatures in the laboratory. Can. Entomol. 1978, 110, 627–632. [Google Scholar] [CrossRef]
- Régnière, J.; Cooke, B.; Béchard, A.; Dupont, A.; Therrien, P. Dynamics and management of rising outbreak spruce budworm populations. Forests 2019, 10, 748. [Google Scholar] [CrossRef]
- Baklanov, A.A.; Grisogono, B.; Bornstein, R.; Zilitinkevich, S.S.; Taylor, P.; Larsen, S.E.; Rotach, M.W.; Fernando, H.J.S. The nature, theory, and modeling of atmospheric planetary boundary layers. Bull. Am. Meteorol. Soc. 2011, 92, 123–128. [Google Scholar] [CrossRef]
- Angevine, W.M. Transitional, entraining, cloudy, and coastal boundary layers. Acta Geophysica 2008, 56, 2–20. [Google Scholar] [CrossRef]
- Mahrt, L. Stably stratified atmospheric boundary layers. Ann. Rev. Fluid Mech. 2014, 46, 23–45. [Google Scholar] [CrossRef]
- Mahrt, L. The early evening boundary layer transition. Quart. J. R. Meteorol. Soc. 1981, 107, 329–343. [Google Scholar] [CrossRef]
- Mahrt, L. Nocturnal boundary-layer regimes. Bound. Layer Meteorol. 1998, 88, 255–278. [Google Scholar] [CrossRef]
- Mahrt, L. The near-surface evening transition. Quart. J. R. Meteorol. Soc. 2017, 143, 2940–2948. [Google Scholar] [CrossRef]
- Acevedo, O.C.; Fitzjarrald, D.R. The early evening surface-layer transition: Temporal and spatial variability. J. Atmos. Sci. 2001, 58, 2650–2667. [Google Scholar] [CrossRef]
- Sastre, M.; Yagüe, C.; Román-Cascón, C.; Maqueda, G. Atmospheric boundary-layer evening transitions: A comparison between two different experimental sites. Bound. Layer Meteorol. 2015, 157, 375–399. [Google Scholar] [CrossRef]
- Angevine, W.M.; Tjernström, M.; Žagar, M. Modeling of the coastal boundary layer and pollutant transport in New England. J. Appl. Meteorol. Climatol. 2006, 45, 137–154. [Google Scholar] [CrossRef]
- Nieuwstadt, F.T.M. The turbulent structure of the stable, nocturnal boundary layer. J. Atmos. Sci. 1984, 41, 2202–2216. [Google Scholar] [CrossRef]
- Acevedo, O.C.; Mahrt, L.; Puhales, F.S.; Costa, F.D.; Medeiros, L.E.; Degrazia, G.A. Contrasting structures between the decoupled and coupled states of the stable boundary layer. Quart. J. R. Meteorol. Soc. 2016, 142, 693–702. [Google Scholar] [CrossRef]
- Mahrt, L. Microfronts in the nocturnal boundary layer. Quart. J. R. Meteorol. Soc. 2019, 145, 546–562. [Google Scholar] [CrossRef]
- Reynolds, A.M.; Reynolds, D.R.; Smith, A.D.; Chapman, J.W. Orientation cues for high-flying nocturnal insect migrants: Do turbulence-induced temperature and velocity fluctuations indicate the mean wind flow? PLoS ONE 2010, 5, e15758. [Google Scholar] [CrossRef]
- Riley, J.R.; Reynolds, D.R.; Rainey, R.C. Radar-based studies of the migratory flight of grasshoppers in the middle Niger area of Mali. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1979, 204, 67–82. [Google Scholar] [CrossRef]
- Rennie, S.J. Common orientation and layering of migrating insects in southeastern Australia observed with a Doppler weather radar. Meteorol. Appl. 2014, 21, 218–229. [Google Scholar] [CrossRef]
- Feng, H.; Wu, X.; Wu, B.; Wu, K. Seasonal migration of Helicoverpa armigera (Lepidoptera: Noctuidae) over the Bohai Sea. J. Econ. Entomol. 2009, 102, 95–104. [Google Scholar] [CrossRef]
- Fu, X.; Zhao, X.; Xie, B.; Ali, A.; Wu, K. Seasonal pattern of Spodoptera litura (Lepidoptera: Noctuidae) migration across the Bohai Strait in northern China. J. Econ. Entomol. 2015, 108, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.R.; Chapman, J.W.; Reynolds, D.R.; Barlow, J.F.; Smith, A.D.; Woiwod, I.P. The influence of the atmospheric boundary layer on nocturnal layers of noctuids and other moths migrating over southern Britain. Int. J. Biometeorol. 2006, 50, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.R.; Reynolds, D.R.; Wells, P.M.; Barlow, J.F.; Woiwod, I.P.; Chapman, J.W. Flight periodicity and the vertical distribution of high-altitude moth migration over southern Britain. Bull. Entomol. Res. 2009, 99, 525–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, C.R.; Clark, S.J.; Barlow, J.F.; Chapman, J.W. Layers of nocturnal insect migrants at high-altitude: The influence of atmospheric conditions on their formation. Agric. For. Entomol. 2010, 12, 113–121. [Google Scholar] [CrossRef]
- Dreyer, D.; El Jundi, B.; Kishkinev, D.; Suchentrunk, C.; Campostrini, L.; Frost, B.J.; Zechmeister, T.; Warrant, E.J. Evidence for a southward autumn migration of nocturnal noctuid moths in central Europe. J. Exp. Biol. 2018, 221, 179218. [Google Scholar] [CrossRef]
- Wang, H.-H.; Grant, W.E.; Elliott, N.C.; Brewer, M.J.; Koralewski, T.E.; Westbrook, J.K.; Alves, T.M.; Sword, G.A. Integrated modelling of the life cycle and aeroecology of wind-borne pests in temporally-variable spatially-heterogeneous environment. Ecol. Model. 2019, 399, 23–38. [Google Scholar] [CrossRef]
- Reynolds, A.M.; Reynolds, D.R.; Smith, A.D.; Chapman, J.W. A single wind-mediated mechanism explains high-altitude “non-goal oriented” headings and layering of nocturnally migrating insects. Proc. R. Soc. Lond. B Biol. Sci. 2010, 277, 765–772. [Google Scholar] [CrossRef]
- Aralimarad, P.; Reynolds, A.M.; Lim, K.S.; Reynolds, D.R.; Chapman, J.W. Flight altitude selection increases orientation performance in high-flying nocturnal insect migrants. Anim. Behav. 2011, 82, 1221–1225. [Google Scholar] [CrossRef]
- Riley, J.R. Collective orientation in night-flying insects. Nature 1975, 253, 113. [Google Scholar] [CrossRef]
- Reynolds, A.M.; Reynolds, D.R.; Riley, J.R. Does a “turbophoretic” effect account for layer concentrations of insects migrating in the stable night-time atmosphere? J. R. Soc. Interface 2009, 6, 87–95. [Google Scholar] [CrossRef]
- Holtslag, A.A.M.; Svensson, G.; Baas, P.; Basu, S.; Beare, B.; Beljaars, A.C.M.; Bosveld, F.C.; Cuxart, J.; Lindvall, J.; Steeneveld, G.J.; et al. Stable atmospheric boundary layers and diurnal cycles: Challenges for weather and climate models. Bull. Am. Meteorol. Soc. 2013, 94, 1691–1706. [Google Scholar] [CrossRef]
- Davy, R. The climatology of the atmospheric boundary layer in contemporary global climate models. J. Clim. 2018, 31, 9151–9173. [Google Scholar] [CrossRef]
- McNider, R.T.; Steeneveld, G.J.; Holtslag, A.A.M.; Pielke, R.A.; Mackaro, S.; Pour-Biazar, A.; Walters, J.; Nair, U.; Christy, J. Response and sensitivity of the nocturnal boundary layer over land to added longwave radiative forcing. J. Geophys. Res. Atmos. 2012, 117, D14106. [Google Scholar] [CrossRef]
- Horvath, K.; Koracin, D.; Vellore, R.; Jiang, J.; Belu, R. Sub-kilometer dynamical downscaling of near-surface winds in complex terrain using WRF and MM5 mesoscale models. J. Geophys. Res. Atmos. 2012, 117, D11111. [Google Scholar] [CrossRef]
| Parameter | Calibration Value | Equation | Regression Statistics |
|---|---|---|---|
| p1 | −3.8 ± 0.7 h | (11) | F = 22.5; df = 1,20; R2 = 0.529; p < 0.001 |
| p2 | 0.145 ± 0.031 h/°C | ||
| p3 | −1.267 ± 0.146 h | (12) | F = 4.5; df = 1,20; R2 = 0.183; p < 0.047 |
| p4 | −0.397 ± 0.187 | ||
| p5 | −2.465 ± 0.152 | (13) | |
| kf | 1.35 ± 0.025 | (14) | R2 = 0.775 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Régnière, J.; Garcia, M.; Saint-Amant, R. Modeling Migratory Flight in the Spruce Budworm: Circadian Rhythm. Forests 2019, 10, 877. https://doi.org/10.3390/f10100877
Régnière J, Garcia M, Saint-Amant R. Modeling Migratory Flight in the Spruce Budworm: Circadian Rhythm. Forests. 2019; 10(10):877. https://doi.org/10.3390/f10100877
Chicago/Turabian StyleRégnière, Jacques, Matthew Garcia, and Rémi Saint-Amant. 2019. "Modeling Migratory Flight in the Spruce Budworm: Circadian Rhythm" Forests 10, no. 10: 877. https://doi.org/10.3390/f10100877





