Geographical Distribution and Environmental Correlates of Eleutherosides and Isofraxidin in Eleutherococcus senticosus from Natural Populations in Forests at Northeast China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
2.3. Bioactive Compound Analysis
2.4. Statistical Analysis
3. Results
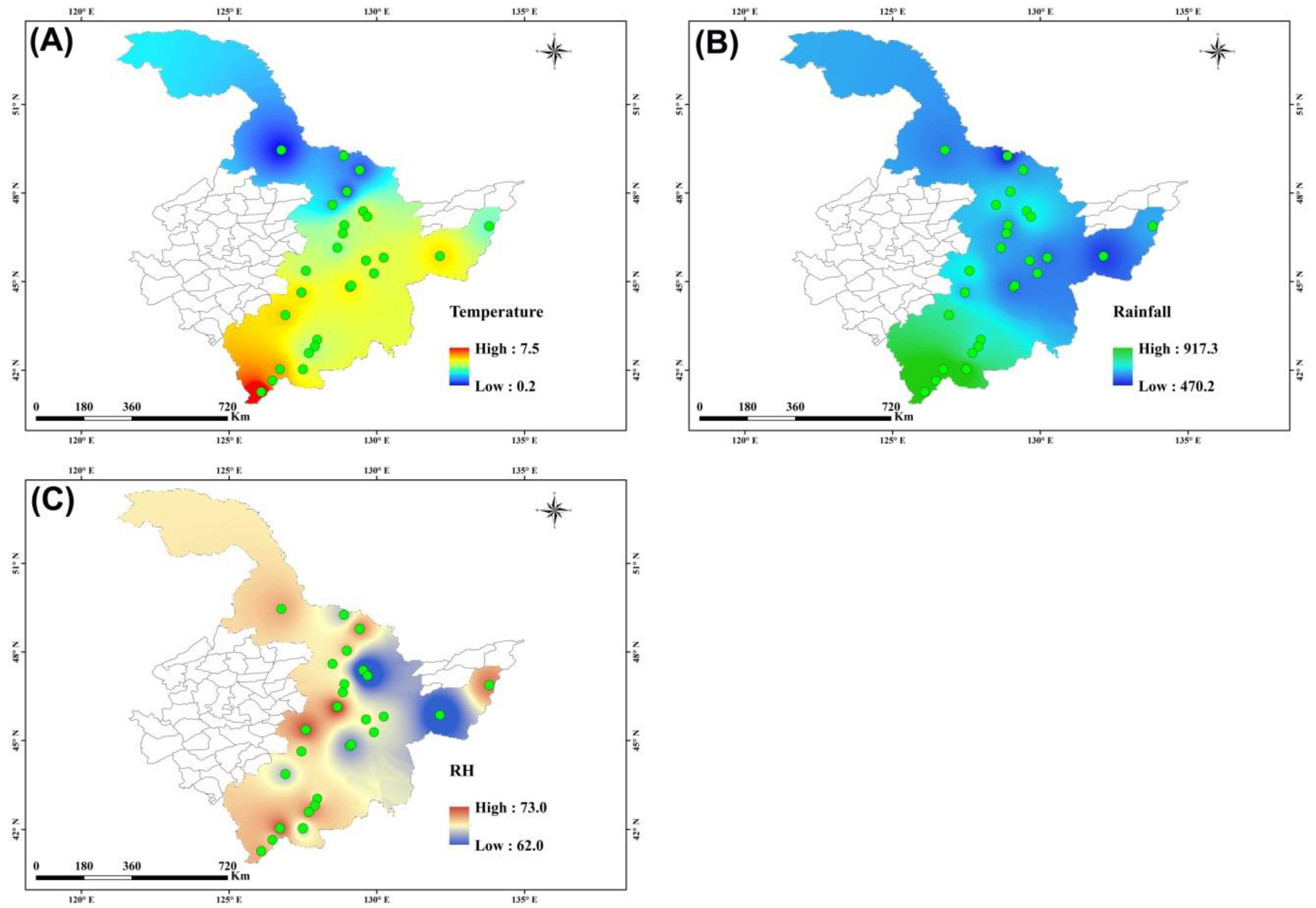
3.1. Spatial Distribution of Climatic Factors
3.2. Spatial Distribution of Bioactive Compounds
3.3. Relationship Between Parameters About Climate and Topography
3.4. Relationship Between Abiotic Factors and Bioactive Compounds
3.5. Regression of Multiple Varialbles of Abiotic Factors with Bioactive Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Walsh, E.S.; Vierling, K.T.; Strand, E.; Bartowitz, K.; Hudiburg, T.W. Climate change, woodpeckers, and forests: Current trends and future modeling needs. Ecol. Evol. 2019, 9, 2305–2319. [Google Scholar] [CrossRef] [PubMed]
- Lemordant, L.; Gentine, P. Vegetation response to rising co2 impacts extreme temperatures. Geophys. Res. Lett. 2019, 46, 1383–1392. [Google Scholar] [CrossRef]
- Park, H.; Jeong, S.J.; Ho, C.H.; Kim, J.; Brown, M.E.; Schaepman, M.E. Nonlinear response of vegetation green-up to local temperature variations in temperate and boreal forests in the northern hemisphere. Remote Sens. Environ. 2015, 165, 100–108. [Google Scholar] [CrossRef]
- Wang, G.; Wang, P.; Wang, T.Y.; Zhang, Y.C.; Yu, J.J.; Ma, N.; Frolova, N.L.; Liu, C.M. Contrasting changes in vegetation growth due to different climate forcings over the last three decades in the selenga-baikal basin. Remote Sens. 2019, 11, 17. [Google Scholar] [CrossRef]
- Ahlstrand, N.I.; Reghev, N.H.; Markussen, B.; Hansen, H.C.B.; Eiriksson, F.F.; Thorsteinsdottir, M.; Ronsted, N.; Barnes, C.J. Untargeted metabolic profiling reveals geography as the strongest predictor of metabolic phenotypes of a cosmopolitan weed. Ecol. Evol. 2018, 8, 6812–6826. [Google Scholar] [CrossRef]
- Jochum, G.M.; Mudge, K.W.; Thomas, R.B. Elevated temperatures increase leaf senescence and root secondary metabolite concentrations in the understory herb panax quinquefolius (araliaceae). Am. J. Bot. 2007, 94, 819–826. [Google Scholar] [CrossRef]
- Souther, S.; McGraw, J.B. Evidence of local adaptation in the demographic response of american ginseng to interannual temperature variation. Conserv. Biol. 2011, 25, 922–931. [Google Scholar] [CrossRef]
- Vezza, M.; Nepi, M.; Guarnieri, M.; Artese, D.; Rascio, N.; Pacini, E. Ivy (hedera helix l.) flower nectar and nectary ecophysiology. Int. J. Plant Sci. 2006, 167, 519–527. [Google Scholar] [CrossRef]
- Abraham, E.M.; Theodoropoulos, K.; Eleftheriadou, E.; Ragkos, A.; Kyriazopoulos, A.P.; Parissi, Z.M.; Arabatzisa, G.; Soutsas, K. Non-wood forest products from the understory and implications for rural development: The case of a broadleaf deciduous oak forest (quercus frainetto t e n.) in chalkidiki, greece. J. Environ. Prot. Ecol. 2015, 16, 1024–1032. [Google Scholar]
- FAO Towards a harmonized definition of non-wood forest products. Unasylva 1999, 198, 63–66.
- Huber, P.; Hujala, T.; Kurttila, M.; Wolfslehner, B.; Vacik, H. Application of multi criteria analysis methods for a participatory assessment of non-wood forest products in two european case studies. For. Policy Econ. 2019, 103, 103–111. [Google Scholar] [CrossRef]
- Huber, F.K.; Ineichen, R.; Yang, Y.P.; Weckerle, C.S. Livelihood and conservation aspects of non-wood forest product collection in the shaxi valley, southwest china(1). Econ. Bot. 2010, 64, 189–204. [Google Scholar] [CrossRef]
- Wei, H.X.; Zhao, H.T.; Chen, X. Foliar n:P stoichiometry in aralia elata distributed on different slope degrees. Not. Bot. Horti. Agrobo. 2018, 47, 887–895. [Google Scholar] [CrossRef]
- Gao, Z.; Khalid, M.; Jan, F.; Saeed ur, R.; Jiang, X.; Yu, X. Effects of light-regulation and intensity on the growth, physiological and biochemical properties of aralia elata (miq.) seedlings. South Afr. J. Bot. 2019, 121, 456–462. [Google Scholar] [CrossRef]
- Nadeau, I.; Olivier, A. The biology and forest cultivation of american ginseng (panax quinquefolius l.) in canada. Can. J. Plant Sci. 2003, 83, 877–891. [Google Scholar]
- Yu, X.H.; Gao, Z.L.; Juan, J.X.; Cheng, Y.; Zhao, M.L.; Ma, D.N.; Xu, X.B.; Jiang, X.M. Digital analysis on cone-thorn density and morphology in mutant aralia elata seedlings induced by ethyl methanesulfonate. Int. J. Agric. Biol. 2019. [Google Scholar]
- Yang, X.; Skidmore, A.K.; Melick, D.R.; Zhou, Z.; Xu, J. Mapping non-wood forest product (matsutake mushrooms) using logistic regression and a gis expert system. Ecol. Model. 2006, 198, 208–218. [Google Scholar] [CrossRef]
- Choi, S.; Kwon, Y.R.; Hossain, M.A.; Hong, S.W.; Lee, B.H.; Lee, H. A mutation in ela1, an age-dependent negative regulator of pap1/myb75, causes uv- and cold stress-tolerance in arabidopsis thaliana seedlings. Plant Sci. 2009, 176, 678–686. [Google Scholar] [CrossRef]
- Jung, C.H.; Ahn, J.; Heo, S.H.; Ha, T.-Y. Eleutheroside e, an active compound from eleutherococcus senticosus, regulates adipogenesis in 3t3-l1 cells. Food Sci. Biotechnol. 2014, 23, 889–893. [Google Scholar] [CrossRef]
- Wu, K.X.; Liu, J.; Liu, Y.; Guo, X.R.; Mu, L.Q.; Hu, X.H.; Tang, Z.H. A comparative metabolomics analysis reveals the tissue-specific phenolic profiling in two acanthopanax species. Molecules 2018, 23, 14. [Google Scholar] [CrossRef]
- Sun, H.; Liu, J.H.; Zhang, A.H.; Zhang, Y.; Meng, X.C.; Han, Y.; Zhang, Y.Z.; Wang, X.J. Characterization of the multiple components of acanthopanax senticosus stem by ultra high performance liquid chromatography with quadrupole time-of-flight tandem mass spectrometry. J. Sep. Sci. 2016, 39, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Sun, Q.; Bai, Y.; Bao, S.; Li, X.; Yan, G.; Liu, S. Characterization of eleutheroside b metabolites derived from an extract of acanthopanax senticosus harms by high-resolution liquid chromatography/quadrupole time-of-flight mass spectrometry and automated data analysis. Biomed. Chromatogr. 2012, 26, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Lihavainen, J.; Keinanen, M.; Keski-Saari, S.; Kontunen-Soppela, S.; Sober, A.; Oksanen, E. Artificially decreased vapour pressure deficit in field conditions modifies foliar metabolite profiles in birch and aspen. J. Exp. Bot. 2016, 67, 4367–4378. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Son, D.; Ryu, J.; Lee, Y.S.; Jung, S.H.; Kang, J.I.; Lee, S.Y.; Kim, H.S.; Shin, K.H. Anti-oxidant activities of acanthopanax senticosus stems and their lignan components. Arch. Pharm. Res. 2004, 27, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.F.; Xing, W.; Li, W.F.; Fan, T.; Hu, H.; Li, Y.M. Isofraxidin exhibited anti-inflammatory effects in vivo and inhibited tnf-alpha production in lps-induced mouse peritoneal macrophages in vitro via the mapk pathway. Int. Immunopharmacol. 2012, 14, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.L.; Zhang, D.H.; Wei, H.Y.; Zhao, Y.N.; Cao, Y.B.; Yu, T.; Wang, Y.; Yan, X.F. Climatic factors shape the spatial distribution of concentrations of triterpenoids in barks of white birch (betula platyphylla suk.) trees in northeast china. Forests 2017, 8, 12. [Google Scholar] [CrossRef]
- Shen, X.J.; Liu, B.H.; Xue, Z.S.; Jiang, M.; Lu, X.G.; Zhang, Q. Spatiotemporal variation in vegetation spring phenology and its response to climate change in freshwater marshes of northeast china. Sci. Total Environ. 2019, 666, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.M.; Shao, G.F.; Xiao, B.Y. Ecological classification for mountain forest sustainability in northeast china. For. Chron. 2003, 79, 233–236. [Google Scholar] [CrossRef]
- Guo, L.; Wang, S.; Zhang, J.; Yang, G.; Zhao, M.; Ma, W.; Zhang, X.; Li, X.; Han, B.; Chen, N.; et al. Effects of ecological factors on secondary metabolites and inorganic elements of scutellaria baicalensis and analysis of geoherblism. Sci. China Life Sci. 2013, 56, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.Z.; Wang, C.J.; Yu, J.H.; Nie, S.M.; Han, S.J.; Liu, J.Z.; Zu, Y.G.; Wang, Q.G. Developing conservation strategies for pinus koraiensis and eleutherococcus senticosus by using model-based geographic distributions. J. For. Res. 2016, 27, 389–400. [Google Scholar] [CrossRef]
- Shohael, A.M.; Ali, M.B.; Yu, K.W.; Hahn, E.J.; Paek, K.Y. Effect of temperature on secondary metabolites production and antioxidant enzyme activities in eleutherococcus senticosus somatic embryos. Plant Cell Tissue Organ. Cult. 2006, 85, 219–228. [Google Scholar] [CrossRef]
- Schmidt, S.; Zietz, M.; Schreiner, M.; Rohn, S.; Kroh, L.W.; Krumbein, A. Genotypic and climatic influences on the concentration and composition of flavonoids in kale (brassica oleracea var. Sabellica). Food Chem. 2010, 119, 1293–1299. [Google Scholar] [CrossRef]
- Shivanna, M.B.; Achar, K.G.S.; Vasanthakumari, M.M.; Mahishi, P. Phoma leaf spot disease of tinospora cordifolia and its effect on secondary metabolite production. J. Phytopathol. 2014, 162, 302–312. [Google Scholar] [CrossRef]
- Jaakola, L.; Hohtola, A. Effect of latitude on flavonoid biosynthesis in plants. Plant Cell Environ. 2010, 33, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Han, S.; Zhou, Y.; Zheng, X.; Yu, D.; Zheng, J.; Dai, G.; Li, M.-H. Needle δ13c and mobile carbohydrates in pinus koraiensis in relation to decreased temperature and increased moisture along an elevational gradient in ne china. Trees 2012, 27, 389–399. [Google Scholar] [CrossRef]
- Botha, L.E.; Prinsloo, G.; Deutschländer, M.S. Variations in the accumulation of three secondary metabolites in euclea undulata thunb. Var. Myrtina as a function of seasonal changes. South Afr. J. Bot. 2018, 117, 34–40. [Google Scholar] [CrossRef]
- Sampaio, B.L.; Edrada-Ebel, R.; Da Costa, F.B. Effect of the environment on the secondary metabolic profile of tithonia diversifolia: A model for environmental metabolomics of plants. Sci. Rep. 2016, 6, 29265. [Google Scholar] [CrossRef]




| Plot Number | Latitude | Longitude | Elevation (m) | Slope (°) | Forest Type | CD 1 (%) | Shoot Height (cm) | RCD 2 (cm) |
|---|---|---|---|---|---|---|---|---|
| 1 | 49°28ʹ41″ | 126°46ʹ29″ | 535 | 5 | Broadleaf-conifer | 0.5 | 161.67 ± 10.41 | 1.13 ± 0.06 |
| 2 | 49°16ʹ33″ | 128°53ʹ30″ | 198 | 15 | Mongolian oak 3 | 0.9 | 168.33 ± 7.64 | 1.33 ± 0.15 |
| 3 | 48°47ʹ24″ | 129°25ʹ29″ | 317 | 10 | Broadleaf-conifer | 0.4 | 193.33 ± 36.17 | 1.57 ± 0.38 |
| 4 | 48°3ʹ24″ | 128°59ʹ16″ | 346 | 8 | Broadleaf-conifer | 0.65 | 203.33 ± 27.54 | 1.87 ± 0.23 |
| 5 | 47°23ʹ57″ | 129°32ʹ30″ | 345 | 10 | Secondary forest | 0.2 | 158.33 ± 16.07 | 1.77 ± 0.15 |
| 6 | 45°49ʹ58″ | 130°14ʹ24″ | 301 | 15 | Secondary forest | 0.5 | 166.67 ± 55.08 | 1.20 ± 0.35 |
| 7 | 45°43ʹ23″ | 129°38ʹ38″ | 164 | 0 | Larch 4 | 0.3 | 178.33 ± 25.17 | 1.30 ± 0.20 |
| 8 | 47°36ʹ31″ | 128°30ʹ11″ | 322 | 5 | Broadleaf-conifer | 0.65 | 196.00 ± 20.81 | 1.33 ± 0.21 |
| 9 | 47°12ʹ35″ | 129°41ʹ21″ | 412 | 4 | Secondary forest | 0.45 | 195.00 ± 21.79 | 1.43 ± 0.32 |
| 10 | 46°55ʹ57″ | 128°54ʹ37″ | 439 | 10 | Broadleaf-conifer | 0.7 | 170.00 ± 5.00 | 1.20 ± 0.09 |
| 11 | 46°38ʹ35″ | 128°51ʹ35″ | 327 | 13 | Broadleaf-conifer | 0.7 | 170.00 ± 10.00 | 1.68 ± 0.16 |
| 12 | 46°9ʹ10″ | 128°40ʹ2″ | 168 | 5 | Secondary forest | 0.55 | 168.33 ± 10.41 | 1.41 ± 0.12 |
| 13 | 45°22ʹ45″ | 127°36ʹ18″ | 363 | 13 | Broadleaf-conifer | 0.5 | 168.33 ± 7.64 | 1.36 ± 0.21 |
| 14 | 44°49ʹ52″ | 129°5ʹ47″ | 674 | 5 | Fir 5 | 0.7 | 197.33 ± 23.69 | 1.33 ± 0.20 |
| 15 | 44°52ʹ22″ | 129°8ʹ16″ | 540 | 10 | Broadleaf-conifer | 0.67 | 150.00 ± 10.00 | 1.09 ± 0.08 |
| 16 | 43°2ʹ1″ | 127°59ʹ42″ | 714 | 5 | Broadleaf-conifer | 0.7 | 176.67 ± 11.55 | 1.50 ± 0.20 |
| 17 | 42°48ʹ58″ | 127°54ʹ19″ | 586 | 15 | Secondary forest | 0.7 | 165.00 ± 21.79 | 1.33 ± 0.25 |
| 18 | 42°35ʹ48″ | 127°42ʹ32″ | 791 | 3 | Secondary forest | 0.5 | 173.33 ± 5.77 | 1.47 ± 0.29 |
| 19 | 42°2ʹ41″ | 127°30ʹ44″ | 839 | 3 | Secondary forest | 0.5 | 160.00 ± 17.32 | 7.40 ± 5.39 |
| 20 | 42°2ʹ24″ | 126°43ʹ45″ | 723 | 8 | Broadleaf-conifer | 0.7 | 186.67 ± 11.55 | 1.43 ± 0.15 |
| 21 | 41°39ʹ48″ | 126°28ʹ47″ | 416 | 10 | Secondary forest | 0.7 | 140.00 ± 0.01 | 0.87 ± 0.06 |
| 22 | 41°16ʹ35″ | 126°5ʹ16″ | 726 | 15 | Secondary forest | 0.7 | 173.33 ± 20.82 | 1.23 ± 0.23 |
| 23 | 43°52ʹ48″ | 126°54ʹ16″ | 305 | 33 | Secondary forest | 0.7 | 196.67 ± 15.28 | 1.13 ± 0.15 |
| 24 | 44°38ʹ48″ | 127°27ʹ13″ | 266 | 20 | Secondary forest | 0.7 | 146.67 ± 5.77 | 1.27± 0.16 |
| 25 | 45°52ʹ49″ | 132°08ʹ11″ | 245 | 20 | Broadleaf forest 6 | 0.6 | 176.00 ± 14.42 | 1.57 ± 0.15 |
| 26 | 46°53ʹ47″ | 133°48ʹ57″ | 398 | 30 | Broadleaf forest | 0.6 | 165.00 ± 15.00 | 1.60 ± 0.36 |
| 27 | 45°17ʹ22″ | 129°54ʹ28″ | 392 | 25 | Broadleaf-conifer | 0.6 | 171.67 ± 12.58 | 1.43 ± 0.35 |
| Compound | Regression Model | R2 | Independent Range (μg) |
|---|---|---|---|
| Eleutheroside B | Y = 3,970,604.45x − 1316.93 | 0.9997 | 0.0390–2.502 |
| Eleutheroside E | Y = 675,578.96x − 23,980.50 | 0.9997 | 0.0390–2.510 |
| Isofraxidin | Y = 6,040,711.41x − 148,307.39 | 0.9996 | 0.0195%–2.507 |
| Regression Coefficient | Temperature | RH 1 | Rainfall 2 | Longitude | Latitude | Elevation 3 | Slope 4 | |
|---|---|---|---|---|---|---|---|---|
| Temperature | R | 1 | ||||||
| P | ||||||||
| RH | R | 0.22984 | 1 | |||||
| P | 0.2488 | |||||||
| Rainfall | R | −0.14057 | −0.22209 | 1 | ||||
| P | 0.4843 | 0.2655 | ||||||
| Longitude | R | −0.41167 | 0.14211 | −0.11174 | 1 | |||
| P | 0.0329 | 0.4795 | 0.579 | |||||
| Latitude | R | −0.78313 | −0.25705 | 0.08392 | 0.0885 | 1 | ||
| P | <0.0001 | 0.1955 | 0.6773 | 0.6607 | ||||
| Elevation | R | 0.1373 | 0.52385 | −0.05712 | 0.43327 | −0.50833 | 1 | |
| P | 0.4947 | 0.0050 | 0.7772 | 0.0240 | 0.0068 | |||
| Slope | R | 0.07157 | −0.01393 | −0.27039 | 0.36195 | −0.0009 | −0.12076 | 1 |
| P | 0.7228 | 0.945 | 0.1726 | 0.0636 | 0.9964 | 0.5485 |
| Variable | Estimate | Standard Error | Type II SS 1 | F Value | Pr > F |
|---|---|---|---|---|---|
| Stem eleutheroside B | |||||
| Intercept | 28.06726 | 7.82155 | 18.16931 | 12.88 | 0.0015 |
| Temperature | −0.91374 | 0.23469 | 21.38854 | 15.16 | 0.0007 |
| Latitude | −0.43905 | 0.15775 | 10.93055 | 7.75 | 0.0103 |
| Stem eleutheroside E 2 | |||||
| Intercept | 4.39383 | 0.87811 | 0.44527 | 25.04 | <0.0001 |
| Temperature | 0.07197 | 0.02635 | 0.13269 | 7.46 | 0.0116 |
| Latitude | −0.07435 | 0.01771 | 0.31342 | 17.62 | 0.0003 |
| Variable | DF | Estimate | Wald Chi-square | Pr > Chi-square | Estimate | Wald Chi-square | Pr > Chi-square |
|---|---|---|---|---|---|---|---|
| Root eleutheroside B | Root eleutheroside E | ||||||
| Intercept | 1 | −56.9266 | 4.72 | 0.0298 | −53.51 | 1.11 | 0.2929 |
| Temperature | 1 | −0.208 | 0.48 | 0.4897 | −0.0061 | 0 | 0.9916 |
| RH | 1 | 0.17081 | 5.4 | 0.0201 | 0.1321 | 0.86 | 0.3546 |
| Rainfall | 1 | −8.0786 | 0.4 | 0.5247 | −53.2192 | 4.66 | 0.0309 |
| Longitude | 1 | 0.5154 | 9.05 | 0.0026 | 0.6154 | 3.42 | 0.0643 |
| Latitude | 1 | −0.2855 | 1.46 | 0.2271 | −0.2281 | 0.25 | 0.6191 |
| Elevation | 1 | 1.0146 | 0.44 | 0.5092 | −2.4571 | 0.68 | 0.4103 |
| Slope | 1 | −0.1232 | 0.03 | 0.8630 | 0.622 | 0.2 | 0.6537 |
| Scale | 1 | 1.1264 | 2.1869 | ||||
| Stem isofraxidin | Root isofraxidin | ||||||
| Intercept | 1 | 16.0687 | 2.45 | 0.1173 | 1.6175 | 1.74 | 0.1866 |
| Temperature | 1 | 2.1062 | 9.67 | 0.0019 | 0.3094 | 14.64 | 0.0001 |
| RH | 1 | −0.6087 | 4.47 | 0.0346 | 0.1628 | 22.43 | <0.0001 |
| Rainfall | 1 | −1.2718 | 0.9 | 0.3438 | −0.5127 | 10.22 | 0.0014 |
| Longitude | 1 | 0.0076 | 0 | 0.9706 | 0.0485 | 3.85 | 0.0496 |
| Latitude | 1 | 0.0009 | 0 | 0.9546 | −0.0027 | 2.07 | 0.1499 |
| Elevation | 1 | 0.7587 | 4.74 | 0.0295 | 0.0266 | 0.41 | 0.5219 |
| Slope | 1 | −2.2329 | 3.89 | 0.0486 | −0.0963 | 0.51 | 0.4762 |
| Scale | 1 | 5.9059 | 0.705 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.; Wei, H.; Li, J.; Fan, R.; Xu, M.; Chen, X.; Wang, Z. Geographical Distribution and Environmental Correlates of Eleutherosides and Isofraxidin in Eleutherococcus senticosus from Natural Populations in Forests at Northeast China. Forests 2019, 10, 872. https://doi.org/10.3390/f10100872
Guo S, Wei H, Li J, Fan R, Xu M, Chen X, Wang Z. Geographical Distribution and Environmental Correlates of Eleutherosides and Isofraxidin in Eleutherococcus senticosus from Natural Populations in Forests at Northeast China. Forests. 2019; 10(10):872. https://doi.org/10.3390/f10100872
Chicago/Turabian StyleGuo, Shenglei, Hongxu Wei, Junping Li, Ruifeng Fan, Mingyuan Xu, Xin Chen, and Zhenyue Wang. 2019. "Geographical Distribution and Environmental Correlates of Eleutherosides and Isofraxidin in Eleutherococcus senticosus from Natural Populations in Forests at Northeast China" Forests 10, no. 10: 872. https://doi.org/10.3390/f10100872
APA StyleGuo, S., Wei, H., Li, J., Fan, R., Xu, M., Chen, X., & Wang, Z. (2019). Geographical Distribution and Environmental Correlates of Eleutherosides and Isofraxidin in Eleutherococcus senticosus from Natural Populations in Forests at Northeast China. Forests, 10(10), 872. https://doi.org/10.3390/f10100872







