Community Attributes Predict the Relationship between Habitat Invasibility and Land Use Types in an Agricultural and Forest Landscape
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling Design and Data Collection
2.3. Data Analysis
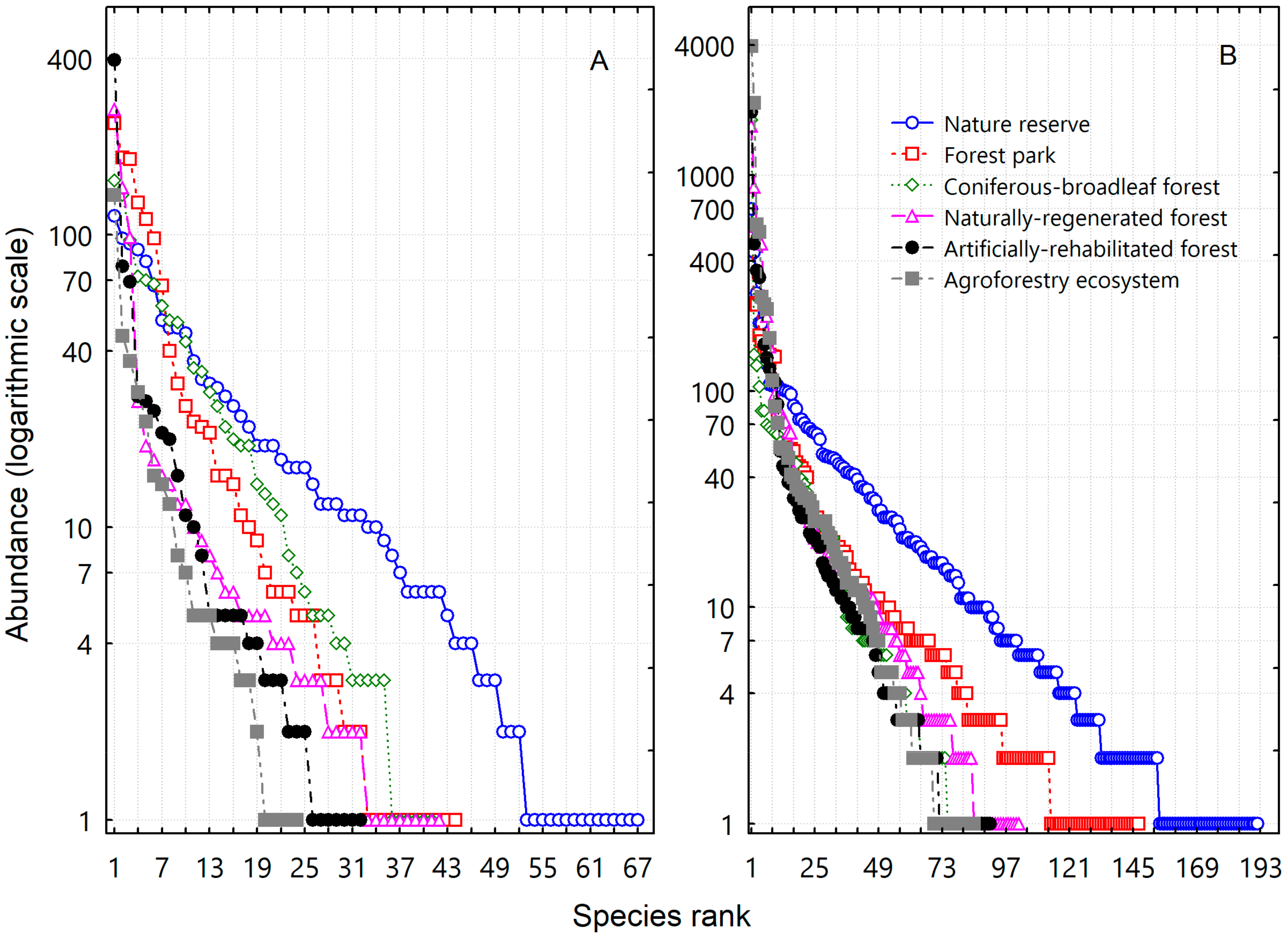
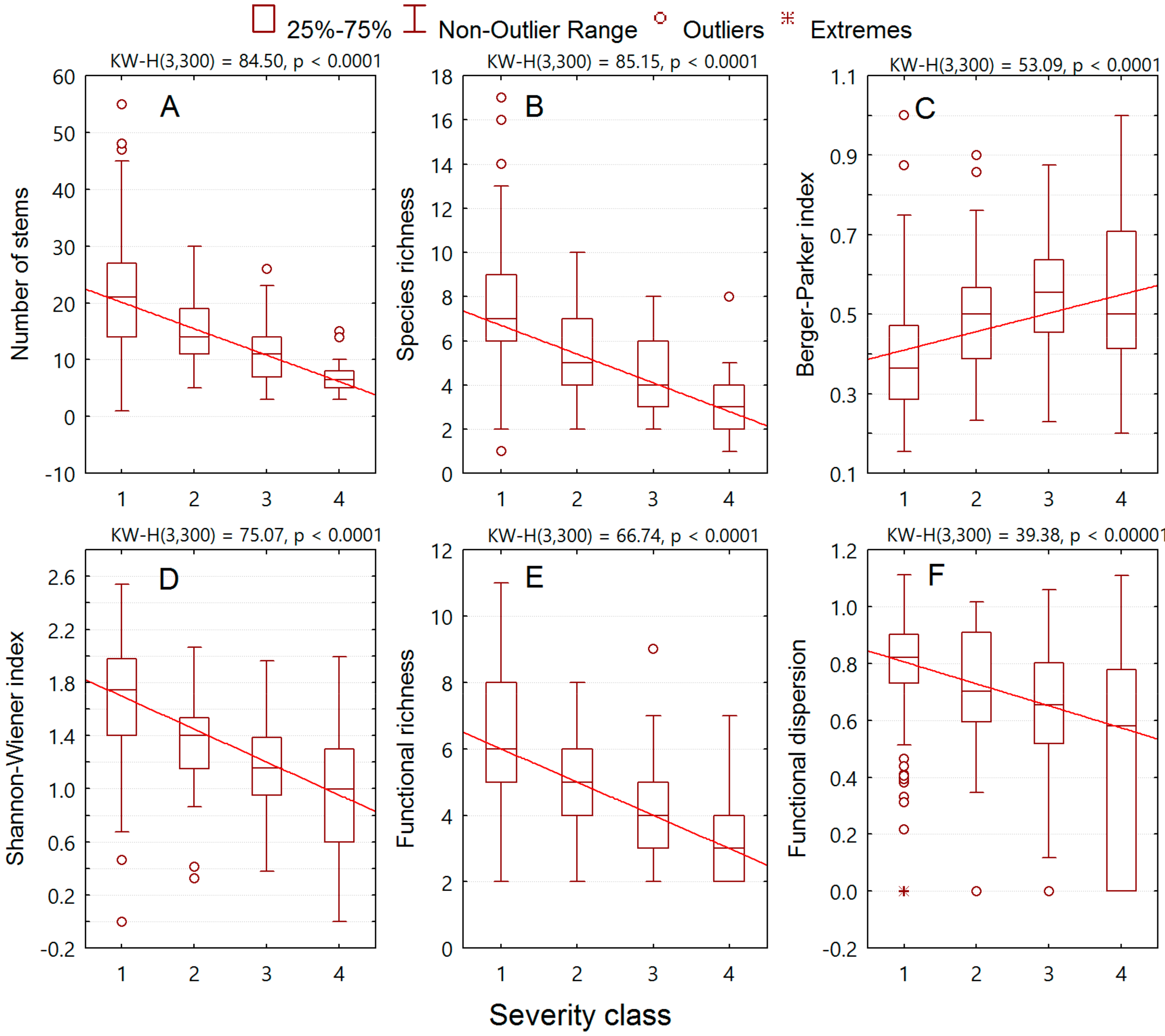
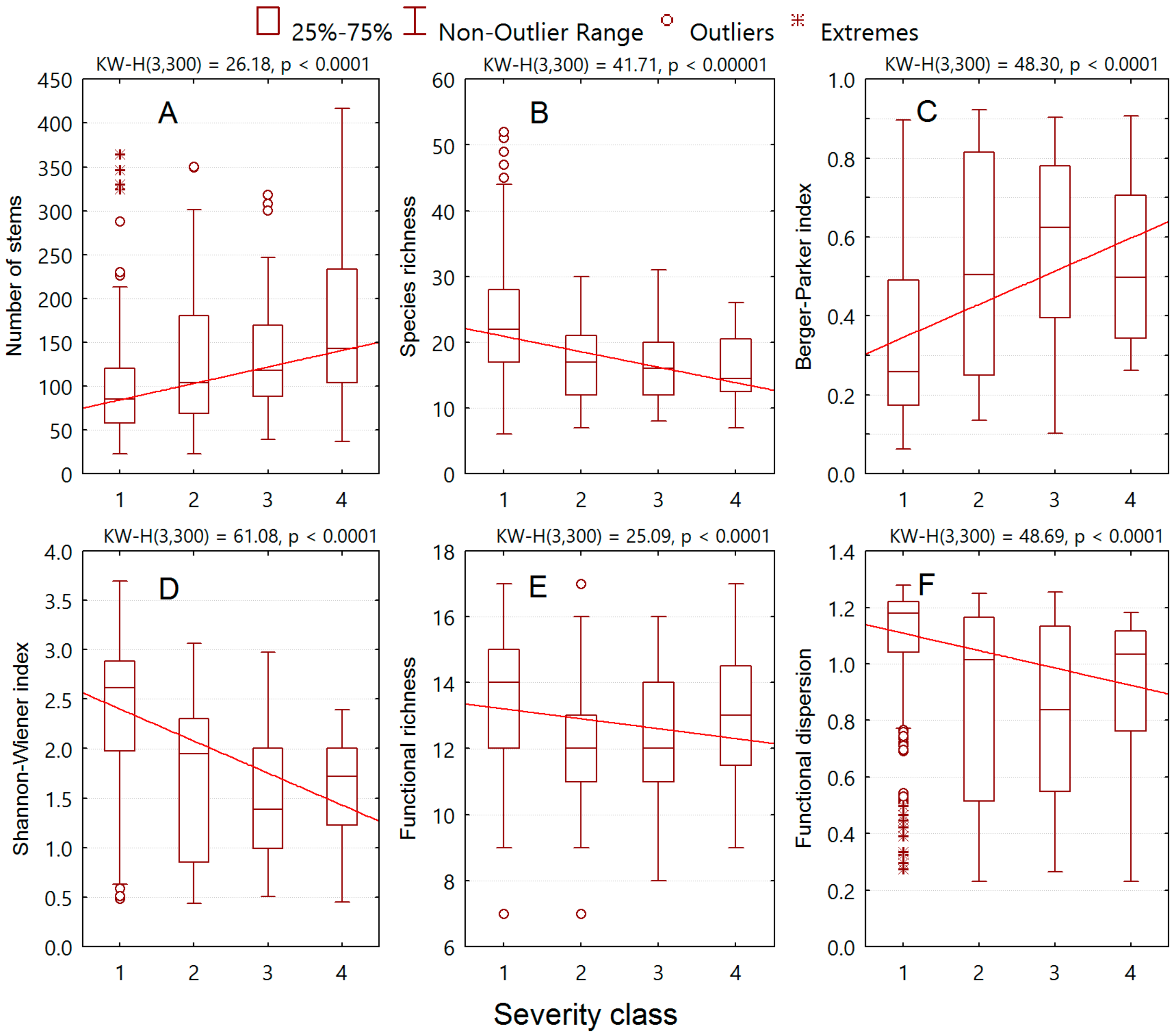
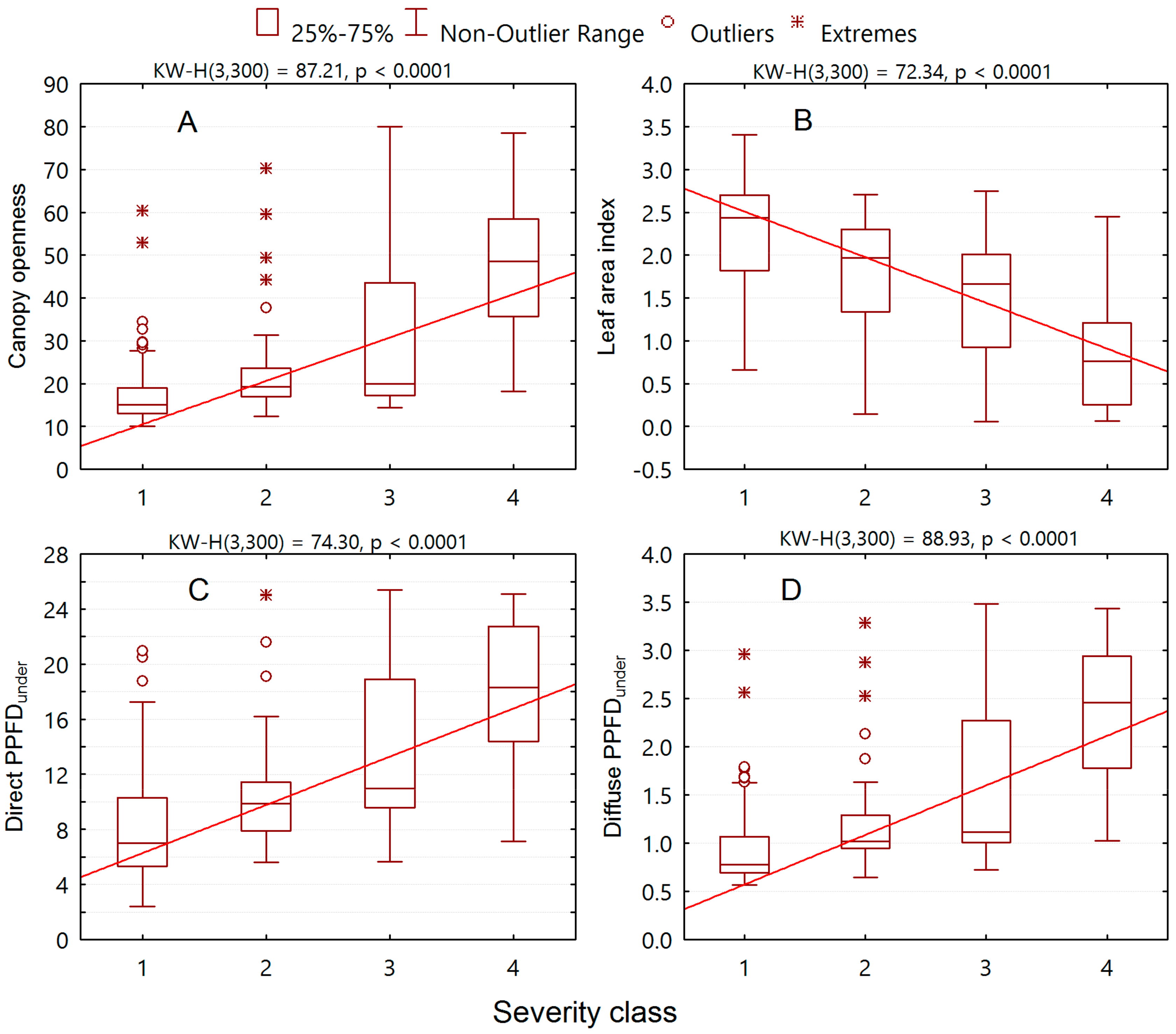
3. Results
3.1. Effect of Land Use and Species Composition on Plant Invasion
3.2. Relationships of Alien Plant Invasion to Community Attributes
3.3. Indicator Species of the Resident Community for Alien Plant Invasion
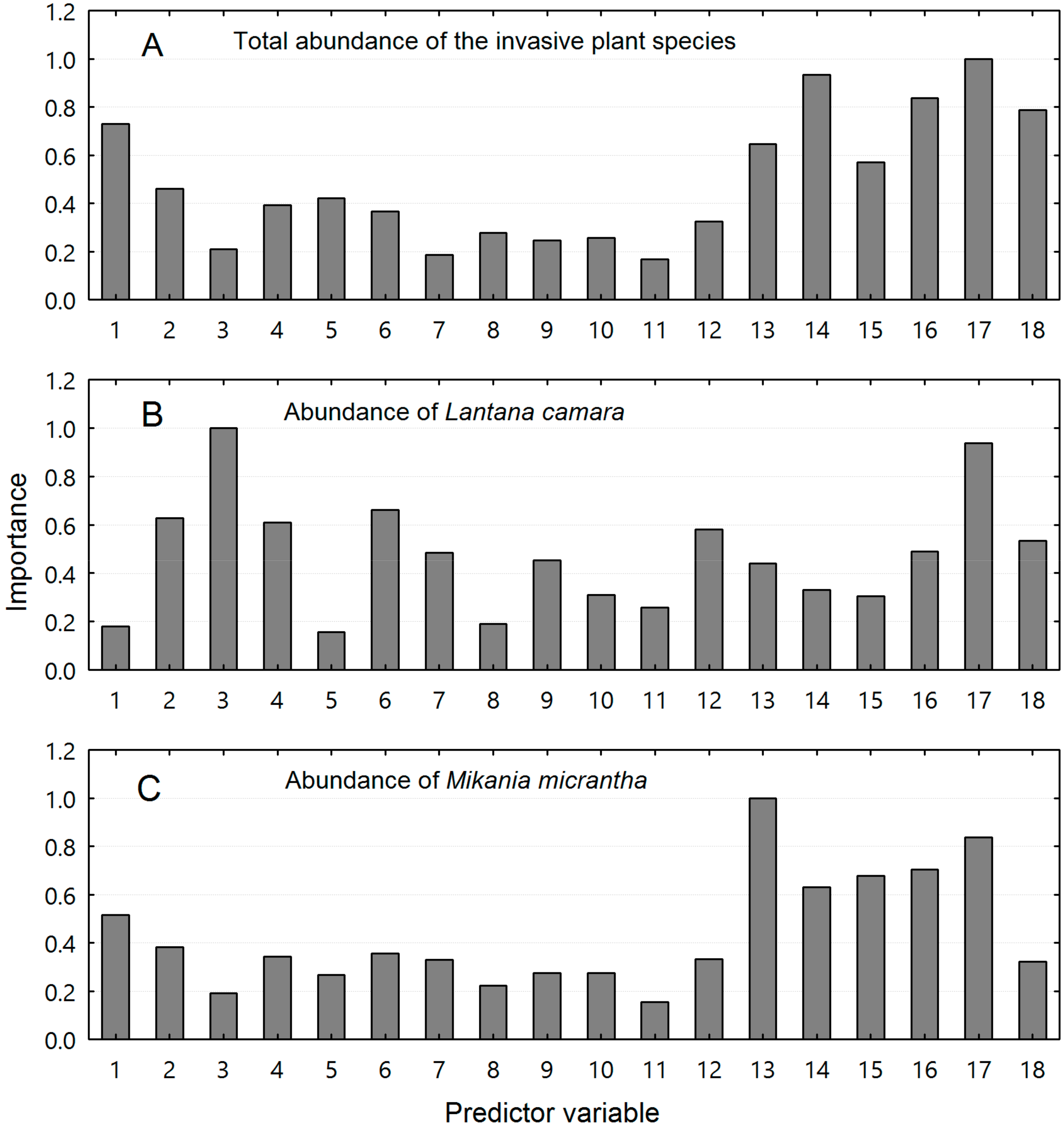
3.4. Major Drivers for the Alien Plant Invasion
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Drake, K.K.; Bowen, L.; Nussear, K.E.; Esque, T.C.; Berger, A.J.; Custer, N.A.; Waters, S.C.; Johnson, J.D.; Miles, A.K.; Lewison, R.L. Negative impacts of invasive plants on conservation of sensitive desert wildlife. Ecosphere 2016, 7, e01531. [Google Scholar] [CrossRef]
- Nickerson, K.; Flory, S.L. Competitive and allelopathic effects of the invasive shrub Schinus terebinthifolius (Brazilian pepper tree). Biol. Invasions 2015, 17, 555–564. [Google Scholar] [CrossRef]
- Shackleton, R.T.; Witt, A.B.R.; Piroris, F.M.; van Wilgen, B.W. Distribution and socio-ecological impacts of the invasive alien cactus Opuntia stricta in eastern Africa. Biol. Invasions 2017, 19, 2427–2441. [Google Scholar] [CrossRef]
- Catford, J.A.; Vesk, P.A.; Richardson, D.M.; Pyšek, P.J.G.C.B. Quantifying levels of biological invasion: Towards the objective classification of invaded and invasible ecosystems. Global Change Biol. 2012, 18, 44–62. [Google Scholar] [CrossRef]
- Milbau, A.; Stout, J.C.; Graae, B.J.; Nijs, I.J.B.I. A hierarchical framework for integrating invasibility experiments incorporating different factors and spatial scales. Biol. Invasions 2009, 11, 941–950. [Google Scholar] [CrossRef]
- Schrama, M.; Bardgett, R.D. Grassland invasibility varies with drought effects on soil functioning. J. Ecol. 2016, 104, 1250–1258. [Google Scholar] [CrossRef]
- Dimitrakopoulos, P.G.; Koukoulas, S.; Galanidis, A.; Delipetrou, P.; Gounaridis, D.; Touloumi, K.; Arianoutsou, M. Factors shaping alien plant species richness spatial patterns across Natura 2000 Special Areas of Conservation of Greece. Sci. Total Environ. 2017, 601, 461–468. [Google Scholar] [CrossRef]
- Menzel, A.; Hempel, S.; Klotz, S.; Moora, M.; Pysek, P.; Rillig, M.C.; Zobel, M.; Kuhn, I. Mycorrhizal status helps explain invasion success of alien plant species. Ecology 2017, 98, 92–102. [Google Scholar] [CrossRef]
- Rembold, K.; Mangopo, H.; Tjitrosoedirdjo, S.; Kreft, H. Plant diversity, forest dependency, and alien plant invasions in tropical agricultural landscapes. Biol. Conserv. 2017, 213, 234–242. [Google Scholar] [CrossRef]
- Stein, A.; Gerstner, K.; Kreft, H. Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecol. Lett. 2014, 17, 866–880. [Google Scholar] [CrossRef]
- Dawud, S.M.; Raulund-Rasmussen, K.; Domisch, T.; Finer, L.; Jaroszewicz, B.; Vesterdal, L. Is tree species diversity or species identity the more important driver of soil carbon stocks, C/N ratio, and pH? Ecosystems 2016, 19, 645–660. [Google Scholar] [CrossRef]
- Arellano-Cataldo, G.; Smith-Ramirez, C. Establishment of invasive plant species in canopy gaps on Robinson Crusoe Island. Plant Ecol. 2016, 217, 289–302. [Google Scholar] [CrossRef]
- Song, X.Y.; Hogan, J.A.; Brown, C.; Cao, M.; Yang, J. Snow damage to the canopy facilitates alien weed invasion in a subtropical montane primary forest in southwestern China. For. Ecol. Manag. 2017, 391, 275–281. [Google Scholar] [CrossRef]
- Liao, H.X.; Luo, W.B.; Peng, S.L.; Callaway, R.M. Plant diversity, soil biota and resistance to exotic invasion. Divers. Distrib. 2015, 21, 826–835. [Google Scholar] [CrossRef]
- Selmants, P.C.; Zavaleta, E.S.; Pasari, J.R.; Hernandez, D.L. Realistic plant species losses reduce invasion resistance in a California serpentine grassland. J. Ecol. 2012, 100, 723–731. [Google Scholar] [CrossRef]
- Levine, J.M.; Adler, P.B.; Yelenik, S.G. A meta-analysis of biotic resistance to exotic plant invasions. Ecol. Lett. 2004, 7, 975–989. [Google Scholar] [CrossRef]
- Grossman, J.J.; Cavenderbares, J.; Hobbie, S.E.; Reich, P.B.; Montgomery, R.A. Species richness and traits predict overyielding in stem growth in an early-successional tree diversity experiment. Ecology 2017, 98, 2601–2614. [Google Scholar] [CrossRef] [PubMed]
- Frankow-Lindberg, B.E. Grassland plant species diversity decreases invasion by increasing resource use. Oecologia 2012, 169, 793–802. [Google Scholar] [CrossRef]
- Whitfeld, T.J.S.; Roth, A.M.; Lodge, A.G.; Eisenhauer, N.; Frelich, L.E.; Reich, P.B. Resident plant diversity and introduced earthworms have contrasting effects on the success of invasive plants. Biol. Invasions 2014, 16, 2181–2193. [Google Scholar] [CrossRef]
- Gentili, R.; Montagnani, C.; Gilardelli, F.; Guarino, M.F.; Citterio, S. Let native species take their course: Ambrosia artemisiifolia replacement during natural or “artificial” succession. Acta Oecol. 2017, 82, 32–40. [Google Scholar] [CrossRef]
- Belote, R.T.; Jones, R.H.; Hood, S.M.; Wender, B.W. Diversity-invasibility across an experimental disturbance gradient in Appalachian forests. Ecology 2008, 89, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.F.; Harrison, S.; Safford, H.D.; Viers, J.H. Productivity alters the scale dependence of the diversity-invasibility relationship. Ecology 2007, 88, 1940–1947. [Google Scholar] [CrossRef] [PubMed]
- Knight, K.S.; Oleksyn, J.; Jagodzinski, A.M.; Reich, P.B.; Kasprowicz, M. Overstorey tree species regulate colonization by native and exotic plants: A source of positive relationships between understorey diversity and invasibility. Divers. Distrib. 2008, 14, 666–675. [Google Scholar] [CrossRef]
- Tabacchi, E.; Planty-Tabacchi, A.M. Exotic and native plant community distributions within complex riparian landscapes: A positive correlation. Ecoscience 2005, 12, 412–423. [Google Scholar] [CrossRef]
- Bhattarai, K.R.; Maren, I.E.; Subedi, S.C. Biodiversity and invasibility: Distribution patterns of invasive plant species in the Himalayas, Nepal. J. Mt. Sci. 2014, 11, 688–696. [Google Scholar] [CrossRef]
- Bart, D.; Davenport, T.; Carpenter, Q. Stress and land-use legacies alter the relationship between invasive- and native- plant richness. J. Veg. Sci. 2015, 26, 80–88. [Google Scholar] [CrossRef]
- Csecserits, A.; Botta-Dukat, Z.; Kroel-Dulay, G.; Lhotsky, B.; Onodi, G.; Redei, T.; Szitar, K.; Halassy, M. Tree plantations are hot-spots of plant invasion in a landscape with heterogeneous land-use. Agric. Ecosyst. Environ. 2016, 226, 88–98. [Google Scholar] [CrossRef]
- Clotet, M.; Basnou, C.; Bagaria, G.; Pino, J. Contrasting historical and current land-use correlation with diverse components of current alien plant invasions in Mediterranean habitats. Biol. Invasions 2016, 18, 2897–2909. [Google Scholar] [CrossRef]
- Chytry, M.; Wild, J.; Pysek, P.; Jarosik, V.; Dendoncker, N.; Reginster, I.; Pino, J.; Maskell, L.C.; Vila, M.; Pergl, J.; et al. Projecting trends in plant invasions in Europe under different scenarios of future land-use change. Global Ecol. Biogeogr. 2012, 21, 75–87. [Google Scholar] [CrossRef]
- Li, S.Y.; Li, X.; Liu, X.P.; Wu, Z.F.; Ai, B.; Wang, F. Simulation of spatial population dynamics based on labor economics and multi-agent systems: A case study on a rapidly developing manufacturing metropolis. Int. J. Geogr. Inf. Sci. 2013, 27, 2410–2435. [Google Scholar] [CrossRef]
- Liu, Z.J.; Huang, H.Q.; Werners, S.E.; Yan, D. Construction area expansion in relation to economic-demographic development and land resource in the Pearl River Delta of China. J. Geogr. Sci. 2016, 26, 188–202. [Google Scholar] [CrossRef]
- Fang, C.L.; Ma, H.T.; Wang, J. A regional categorization for "new-type urbanization" in China. PLoS ONE 2015, 10, e0134253. [Google Scholar] [CrossRef] [PubMed]
- Liao, F.H.F.; Wei, Y.H.D. Modeling determinants of urban growth in Dongguan, China: A spatial logistic approach. Stoch. Environ. Res. Risk A 2014, 28, 801–816. [Google Scholar] [CrossRef]
- Huang, G.X.; Sun, J.C.; Zhang, Y.; Chen, Z.Y.; Liu, F. Impact of anthropogenic and natural processes on the evolution of groundwater chemistry in a rapidly urbanized coastal area, south China. Sci. Total Environ. 2013, 463, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.D.; Su, Z.Y.; Ke, X.D.; Li, Z.K. Vascular ground flora in relation to topography, canopy structure and gap light regimes in a subtropical broadleaved forest (south China). Pol. J. Ecol. 2012, 60, 463–478. [Google Scholar]
- Beaudet, M.; Messier, C. Variation in canopy openness and light transmission following selection cutting in northern hardwood stands: An assessment based on hemispherical photographs. Agric. For. Meteorol. 2002, 110, 217–228. [Google Scholar] [CrossRef]
- Magurran, A.E.; McGill, B.J. Biological Diversity: Frontiers in Measurement and Assessment; Oxford University Press: New York, NY, USA, 2011. [Google Scholar]
- Su, Y.Q.; Tang, Q.M.; Mo, F.Y.; Xue, Y.G. Karst tiankengs as refugia for indigenous tree flora amidst a degraded landscape in southwestern China. Sci. Rep. 2017, 7, 4249. [Google Scholar] [CrossRef]
- He, S.Y.; Zhong, Y.L.; Sun, Y.D.; Su, Z.Y.; Jia, X.R.; Hu, Y.Q.; Zhou, Q. Topography-associated thermal gradient predicts warming effects on woody plant structural diversity in a subtropical forest. Sci. Rep. 2017, 7, 40387. [Google Scholar] [CrossRef]
- McCune, B.; Grace, J.B.; Urban, D.L. Analysis of Ecological Communities; MjM Software Design: Gleneden Beach, OR, USA, 2002. [Google Scholar]
- Breiman, L.; Last, M.; Rice, J. Random forests: Finding quasars. In Statistical Challenges in Astronomy; Springer: New York, NY, USA, 2003; pp. 243–254. [Google Scholar]
- Breiman, L. Random forest. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Prabu, N.R.; Stalin, N.; Swamy, P.S. Ecophysiological attributes of Mikania micrantha, an exotic invasive weed, at two different elevations in the tropical forest regions of the western Ghats, south India. Weed Biol. Manag. 2014, 14, 59–67. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Ye, W.H.; Cao, H.L.; Feng, H.L. Mikania micrantha H.B.K. In China—An overview. Weed Res. 2010, 44, 42–49. [Google Scholar] [CrossRef]
- Ye, H.G.; Peng, S.L. Plant Diversity Inventory of Guangdong; Guangdong World Publishing Corporation: Guangzhou, China, 2006. [Google Scholar]
- Romanuk, T.N.; Kolasa, J. Resource limitation, biodiversity, and competitive effects interact to determine the nvisibility of rock pool microcosms. Biol. Invasions 2005, 7, 711–722. [Google Scholar] [CrossRef][Green Version]
- Annighöfer, P.; Petritan, A.M.; Petritan, I.C.; Ammer, C. Disentangling juvenile growth strategies of three shade-tolerant temperate forest tree species responding to a light gradient. For. Ecol. Manag. 2017, 391, 115–126. [Google Scholar] [CrossRef]
- Chin, A.R.; Sillett, S.C. Leaf acclimation to light availability supports rapid growth in tall Picea sitchensis trees. Tree Physiol. 2017, 37, 1352–1366. [Google Scholar] [CrossRef] [PubMed]
- Oberhuber, W. Soil water availability and evaporative demand affect seasonal growth dynamics and use of stored water in co-occurring saplings and mature conifers under drought. Trees 2017, 31, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Barbier, S.; Gosselin, F.; Balandier, P. Influence of tree species on understory vegetation diversity and mechanisms involved—a critical review for temperate and boreal forests. For. Ecol. Manag. 2008, 254, 1–15. [Google Scholar] [CrossRef]
- Battaglia, M.A.; Mou, P.; Palik, B.; Mitchell, R.J. The effect of spatially variable overstory on the understory light environment of an open-canopied longleaf pine forest. Can. J. For. Res. 2002, 32, 1984–1991. [Google Scholar] [CrossRef]
- Sharma, A.; Jose, S.; Bohn, K.K.; Andreu, M.G. Effects of reproduction methods and overstory species composition on understory light availability in longleaf pine–slash pine ecosystems. For. Ecol. Manag. 2012, 284, 23–33. [Google Scholar] [CrossRef]
- Fargione, J.E.; Tilman, D. Diversity decreases invasion via both sampling and complementarity effects. Ecol. Lett. 2005, 8, 604–611. [Google Scholar] [CrossRef]
- Bresciano, D.; Altesor, A.; Rodríguez, C. The growth form of dominant grasses regulates the invasibility of Uruguayan grasslands. Ecosphere 2016, 5, 1–12. [Google Scholar] [CrossRef]
- Ruijven, J.V.; Deyn, G.B.D.; Berendse, F. Diversity reduces invasibility in experimental plant communities: The role of plant species. Ecol. Lett 2003, 6, 910–918. [Google Scholar] [CrossRef]
- Chen, B.M.; Liao, H.; Chen, W.B.; Wei, H.J.; Peng, S.L. Role of allelopathy in plant invasion and control of invasive plants. Allelopathy J. 2017, 41, 155–166. [Google Scholar] [CrossRef]
- Alnamazi, A.A.; Elbana, M.I.; Bonser, S.P. Competition and facilitation structure plant communities under nurse tree canopies in extremely stressful environments. Ecol. Evol. 2017, 7, 2747–2755. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.Y.; Ke, X.D.; Zhang, S.J. Vascular plants as indicators of organic carbon gradient in subtropical forested soils. Pol. J. Environ. Stud. 2012, 21, 1393–1398. [Google Scholar]
- Clark, G.F.; Johnston, E.L. Temporal change in the diversity–invasibility relationship in the presence of a disturbance regime. Ecol. Lett. 2011, 14, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.A.; Matlack, G.R. Agricultural history drives structure and tree species composition of second growth forest over 100 years in southeastern Ohio, USA. J. Veg. Sci. 2017, 28, 736–746. [Google Scholar] [CrossRef]
- Schmidt, M.; Veldkamp, E.; Corre, M.D. Tree species diversity effects on productivity, soil nutrient availability and nutrient response efficiency in a temperate deciduous forest. For. Ecol. Manag. 2015, 338, 114–123. [Google Scholar] [CrossRef]
- Hu, L.; Li, M.; Li, Z. Geographical and environmental gradients of lianas and vines in China. Global Ecol. Biogeogr. 2010, 19, 554–561. [Google Scholar] [CrossRef]
- Haeuser, E.; Dawson, W.; Van Kleunen, M. The effects of climate warming and disturbance on the colonization potential of ornamental alien plant species. J. Ecol. 2017, 105, 1698–1708. [Google Scholar] [CrossRef]
- Lembrechts, J.J.; Pauchard, A.; Lenoir, J.; Nuñez, M.A.; Geron, C.; Ven, A.; Bravomonasterio, P.; Teneb, E.; Nijs, I.; Milbau, A. Disturbance is the key to plant invasions in cold environments. Proc. Natl. Acad. Sci. USA 2016, 113, 14061. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Acosta, N.; Kowaljow, E.; Aguilar, R. Reproductive performance of the invasive tree Ligustrum lucidum in a subtropical dry forest: Does habitat fragmentation boost or limit invasion? Biol. Invasions 2014, 16, 1397–1410. [Google Scholar] [CrossRef]
| Attribute | Ecosystem | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Location | 114°13′23″ E 22°53′09″ N | 114°14′40″ E 22°53′12″ N | 113°48′06″ E 22°55′20″ N | 113°46′18″ E 22°51′41″ N | 113°47′50″ E 22°50′51″ N | 113°46′53″ E 22°50′42″ N |
| Land use types | Nature reserve | Forest park | Coniferous-broadleaf forest | Naturally- regenerated forest | Artificially- rehabilitated forest | Agroforestry ecosystem |
| Management regime | Natural | Natural | Semi-natural | Semi-natural | Managed | Managed |
| Disturbance regime | Strictly protected |  |  |  |  |  |
| Elevation (m) | 246.4 | 581.2 | 59.6 | 62.5 | 57.2 | 68.7 |
| Aspect | NW | NE | SW | SW | SW | NW |
| Slope (°) | 25 | 15 | 7 | 5 | 6 | 8 |
| Plot area (m2) | 5000 | 5000 | 5000 | 5000 | 5000 | 5000 |
| Number of quadrats | 50 | 50 | 50 | 50 | 50 | 50 |
| Attribute | Ecosystem | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Number of species | ||||||
| Overstory | 68 | 45 | 42 | 43 | 33 | 25 |
| Understory | 192 | 147 | 90 | 102 | 91 | 85 |
| Number of stems | ||||||
| Overstory | 1292 | 1315 | 1116 | 737 | 762 | 367 |
| Understory | 5723 | 3645 | 3685 | 6242 | 4946 | 9446 |
| Number of subplots with invasive plants | uninvaded | 2 | 5 | 8 | 30 | 45 |
| Invasive plant species | None | EUPCAT LANCAM | LANCAM MIKMIC | MIKMIC | LANCAM MIKMIC | LANCAM MIKMIC RHYREP |
| Total number of invasive plant individuals | None | 2 | 9 | 18 | 62 | 286 |
| Groups Compared | Overstory | Understory | ||||
|---|---|---|---|---|---|---|
| T | A | P | T | A | P | |
| Overall comparison | −36.170 | 0.048 | <10−7 | −22.590 | 0.029 | <10−7 |
| Pairwise comparison | ||||||
| Uninvaded vs. Low | −12.478 | 0.012 | <10−6 | −10.422 | 0.010 | <10−5 |
| Uninvaded vs. Medium | −24.299 | 0.021 | <10−7 | −20.265 | 0.018 | <10−7 |
| Uninvaded vs. High | −38.944 | 0.036 | <10−7 | −17.436 | 0.016 | <10−7 |
| Low vs. Medium | −1.053 | 0.005 | 0.131 | −0.086 | 0.0004 | 0.380 |
| Low vs. High | −14.579 | 0.111 | <10−5 | −5.013 | 0.030 | 0.001 |
| Medium vs. High | −7.709 | 0.049 | 0.001 | −3.134 | 0.016 | 0.011 |
| Invasion Severity Class | Species | Growth Form | IV | P |
|---|---|---|---|---|
| Overstory | ||||
| Medium | Acacia mangium | Tree | 22.8 | 0.0019 |
| High | Dimocarpus longan | Tree | 55.1 | 0.0001 |
| Understory | ||||
| Uninvaded | Adiantum flabellulatum | Fern | 34.7 | 0.0028 |
| Uninvaded | Wikstroemia nutans | Shrub | 26.1 | 0.0005 |
| Uninvaded | Tricalysia dubia | Shrub | 23.6 | 0.005 |
| Uninvaded | Rapanea neriifolia | Shrub | 22.5 | 0.0064 |
| Uninvaded | Itea chinensis | Tree seedling | 21.7 | 0.0065 |
| Uninvaded | Styrax suberifolia | Tree seedling | 21.4 | 0.0039 |
| Uninvaded | Gardenia jasminoides | Shrub | 20.6 | 0.0166 |
| Uninvaded | Smilax hypoglauca | Climber shrub | 20 | 0.0037 |
| Low | Psychotria rubra | Tree seedling | 29.1 | 0.0184 |
| Low | Litsea glutinosa | Tree seedling | 28.6 | 0.0029 |
| Low | Ficus hirta | Shrub | 24.7 | 0.0348 |
| Medium | Aporosa dioica | Tree seedling | 28.8 | 0.0077 |
| High | Lygodium japonicum | Fern | 58.7 | 0.0001 |
| High | Miscanthus sinensis | graminoid | 58.3 | 0.0001 |
| High | Blechnum orientale | Fern | 42.2 | 0.0002 |
| High | Hedyotis hedyotidea | Climber shrub | 34.3 | 0.0001 |
| High | Microstegium vagans | Graminoid | 32.2 | 0.0002 |
| High | Lindsaea heterophyllum | Fern | 21.5 | 0.0019 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Su, Y.; Zhong, Y.; Xie, P.; Xu, M.; Su, Z. Community Attributes Predict the Relationship between Habitat Invasibility and Land Use Types in an Agricultural and Forest Landscape. Forests 2019, 10, 867. https://doi.org/10.3390/f10100867
Zhou Y, Su Y, Zhong Y, Xie P, Xu M, Su Z. Community Attributes Predict the Relationship between Habitat Invasibility and Land Use Types in an Agricultural and Forest Landscape. Forests. 2019; 10(10):867. https://doi.org/10.3390/f10100867
Chicago/Turabian StyleZhou, Yi, Yuqiao Su, Yonglin Zhong, Peiyun Xie, Mingfeng Xu, and Zhiyao Su. 2019. "Community Attributes Predict the Relationship between Habitat Invasibility and Land Use Types in an Agricultural and Forest Landscape" Forests 10, no. 10: 867. https://doi.org/10.3390/f10100867
APA StyleZhou, Y., Su, Y., Zhong, Y., Xie, P., Xu, M., & Su, Z. (2019). Community Attributes Predict the Relationship between Habitat Invasibility and Land Use Types in an Agricultural and Forest Landscape. Forests, 10(10), 867. https://doi.org/10.3390/f10100867





