Improving Mountain Pine Beetle Survival Predictions Using Multi-Year Temperatures Across the Western USA
Abstract
:1. Introduction
2. Methods
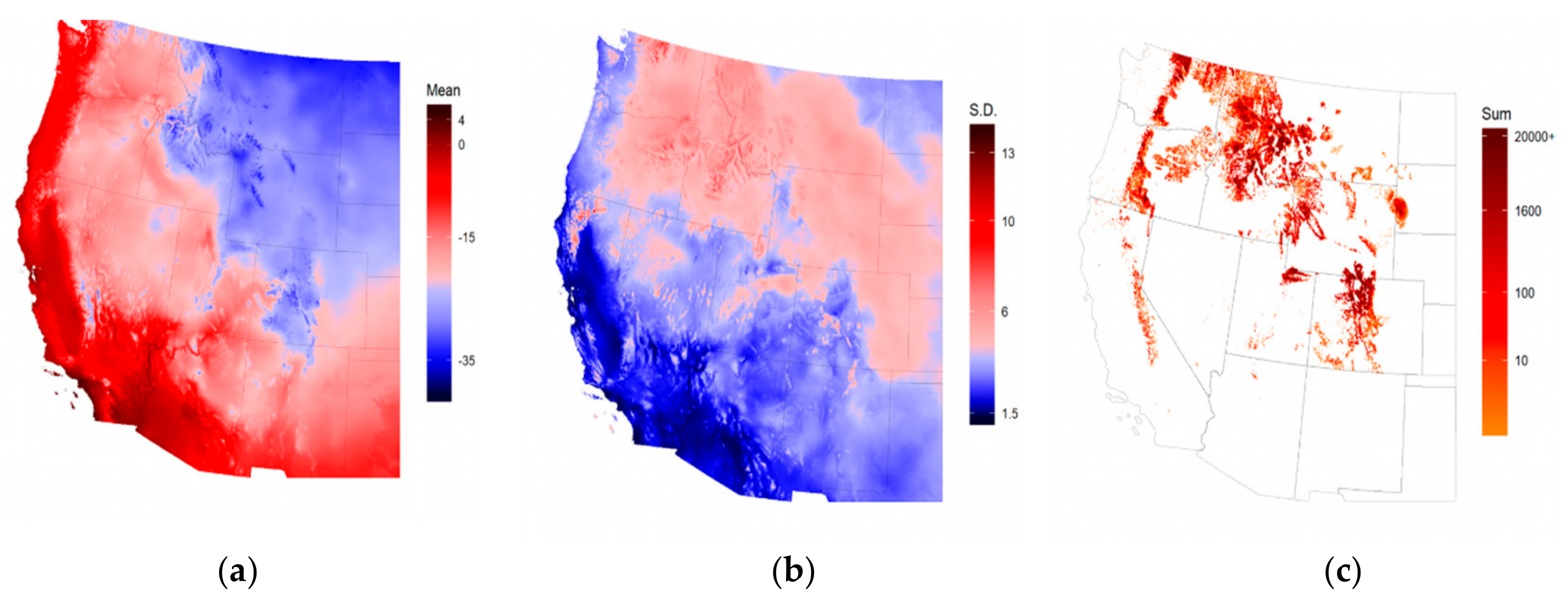
2.1. Study Area and Data
2.2. MPB Overwinter Survival Model
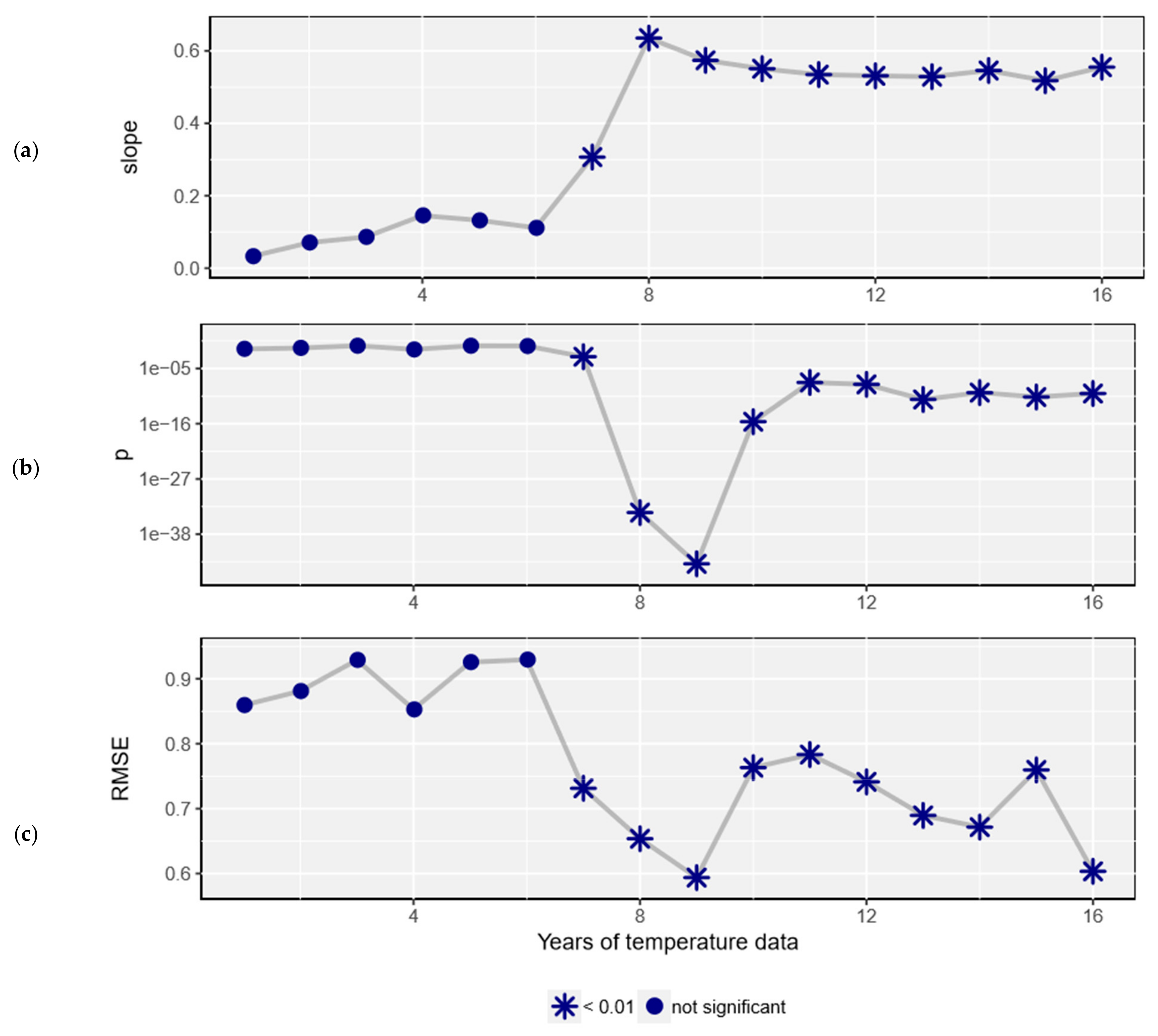
2.3. Analysis
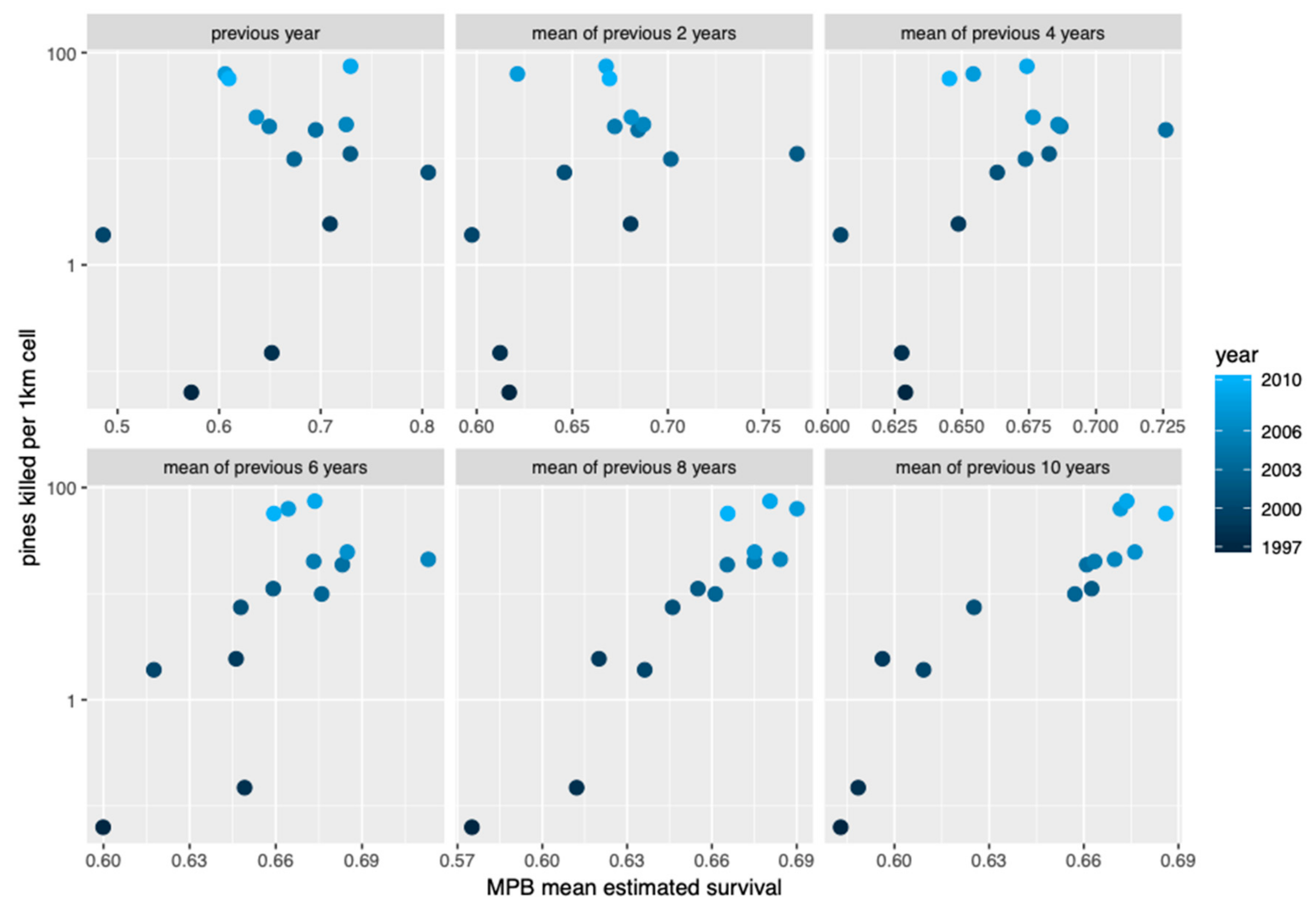
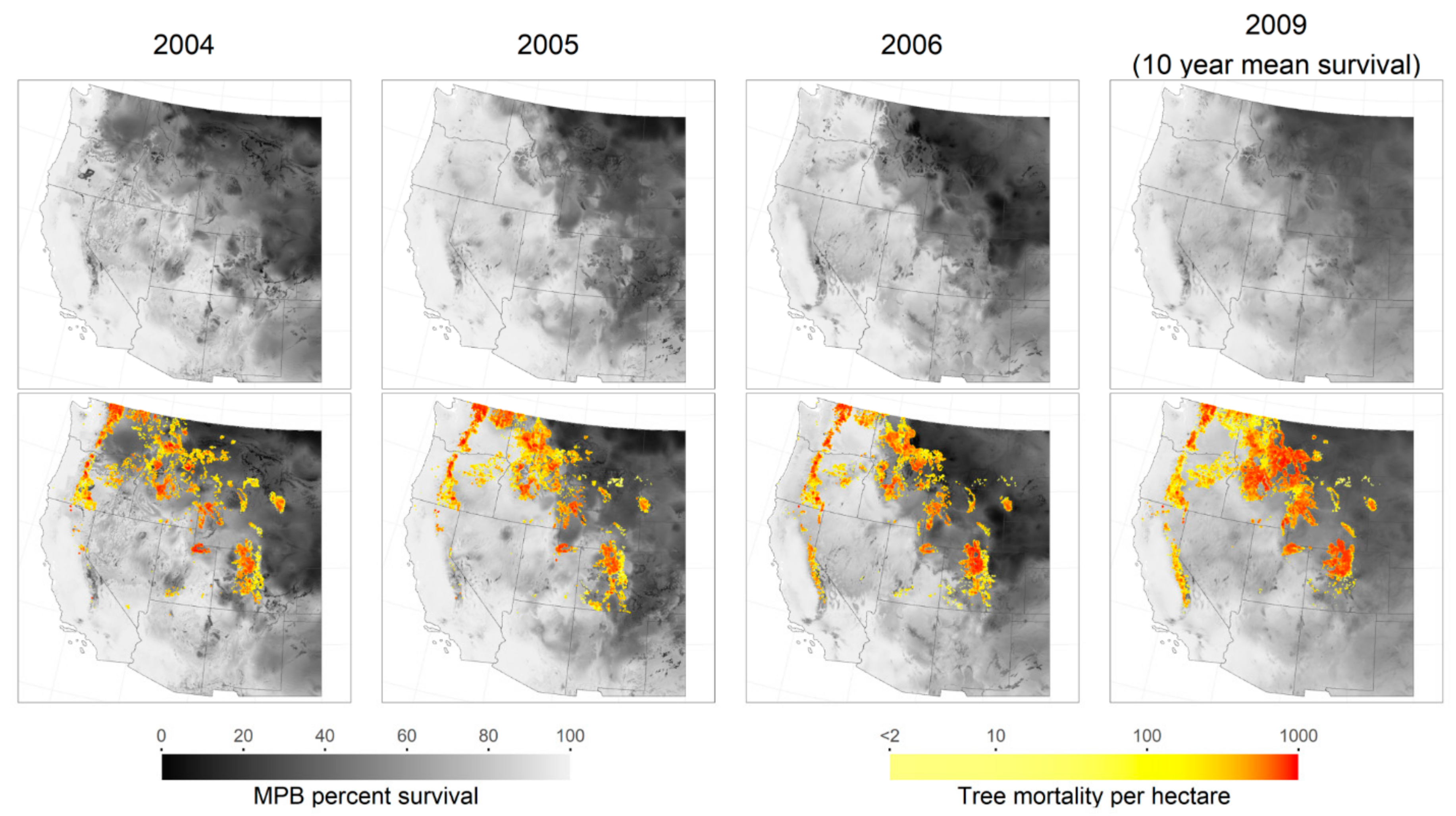
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bentz, B.J.; Régnière, J.; Fettig, C.J.; Hansen, E.M.; Hayes, J.L.; Hicke, J.A.; Kelsey, R.G.; Negrón, J.F.; Seybold, S.J. Climate change and bark beetles of the western United States and Canada: Direct and indirect effects. Bioscience 2010, 60, 602–613. [Google Scholar] [CrossRef]
- Safranyik, L.; Carroll, A.; Régnière, J.; Langor, D.; Riel, W.; Shore, T.; Peter, B.; Cooke, B.; Nealis, V.; Taylor, S. Potential for range expansion of mountain pine beetle into the boreal forest of North America. Can. Entomol. 2010, 142, 415–442. [Google Scholar] [CrossRef]
- MacDonald, G.M. Climate change and water in Southwestern North America special feature: Water, climate change, and sustainability in the southwest. Proc. Natl. Acad. Sci. USA 2010, 107, 21256–21262. [Google Scholar] [CrossRef] [PubMed]
- Weed, A.S.; Ayres, M.P.; Hicke, J.A. Consequences of climate change for biotic disturbances in North American forests. Ecol. Monogr. 2013, 83, 441–470. [Google Scholar] [CrossRef]
- Schlyter, P.; Stjernquist, I.; Bärring, L.; Jönsson, A.; Nilsson, C. Assessment of the impacts of climate change and weather extremes on boreal forests in northern Europe, focusing on Norway spruce. Clim. Res. 2006, 31, 75–84. [Google Scholar] [CrossRef]
- Bolte, A.; Ammer, C.; Löf, M.; Madsen, P.; Nabuurs, G.-J.; Schall, P.; Spathelf, P.; Rock, J. Adaptive forest management in central Europe: Climate change impacts, strategies and integrative concept. Scand. J. For. Res. 2009, 24, 473–482. [Google Scholar] [CrossRef]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Seidl, R.; Schelhaas, M.-J.; Rammer, W.; Verkerk, P.J. Increasing forest disturbances in Europe and their impact on carbon storage. Nat. Clim. Chang. 2014, 4, 806–810. [Google Scholar] [CrossRef] [Green Version]
- Morris, J.L.; Cottrell, S.; Fettig, C.J.; DeRose, R.J.; Mattor, K.M.; Carter, V.A.; Clear, J.; Clement, J.; Hansen, W.D.; Hicke, J.A.; et al. Bark beetles as agents of change in social-ecological systems. Front Ecol. Environ. 2018, 16, S34–S43. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Mooney, H.A.; Agard, J.; Capistrano, D.; Defries, R.S.; Díaz, S.; Dietz, T.; Duraiappah, A.K.; Oteng-Yeboah, A.; Pereira, H.M.; et al. Science for managing ecosystem services: Beyond the millennium ecosystem assessment. Proc. Natl. Acad. Sci. USA 2009, 106, 1305–1312. [Google Scholar] [CrossRef]
- Corbett, L.J.; Withey, P.; Lantz, V.A.; Ochuodho, T.O. The economic impact of the mountain pine beetle infestation in British Columbia: Provincial estimates from a CGE analysis. Forestry 2016, 89, 100–105. [Google Scholar] [CrossRef]
- Klutsch, J.G.; Negron, J.F.; Costello, S.L.; Rhoades, C.C.; West, D.R.; Popp, J.; Caissie, R. Stand characteristics and downed woody debris accumulations associated with a mountain pine beetle (Dendroctonus ponderosae Hopkins) outbreak in Colorado. For. Ecol. Manag. 2009, 258, 641–649. [Google Scholar] [CrossRef]
- Johnstone, J.F.; Allen, C.D.; Franklin, J.F.; Frelich, L.E.; Harvey, B.J.; Higuera, P.E.; Mack, M.C.; Meentemeyer, R.K.; Metz, M.R.; Perry, G.L.; et al. Changing disturbance regimes, ecological memory, and forest resilience. Front. Ecol. Environ. 2016, 14, 369–378. [Google Scholar] [CrossRef]
- Pelz, K.A.; Rhoades, C.C.; Hubbard, R.M.; Smith, F.W. Severity of overstory mortality influences conifer recruitment and growth in mountain pine beetle-affected forests. Forests 2018, 9, 536. [Google Scholar] [CrossRef]
- Logan, J.A.; Powell, J.A. Ghost forests, global warming, and the mountain pine beetle (Coleoptera: Scolytidae). Am. Entomol. 2001, 47, 160–173. [Google Scholar] [CrossRef]
- Buotte, P.C.; Hicke, J.A.; Preisler, H.K.; Abatzoglou, J.T.; Raffa, K.F.; Logan, J.A. Climate influences on whitebark pine mortality from mountain pine beetle in the Greater Yellowstone Ecosystem. Ecol. Appl. 2016, 26, 2507–2524. [Google Scholar] [CrossRef]
- Sambaraju, K.R.; Carroll, A.L.; Aukema, B.H. Multiyear weather anomalies associated with range shifts by the mountain pine beetle preceding large epidemics. For. Ecol. Manag. 2019, 438, 86–95. [Google Scholar] [CrossRef]
- Safranyik, L.; Carroll, A. The biology and epidemiology of the mountain pine beelte in lodgepole pine forests. In The Mountain Pine Beetle: A Synthesis of Biology, Management, and Impacts on Lodgepole Pine; Safranyik, L., Wilson, W., Eds.; Canadian Forest Service Publications: Victoria, BC, Canada, 2006; pp. 3–66. [Google Scholar]
- Stahl, K.; Moore, R.; McKendry, I. Climatology of winter cold spells in relation to mountain pine beetle mortality in British Columbia, Canada. Clim. Res. 2006, 32, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Preisler, H.K.; Hicke, J.A.; Ager, A.A.; Hayes, J.L. Climate and weather influences on spatial temporal patterns of mountain pine beetle populations in Washington and Oregon. Ecology 2012, 93, 2421–2434. [Google Scholar] [CrossRef]
- Bentz, B.J.; Mullins, D.E. Ecology of mountain pine beetle (Coleoptera: Scolytidae) cold hardening in the intermountain west. Environ. Entomol. 1999, 28, 577–587. [Google Scholar] [CrossRef]
- Carroll, A.L.; Raffa, K.F.; Aukema, B.H.; Bentz, B.J.; Hicke, J.A.; Turner, M.G.; Romme, W.H. Cross-scale drivers of natural disturbances prone to anthropogenic amplification: The dynamics of bark beetle eruptions. Bioscience 2008, 58, 501–517. [Google Scholar]
- Logan, J.; Amman, G. A distribution model for egg development in mountain pine beetle. Can. Entomol. 1986, 118, 361–372. [Google Scholar] [CrossRef]
- Bentz, B.J.; Logan, J.A.; Amman, G.D. Temperature-dependent development of the mountain pine beetle (Coleoptera: Scolytidae) and simulation of its phenology. Can. Entomol. 1991, 123, 1083–1094. [Google Scholar] [CrossRef]
- Bolstad, P.V.; Bentz, B.J.; Logan, J.A. Modelling micro-habitat temperature for dendroctonus ponderosae (Coleoptera: Scolytidae). Ecol. Model. 1997, 94, 287–297. [Google Scholar] [CrossRef]
- Logan, J.A.; Bentz, B.J. Model analysis of mountain pine beetle (Coleoptera: Scolytidae) seasonality. Environ. Entomol. 1999, 28, 924–934. [Google Scholar] [CrossRef]
- Powell, J.; Jenkins, J.L.; Logan, J.A.; Bentz, B.J. Seasonal temperature alone can synchronize life cycles. Bull. Math. Boil. 2000, 62, 977–998. [Google Scholar] [CrossRef]
- Perkins, D.L.; Roberts, D.W. Predictive models of whitebark pine mortality from mountain pine beetle. For. Ecol. Manag. 2003, 174, 495–510. [Google Scholar] [CrossRef]
- Aukema, B.H.; Carroll, A.L.; Zheng, Y.; Zhu, J.; Raffa, K.F.; Moore, R.D.; Stahl, K.; Taylor, S.W. Movement of outbreak populations of mountain pine beetle: Influences of spatiotemporal patterns and climate. Ecography 2008, 31, 348–358. [Google Scholar] [CrossRef]
- Mitchell, R.G.; Waring, R.H.; Pitman, G.B. Thinning lodgepole pine increases tree vigor and resistance to mountain pine beetle. For. Sci. 1983, 29, 204–211. [Google Scholar]
- Régnière, J.; Bentz, B. Modeling cold tolerance in the mountain pine beetle, Dendroctonus ponderosae. J. Insect Physiol. 2007, 53, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Thornton, P.E.; Thornton, M.; Mayer, B.W.; Wei, Y.; Devarakonda, R.; Vose, R.S.; Cook, R.B. Daymet: Daily surface weather data on a 1-km grid for North America, Version 2. In Proceedings of the American Geophysical Union Fall Meeting, San Francisco, CA, USA, 15–19 December 2014. [Google Scholar]
- Meddens, A.J.; Hicke, J.A. Spatial and temporal patterns of Landsat-based detection of tree mortality caused by a mountain pine beetle outbreak in Colorado, USA. For. Ecol. Manag. 2014, 322, 78–88. [Google Scholar] [CrossRef]
- USDA Forest Service Pest Portal. Available online: https://www.fs.fed.us/foresthealth/applied-sciences/mapping-reporting/data-app-development.shtml (accessed on 5 January 2019).
- Little, E. Atlas of United States Trees, Conifers and Important Hardwoods; US Department of Agriculture Miscellaneous Publication: Washington, DC, USA, 1971. [Google Scholar]
- USDA Forest Service Geodata Clearinghouse. Available online: https://data.fs.usda.gov/geodata/ (accessed on 5 January 2019).
- Creeden, E.P.; Hicke, J.A.; Buotte, P.C. Climate, weather, and recent mountain pine beetle outbreaks in the western United States. For. Ecol. Manag. 2014, 312, 239–251. [Google Scholar] [CrossRef]
- Waring, R.H.; Pitman, G.B. Modifying lodgepole pine stands to change susceptibility to mountain pine beetle attack. Ecology 1985, 66, 889–897. [Google Scholar] [CrossRef]
- Christiansen, E.; Waring, R.H.; Berryman, A.A. Resistance of conifers to bark beetle attack: Searching for general relationships. For. Ecol. Manag. 1987, 22, 89–106. [Google Scholar] [CrossRef]
- Romo, C.; Bader, M.-F.; Pawson, S. Inner log temperatures vary with log direction and forest cover: Implications for predicting the phenology of saproxylic insects. Agric. For. Meteorol. 2019, 275, 329–339. [Google Scholar] [CrossRef]

| Location | Model | Reference | Difference |
|---|---|---|---|
| Fairfield Ranger Station | 0.353 | 0.408 | 0.055 |
| Ketchum Ranger Station | 0.556 | 0.55 | −0.006 |
| Stanley | 0.184 | 0.205 | 0.021 |
| Banner Summit | 0.51 | 0.602 | 0.092 |
| Galena | 0.547 | 0.533 | −0.014 |
| Dollarhide Summit | 0.698 | 0.777 | 0.079 |
| Galena Summit | 0.613 | 0.652 | 0.039 |
| Vienna MINE | 0.61 | 0.623 | 0.013 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bone, C.; Nelson, M.F. Improving Mountain Pine Beetle Survival Predictions Using Multi-Year Temperatures Across the Western USA. Forests 2019, 10, 866. https://doi.org/10.3390/f10100866
Bone C, Nelson MF. Improving Mountain Pine Beetle Survival Predictions Using Multi-Year Temperatures Across the Western USA. Forests. 2019; 10(10):866. https://doi.org/10.3390/f10100866
Chicago/Turabian StyleBone, Christopher, and Michael France Nelson. 2019. "Improving Mountain Pine Beetle Survival Predictions Using Multi-Year Temperatures Across the Western USA" Forests 10, no. 10: 866. https://doi.org/10.3390/f10100866
APA StyleBone, C., & Nelson, M. F. (2019). Improving Mountain Pine Beetle Survival Predictions Using Multi-Year Temperatures Across the Western USA. Forests, 10(10), 866. https://doi.org/10.3390/f10100866




