Thermal Time and Cardinal Temperatures for Germination of Cedrela odorata L.
Abstract
1. Introduction
2. Materials and Methods
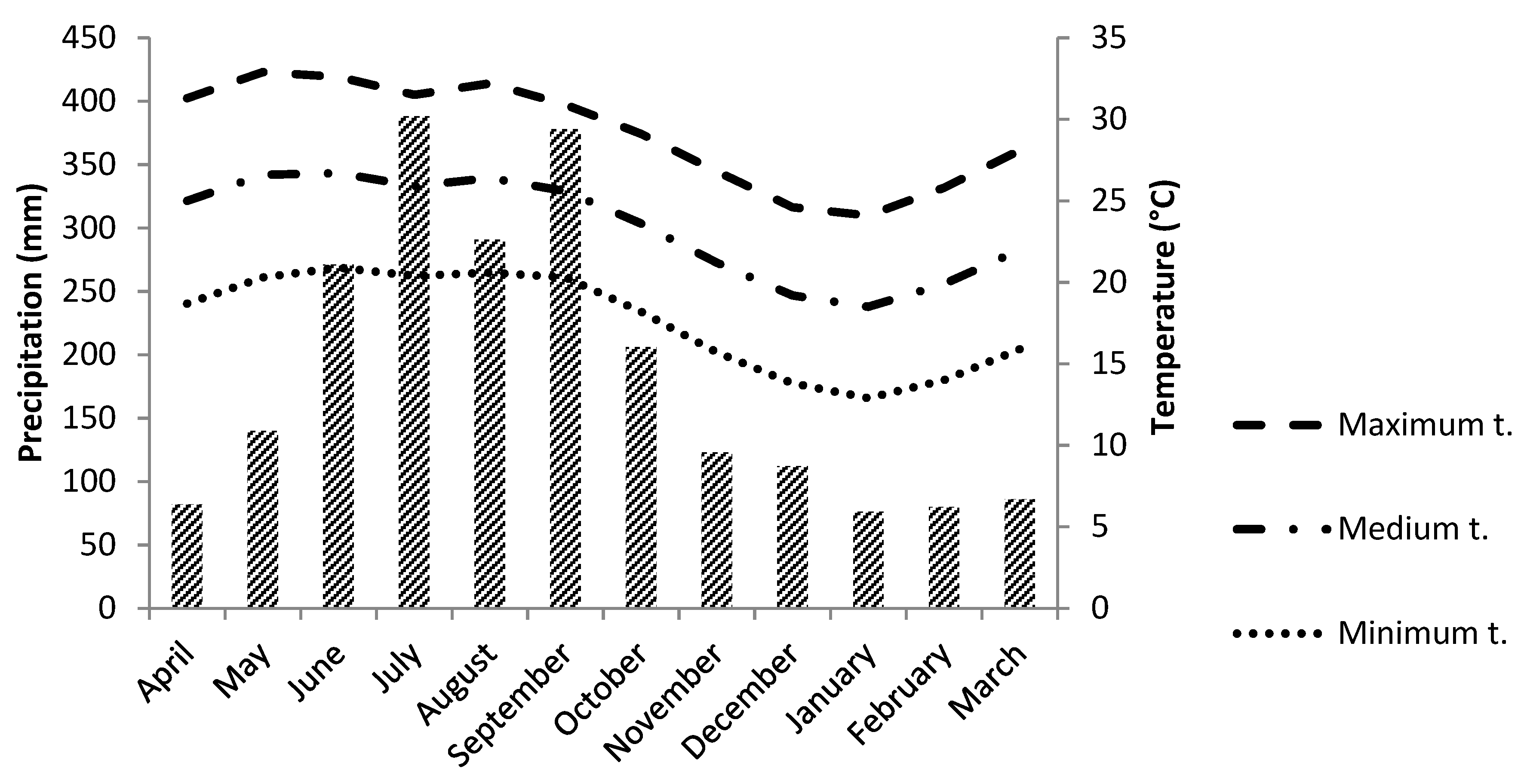
2.1. Seed Collection
2.2. Disinfection
2.3. Initial Germination Test
2.4. Effect of Temperature on Germination
2.5. Variables Evaluated
2.5.1. Total Germination
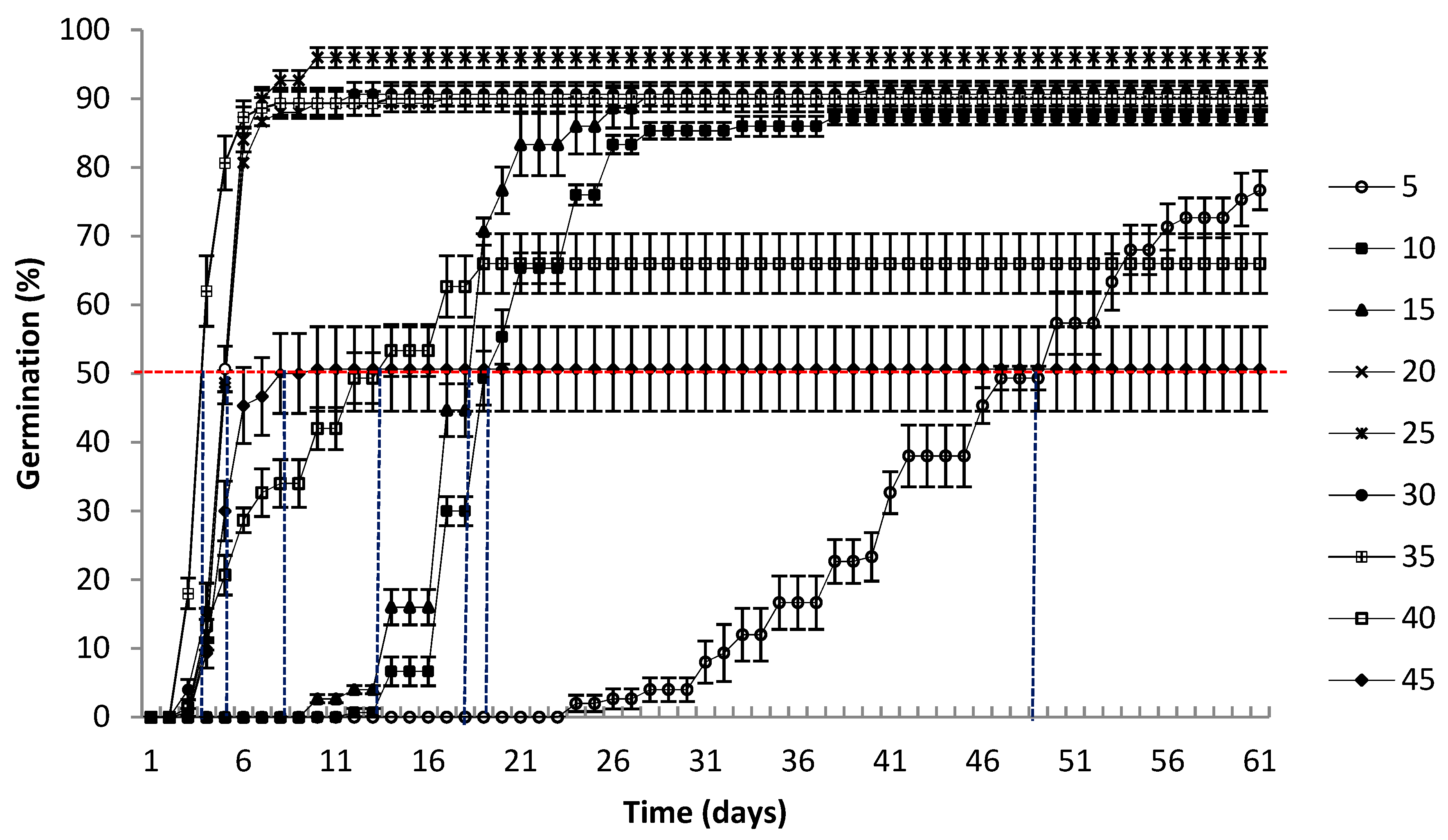
2.5.2. Median Germination Time (t50)
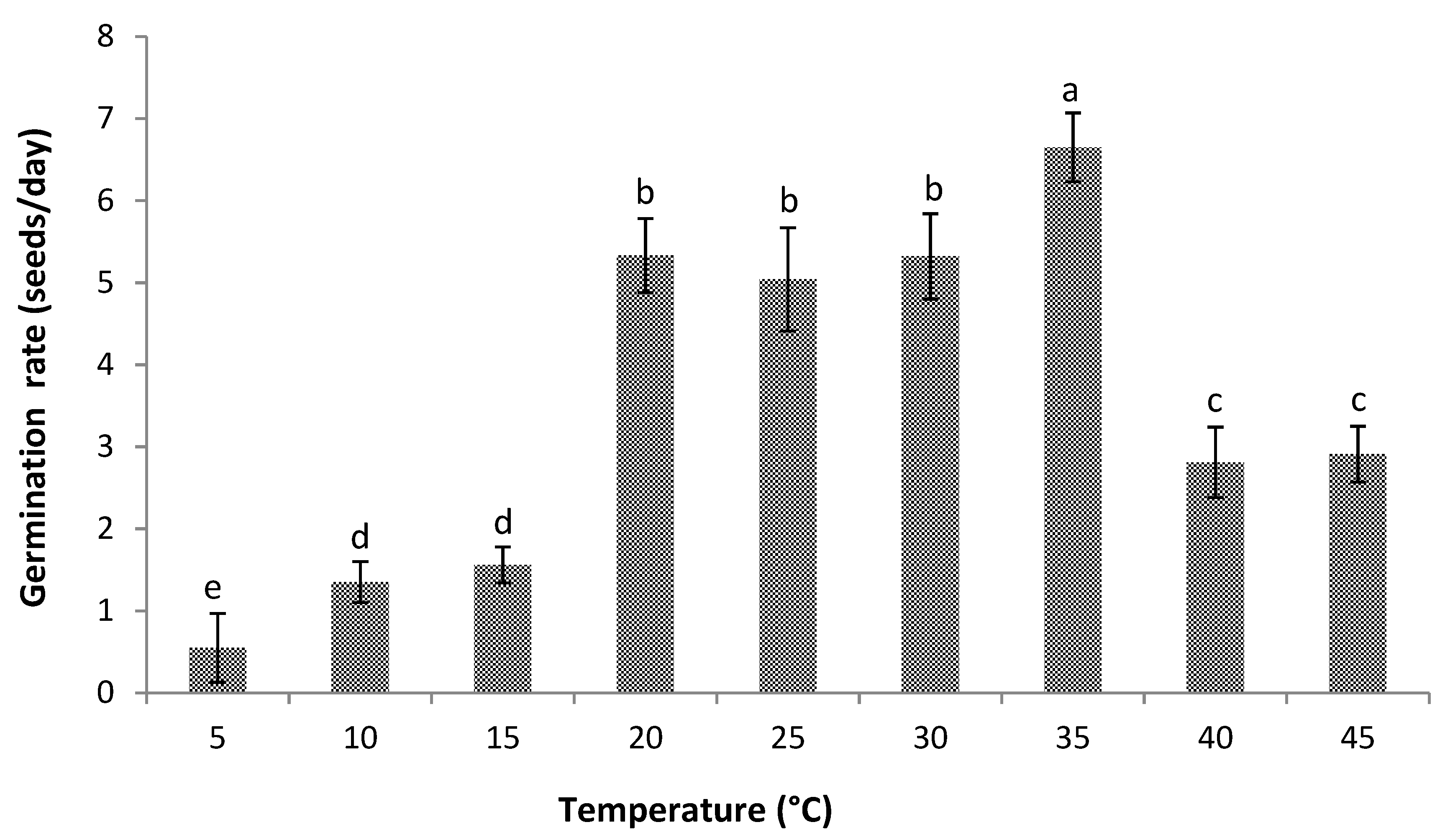
2.5.3. Germination Rate
2.5.4. Base Temperature (Tb)
2.5.5. Upper Threshold Temperature (Tc)
2.5.6. Optimal Temperature (To)
2.5.7. Thermal Time (θ, °C)
2.5.8. Climate Change Scenarios
2.6. Experimental Design and Analyses
3. Results
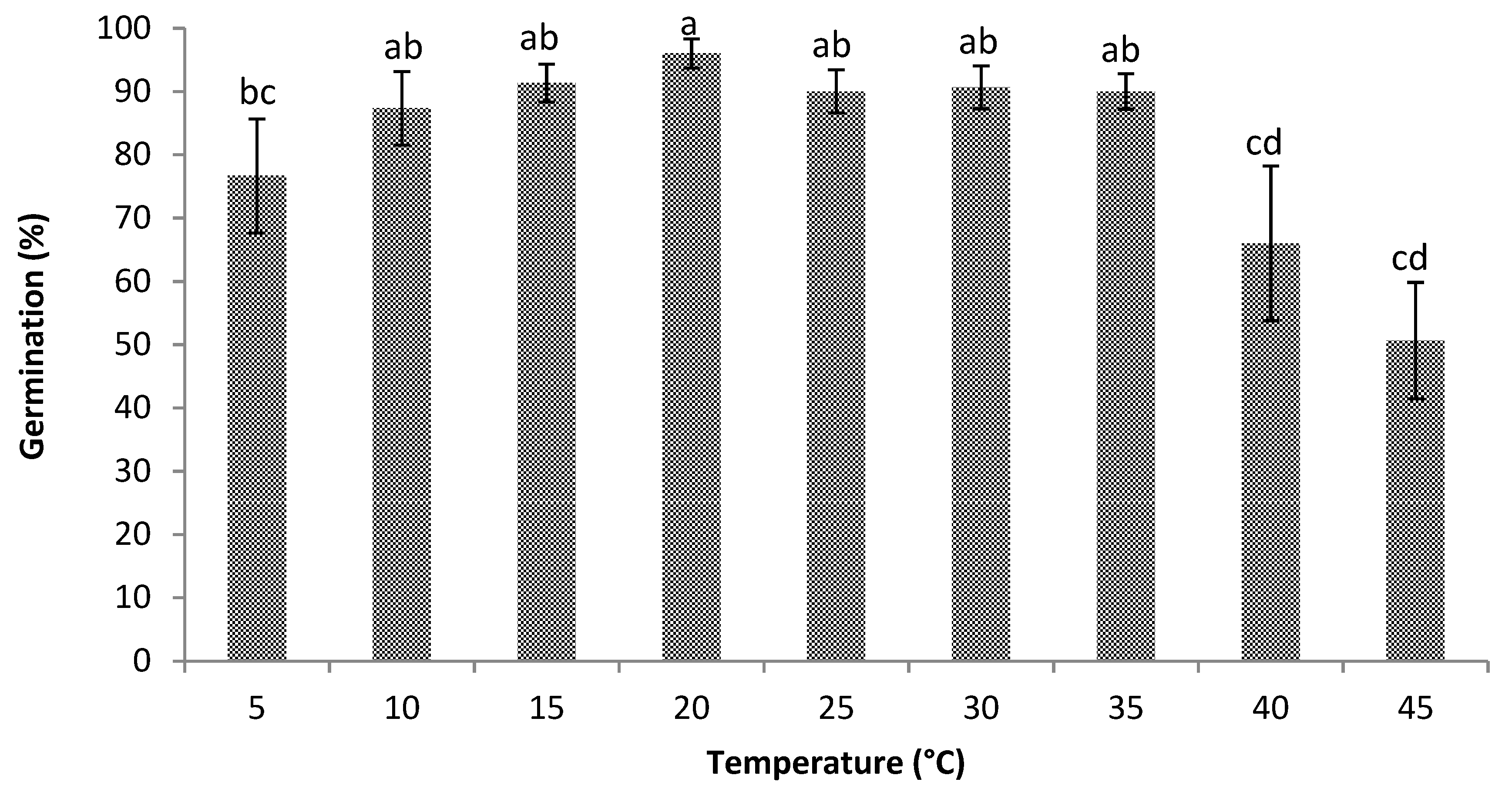
3.1. Germination
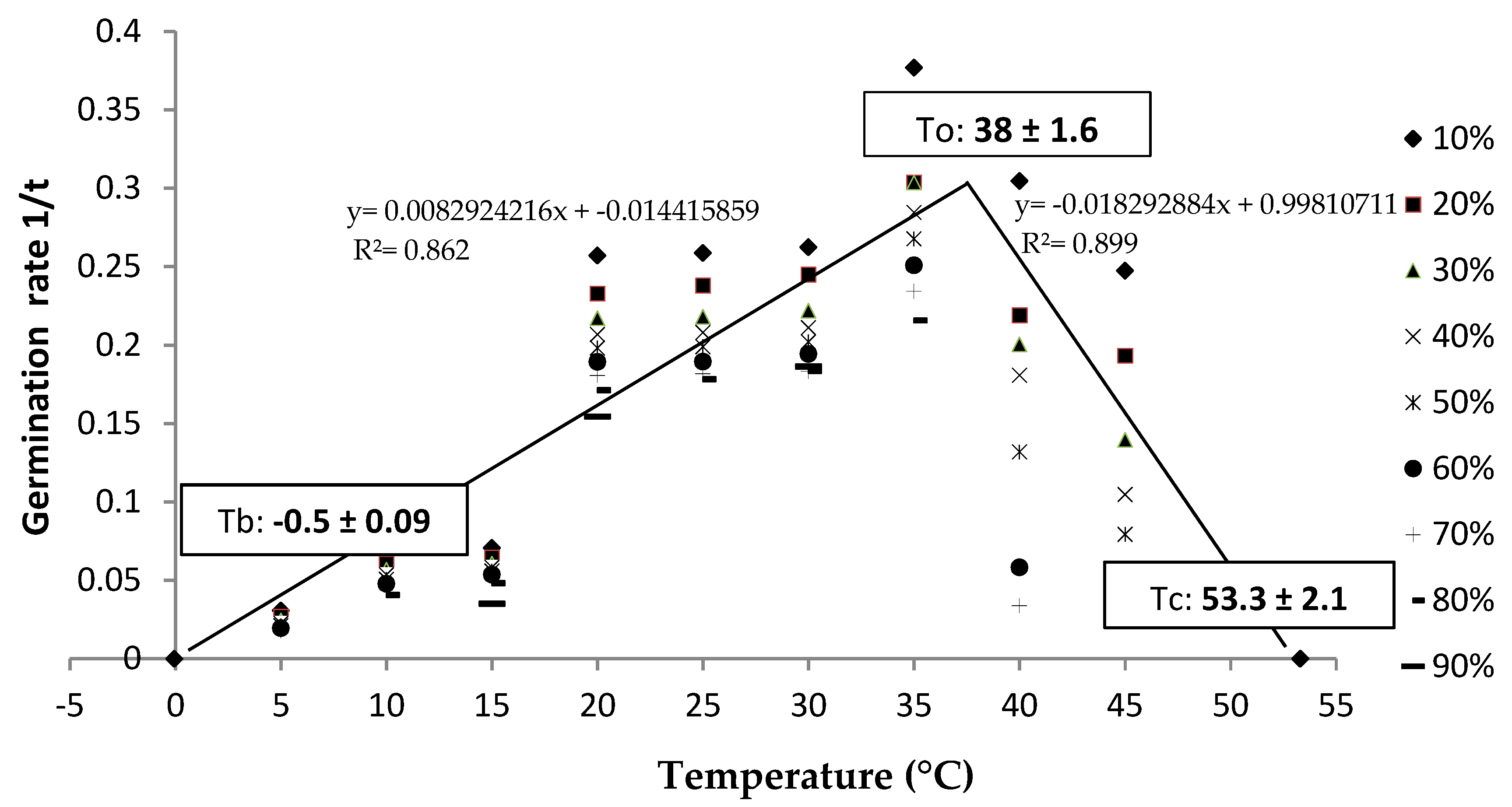
3.2. Cardinal Temperatures
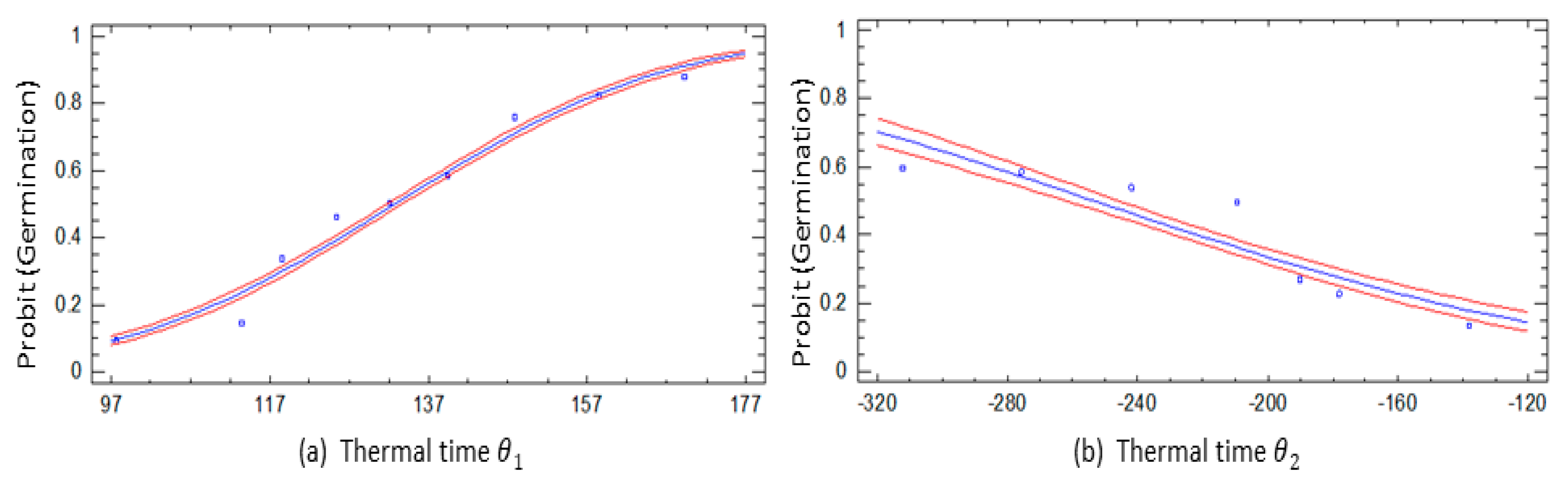
3.3. Thermal Time
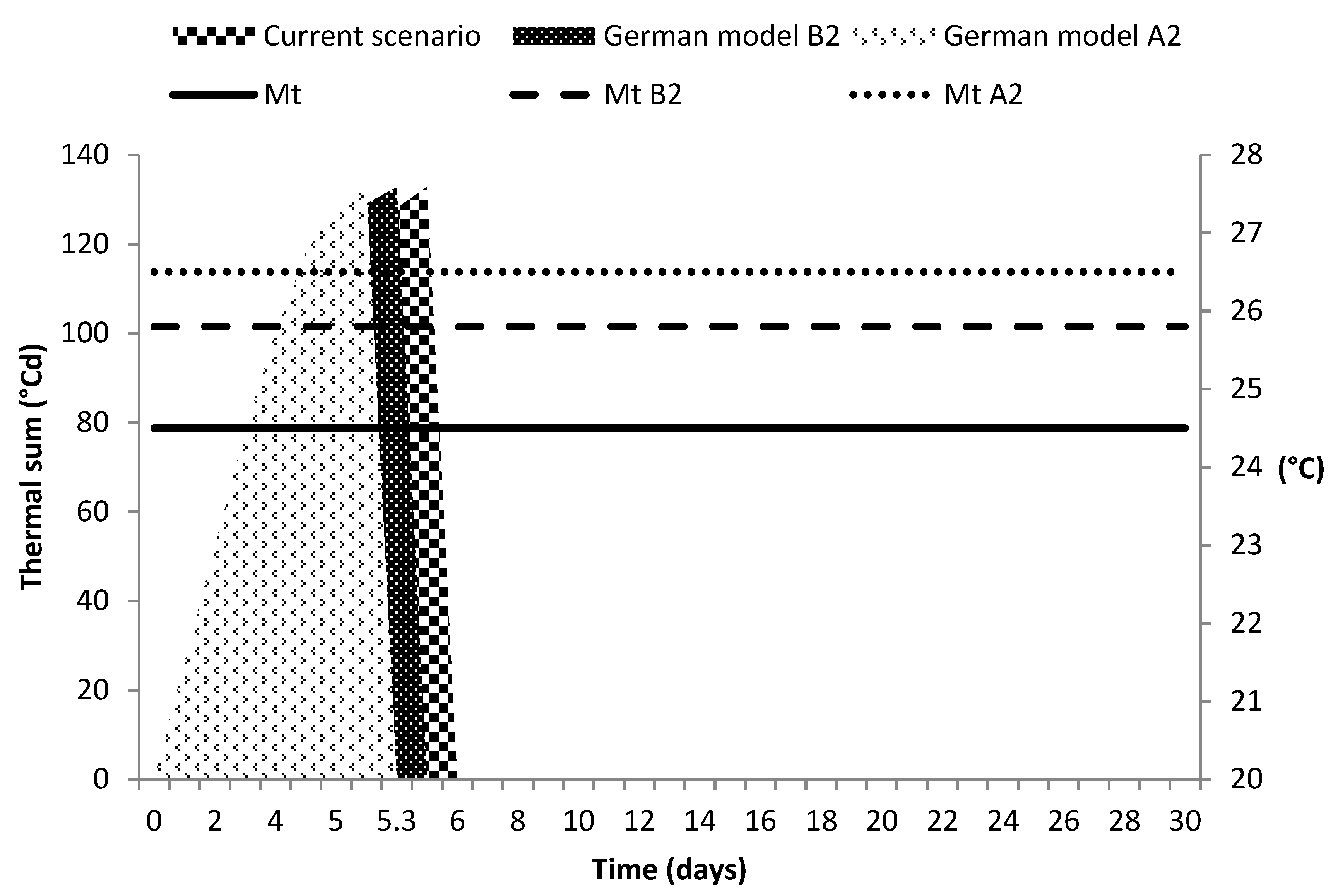
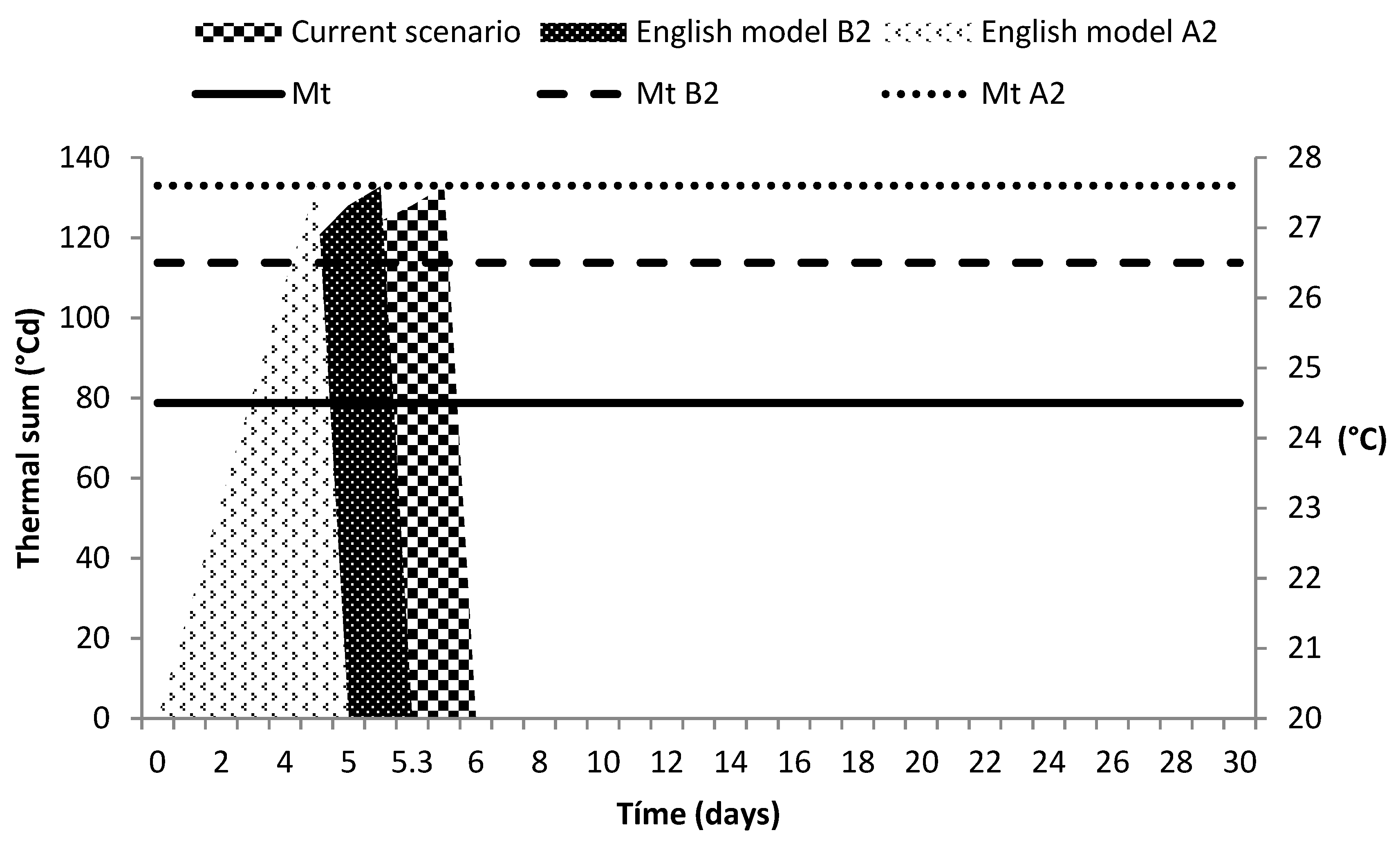
3.4. Climate Change Scenarios
4. Discussion
4.1. Germination
4.2. Cardinal Temperatures
4.3. Thermal Time
4.4. Climate Change Scenarios
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pennington, T.D.; Sarukhán, J. Tropical Trees of Mexico: Manual for the Identification of the Main Species, 3rd ed.; University Scientific Text. UNAM: Mexico City, Mexico, 2005; pp. 294–296. [Google Scholar]
- Mendizábal-Hernández, L.C.; Alba-Landa, J.; Márquez-Ramírez, J.; Cruz-Jiménez, H.; Ramírez-García, E.O. Carbon sequestration by Cedrela odorata L. in a genetic trial. Revista Mexicana de Ciencias Forestales 2011, 2, 105–111. [Google Scholar]
- Romo-Lozano, J.L.; Vargas-Hernández, J.J.; López-Upton, J.; Ávila-Angulo, M.L. Estimate of the financial value of timber stocks of red cedar (Cedrela odorata L.) in Mexico. Madera y Bosques 2017, 23, 111–120. [Google Scholar] [CrossRef][Green Version]
- Navarro, C.; Montagnini, F.; Hernández, G. Genetic variability of Cedrela odorata Linnaeus: Results of early performance of provenances and families from Mesoamerica grown in association with coffee. For. Ecol. Manag. 2004, 192, 217–227. [Google Scholar] [CrossRef]
- Ramírez-Garcí, C.; Vera-Castillo, G.; Carrillo-Anzures, F.; Magaña-Torres, O.S. Red cedar (Cedrela odorata L.) as reconversion alternative of agricultural lands in the south of Tamaulipas. Agricultura Técnica en México 2008, 34, 243–256. [Google Scholar]
- Pérez-Salicrup, D.R.; Esquivel, R. Tree infection by Hypsipyla grandella in Swietenia macrophylla and Cedrela odorata (Meliaceae) in Mexico´s southern Yucatan Peninsula. For. Ecol. Manag. 2008, 225, 324–327. [Google Scholar] [CrossRef]
- Sampayo-Maldonado, S.; Jiménez-Casas, M.; López-Upton, J.; Sánchez-Monsalvo, V.; Jasso-Mata, J.; Equihua-Martínez, A.; Castillo-Martínez, C.R. Cedrela odorata L. mini-cutting rooting. Agrociencias 2016, 50, 919–929. [Google Scholar]
- Comisión Nacional Forestal (CONAFOR). Main Timber Species Established in Commercial Forest Plantations, 2000–2014. Available online: http://www.conafor.gob.mx:8080/documentos/ver.aspx?grupo=43&articulo=6022 (accessed on 15 July 2018).
- Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES). In Proceedings of the Fourteenth meeting of the Conference of the Parties, The Hague, The Netherlands, 3–15 June 2007; p. 26.
- Secretaria del Medio Ambiente y Recursos Naturales (SEMARNAT). Norma Oficial Mexicana NOM-059-SEMARNAT-2010, Protección Ambiental-Especies Nativas de México de flora y Fauna Silvestres-Categorías de Riesgo y Especificaciones para su Inclusión, Exclusión o Cambio-Lista de Especies en Riesgo. Available online: https://www.gob.mx/cms/uploads/attachment/file/134778/35._NORMA_OFICIAL_MEXICANA_NOM-059-SEMARNAT-2010.pdf (accessed on 25 July 2018).
- Gómez-Díaz, J.D.; Monterroso-Rivas, A.I.; Tinoco-Rueda, J.A. Distribution of red cedar (Cedrela odorata L.) in the Hidalgo state, under current conditions and scenarios of climate change. Madera y Bosques 2007, 13, 29–49. [Google Scholar] [CrossRef]
- Hartmann, H.T.; Kester, D.E.; Davies, F.T.; Geneve, R.L. Plant Propagation: Principles and Practices, 8th ed.; Prentice-Hall: Upper Saddle River, NJ, USA, 2013; 913p. [Google Scholar]
- González, J.E. Collection and germination of seeds from 26 species of tree in a tropical moist forest. J. Biol. Trop. 1991, 39, 47–51. [Google Scholar] [CrossRef]
- Dewan, S.; Vander-Mijnsbrugge, K.; De Frene, P.; Steenackers, M.; Michiels, B.; Verheyen, K. Maternal temperature during seed maturation affects seed germination and timing of bud set in seedlings of European black poplar. For. Ecol. Manag. 2008, 410, 126–135. [Google Scholar] [CrossRef]
- Quinto, L.; Martínez-Hernández, P.A.; Pimentel-Bribiesca, L.; Rodríguez-Trejo, D.A. Alternatives to improve seed germination in three tropical trees. Revista Chapingo Serie Ciencias Forestales y del Ambiente 2009, 15, 23–28. [Google Scholar]
- Garcia-Huidobro, J.; Monteith, J.L.; Squire, G.R. Time, temperature and germination of pearl millet (Pennisetum typhoides S.&H.). I. Constant temperature. J. Exp. Bot. 1982, 33, 288–296. [Google Scholar] [CrossRef]
- Ellis, R.H.; Covell, S.; Roberts, E.H.; Summerfield, R.J. The influence of temperature on seed germination rate in grain legumes. II. Intraspecific variation in chickpea (Cicer arietinum L.) at constant temperatures. J. Exp. Bot. 1986, 37, 1503–1515. [Google Scholar] [CrossRef]
- Bradford, K.J. Applications of hydrothermal time to quantifying and modeling seed germination and dormancy. Weed Sci. 2002, 50, 248–260. [Google Scholar] [CrossRef]
- Nakao, E.A.; Cardoso, V.J.M. Analysis of thermal dependence on the germination of braquiarão seeds using the thermal time model. Braz. J. Biol. 2016, 79, 162–168. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Calzada-López, S.G.; Kohashi-Shibata, J.; Uscanga-Mortera, E.; García-Esteva, A.; Yáñez-Jiménez, P. Cardinal temperatures and germination rate in husk tomato cultivars. Revista Mexicana de Ciencias Agrícolas 2014, 8, 1451–1458. [Google Scholar]
- Ruíz-Corral, J.A.; Flores-López, H.E.; Ramírez-Díaz, J.L.; González-Eguiarte, D.R. Cardinal temperatures and length of maturation cycle of maize hybrid H-311 under rainfed conditions. Agrociencia 2002, 36, 569–577. [Google Scholar]
- Muellner, A.N.; Pennington, T.D.; Chase, M.W. Molecular phylogenetics of Neotropical Cedrela mahogany (family, Meliaceae) based on nuclear and plastid DNA sequences reveal multiple origins of “Cedrela odorat”. Mol. Phylogenet. Evol. 2009, 52, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.H.; Wei, X.; Zeng, X.L.; Ye, W.H.; Yin, X.J.; Zhang-Ming, W.; Jiang, Y.S. Seed biology and germination ecophysiology of Camellia nitidissima. For. Ecol. Manag. 2008, 255, 113–118. [Google Scholar] [CrossRef]
- Sánchez-Monsalvo, V.; Salazar-García, J.G.; Vargas-Hernández, J.J.; López-Upton, J.; Jasso-Mata, J. Genetic parameters and response to selection for growth traits in Cedrela odorata L. Fitotecnia Mexicana 2003, 26, 19–27. [Google Scholar]
- Castellanos-Acuña, D.; Vance-Borland, K.W.; St. Clair, J.B.; Hamann, A.; López-Upton, J.; Gómez-Pimeda, E.; Ortega-Rodríguez, J.M.; Sáenz-Romero, C. Climate-based seed zones for Mexico: Guiding reforestation under observed and projected climate change. New For. 2008, 49, 297–309. [Google Scholar] [CrossRef]
- Espitia-Camacho, M.; Araméndiz-Tatis, H.; Cardona-Ayala, C. Viability, morphometric, and anatomical characteristics of Cedrela odorata L. and Cariniana pyriformis Miers seeds. Agron. Mesoam. 2017, 28, 605–617. [Google Scholar] [CrossRef]
- García, E. Modifications to the Climate Classification System of Köppen, 5th ed.; series #6; Instituto de Geografía, Universidad Nacional Autónoma de México: Mexico City, Mexico, 2004; pp. 19–49. [Google Scholar]
- Villarreal-Manzano, L.A.; Herrera-Cabrera, B.E. Water requirement in the vanilla (Vanilla planifolia Jacks. ex Andrews)—Naranjo (Citrus sinensis L.) production system in the Totonacapan region, Veracruz, Mexico. Agroproductividad 2018, 11, 29–36. [Google Scholar]
- Sampayo-Maldonado, S.; Castillo-Martínez, C.R.; Jiménez-Casas, M.; Sánchez-Monsalvo, V.; Jasso-Mata, J.; López-Upton, J. In vitro germination of Cedrela odorata L. seed from extinct genotypes. Agroproductividad 2017, 10, 53–58. [Google Scholar]
- International Seed Testing Association (ISTA). International Rules for Seed Testing; International Seed Testing Association: Zurich, Switzerland, 2005; 243p. [Google Scholar]
- Parmoon, G.; Moosavi, S.A.; Akbari, H.; Ebadi, A. Quantifying cardinal temperatures and thermal time required for germination of Silybum marianum seed. Crop J. 2015, 3, 145–151. [Google Scholar] [CrossRef][Green Version]
- Ordoñez-Salanueva, C.A.; Seal, C.E.; Pritchard, H.W.; Orozco-Segovia, A.; Canales-Martínez, M.; Flores-Ortíz, C.M. Cardinal temperatures and thermal time in Polaskia Beckeb (Cactaceae) species: Effect of projected soil temperature increase and nurse interaction on germination timing. J. Arid Environ. 2015, 115, 73–80. [Google Scholar] [CrossRef]
- Hardegree, S.P. Predicting germination response to temperature. I. Cardinal temperature models and subpopulation-specific regression. Ann. Bot. 2006, 97, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Covell, S.; Ellis, R.H.; Roberts, E.H.; Summerfield, R.J. The influence of temperature on seed germination rate in grain legumes. 1: A comparison of chickpea, lentil, soybean and cowpea at constant temperatures. J. Exp. Bot. 1986, 37, 705–715. [Google Scholar] [CrossRef]
- Gutiérrez, E.; Trejo, I. Effect of climatic change on the potential distribution of five species of temperate forest trees in Mexico. Revista Mexicana de Biodiversidad 2014, 85, 179–188. [Google Scholar] [CrossRef]
- Fernández-Eguiarte, A.; Zavala-Hidalgo, J.; Romero-Centeno, R.; Digital Climatic Atlas of the Mexico. Center of Sciences of the Atmosphere, Universidad Nacional Autónoma de México, México. 2010. Available online: http://uniatmos.atmosfera.unam.mx/ACDM/servmapas (accessed on 13 September 2018).
- Flores-Magdaleno, H.; Flores-Gallardo, H.; Ojeda-Bustamante, W. Phenological prediction of potato crop by means of thermal time. Rev. Fitotec. Mex. 2014, 37, 149–157. [Google Scholar]
- Orrù, M.; Mattana, E.; Pritchard, H.W.; Bacchetta, G. Thermal thresholds as predictors of seed dormancy release and germination timing: Altitude-related risks from climate warming for the wild grapevine Vitis vinifera subsp. Sylvestris. Ann. Bot. 2012, 110, 1651–1660. [Google Scholar] [CrossRef] [PubMed]
- Itoh, A.; Yamakura, T.; Kanzaki, M.; Ohkubo, T.; Palmiotto, P.A.; LaFrankie, J.V.; Kendawang, J.J.; Lee, H.S. Rooting ability of cuttings relates to phylogeny, habitat preference and growth characteristics of tropical rainforest trees. For. Ecol. Manag. 2002, 168, 275–287. [Google Scholar] [CrossRef]
- Syros, T.; Yupsanis, T.; Zafiriadis, H.; Economou, A. Activity and isoforms of peroxidases, lignin and anatomy, during adventitious rooting in cuttings of Ebenus cretica L. J. Plant Physiol. 2004, 161, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Gutiérrez, L.; Vargas-Hernández, J.J.; López-Upton, J.; Soto-Hernández, M. Effect of cutting age and substrate temperature on rooting of Taxus globosa. New For. 2009, 38, 187–196. [Google Scholar] [CrossRef]
- Statistical Analysis System (SAS). Institute SAS/STAT 9.1 Guide for Personal Computers; SAS Institute Inc.: Cary, NC, USA, 2004; 378p. [Google Scholar]
- González-Rivas, B.; Tigabu, M.; Castro-Marín, G.; Odén, P.C. Seed germination and seedling stablishment of Neotropical dry forest species in response to temperature and light conditions. J. For. Res. 2009, 20, 99–104. [Google Scholar] [CrossRef]
- Buttler, T.J.; Celen, A.E.; Webb, S.L.; Krstic, D.; Interrante, S.M. Temperature affects the germination of forage legume seeds. Crop Sciense 2014, 54, 2846–2853. [Google Scholar] [CrossRef]
- Manjul, N.M.J.; Metali, F. Germination and growth of selected tropical pioneers in Brunei Darussalam: Effects of temperature and seed size. Res. J. Seed Sci. 2016, 9, 48–53. [Google Scholar] [CrossRef]
- Rajjou, L.; Duval, M.; Gallardo, K.; Catusse, J.; Bally, J.; Job, C.; Job, D. Seed germination and vigour. Annu. Rev. Plant Biol. 2012, 63, 507–533. [Google Scholar] [CrossRef]
- Duran-Puga, N.; Ruíz-Corral, J.A.; González-Eguiarte, D.R.; Núñez-Hernández, G.; Padilla-Ramírez, F.J.; Contreras-Rodríguez, S.H. Devolopment cardinal temperatures of the planting-emergence stage for 11 forage grasses. Rev. Mex. Cienc. Pecu. 2011, 2, 347–357. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Seed dormancy in trees of climax tropical vegetation types. Trop. Ecol. 2005, 46, 17–28. [Google Scholar]
- Adam, N.R.; Dierig, D.A.; Coffelt, T.A.; Wintermeyer, M.J.; Mackey, B.E.; Wall, G.W. Cardinal temperatures for germination and early growth of two Lesquerella species. Ind. Crops Prod. 2007, 25, 24–33. [Google Scholar] [CrossRef]
- Caroca, R.; Zapata, N.; Vargas, M. Temperature effect on the germination of four peanut genotypes (Arachis hypogaea L.). Chil. J. Agric. Anim. Sci. 2016, 32, 94–101. [Google Scholar] [CrossRef]
- Lindig-Cisneros, R. Ecology of Restoration and Environmental Restoration; Escuela Nacional de Estudios Superiores, Unidad Morelia. Instituto de Investigaciones en Ecosistemas y Sustentabilidad, Universidad Nacional Autónoma de México: Mexico, 2017; pp. 59–60. [Google Scholar]
- Orozco-Segovia, A.; González-Zertuche, L.; Mendoza, A.; Orozco, S. A mathematical model that uses gaussian distribution to analyze the germination of Manfreda brachystachya (Agavaceae) in a thermogradient. Physiol. Plant. 1996, 98, 431–438. [Google Scholar] [CrossRef]
- Grey, T.L.; Beasley, J.P.; Webster, T.M.; Chen, C.Y. Peanut seed vigor evaluation using a thermal gradient. Int. J. Agron. 2011, 7. [Google Scholar] [CrossRef]
- Sánchez-Rendón, J.A.; Suárez-Rodríguez, A.G.; Montejo-Valdés, L.; Muñoz-Garcia, C. Climate change and the seeds from the Cuban native plants. Acta Botánica Cubana 2011, 214, 38–50. [Google Scholar]
- Cóbar-Carranza, A.J.; García, R.A.; Pauchard, A.; Peña, E. Effects of high temperatures in germination and seed survival of the invasive species Pinus contorta and two native species of South Chile. Bosque 2015, 36, 53–60. [Google Scholar] [CrossRef]
- Parra-Coronado, A.; Fischer, G.; Chaves-Cordoba, B. Thermal time for reproductive phenological stages of pineapple guava (Acca sellowiana (O. Berg) Burret). Acta Biol. Colomb. 2015, 20, 163–173. [Google Scholar] [CrossRef]
- Andreucci, M.P.; Moot, D.J.; Blakc, A.D.; Sedcole, R. A comparison of cardinal temperatures estimated by linear and nonlinear models for germination and bulb growth of forage brassicas. Eur. J. Agron. 2016, 81, 52–63. [Google Scholar] [CrossRef]
- Normand, F.; Léchaudel, M. Toward a better interpretation and use of thermal time model. Acta Hortic. 2006, 707, 159–164. [Google Scholar] [CrossRef]
- Funes, G.; Díaz, S.; Venier, P. Temperature as a main factor determining germination in Argentinean dry Chaco species. Ecol. Austral 2009, 19, 129–138. [Google Scholar]
- Asseng, S.; Foster, I.; Turner, N.C. The impact of temperature variability on wheat yields. Glob. Chang. Biol. 2011, 17, 997–1012. [Google Scholar] [CrossRef]
- Colauto-Stenzel, N.M.; Janeiro-Neves, C.S.V.; Jamil-Marur, C.; DosSantos-Scholz, M.B.; Gomes, J.C. Maturation curves and degree-day accumulation for fruits of “Folha Murcha” orange trees. Sci. Agric. 2006, 63, 219–225. [Google Scholar] [CrossRef]
- Intergovernmental Panel of Climate Change (IPCC). Climate Change 2013: The Physical Science Basis; Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel of Climate Change; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; pp. 22–127. [Google Scholar]
- Hernández-Ramos, J.; Reynoso-Santos, R.; Hernández-Ramos, A.; García-Cuevas, X.; Hernández-Máximo, E.; Cob-Uicab, J.V.; Sumano-López, D. Historical, current and future distribution of Cedrela odorata in Mexico. Acta Botánica Mexicana 2018, 124, 117–134. [Google Scholar] [CrossRef]

| Parameters | Sub-Optimal | Supra-Optimal |
|---|---|---|
| R2 | 96.33 | 82.99 |
| K | −4.91 ± 0.53 | −2.02 ± 0.28 |
| 0.125 ± 0.019 | 0.122 ± 0.012 | |
| log | 2.12 ± 0.008 | 2.40 ± 0.001 |
| 132.74 ± 2.60 | 253.31 ± 4.73 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sampayo-Maldonado, S.; Ordoñez-Salanueva, C.A.; Mattana, E.; Ulian, T.; Way, M.; Castillo-Lorenzo, E.; Dávila-Aranda, P.D.; Lira-Saade, R.; Téllez-Valdéz, O.; Rodriguez-Arevalo, N.I.; et al. Thermal Time and Cardinal Temperatures for Germination of Cedrela odorata L. Forests 2019, 10, 841. https://doi.org/10.3390/f10100841
Sampayo-Maldonado S, Ordoñez-Salanueva CA, Mattana E, Ulian T, Way M, Castillo-Lorenzo E, Dávila-Aranda PD, Lira-Saade R, Téllez-Valdéz O, Rodriguez-Arevalo NI, et al. Thermal Time and Cardinal Temperatures for Germination of Cedrela odorata L. Forests. 2019; 10(10):841. https://doi.org/10.3390/f10100841
Chicago/Turabian StyleSampayo-Maldonado, Salvador, Cesar A. Ordoñez-Salanueva, Efisio Mattana, Tiziana Ulian, Michael Way, Elena Castillo-Lorenzo, Patricia D. Dávila-Aranda, Rafael Lira-Saade, Oswaldo Téllez-Valdéz, Norma I. Rodriguez-Arevalo, and et al. 2019. "Thermal Time and Cardinal Temperatures for Germination of Cedrela odorata L." Forests 10, no. 10: 841. https://doi.org/10.3390/f10100841
APA StyleSampayo-Maldonado, S., Ordoñez-Salanueva, C. A., Mattana, E., Ulian, T., Way, M., Castillo-Lorenzo, E., Dávila-Aranda, P. D., Lira-Saade, R., Téllez-Valdéz, O., Rodriguez-Arevalo, N. I., & Flores-Ortíz, C. M. (2019). Thermal Time and Cardinal Temperatures for Germination of Cedrela odorata L. Forests, 10(10), 841. https://doi.org/10.3390/f10100841






