Adaptation Capacity of Norway Spruce Provenances in Western Latvia
Abstract
1. Introduction
2. Materials and Methods
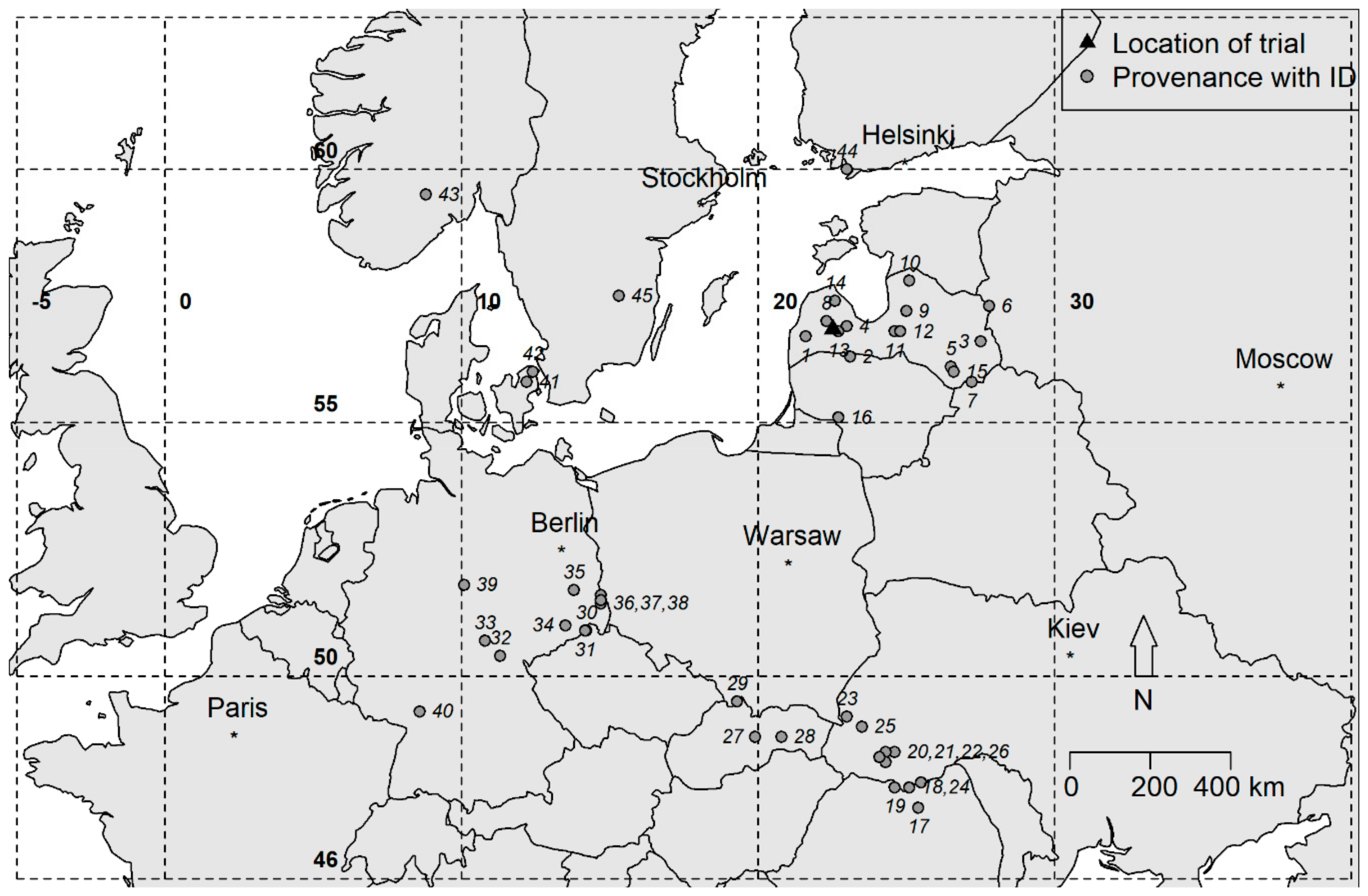
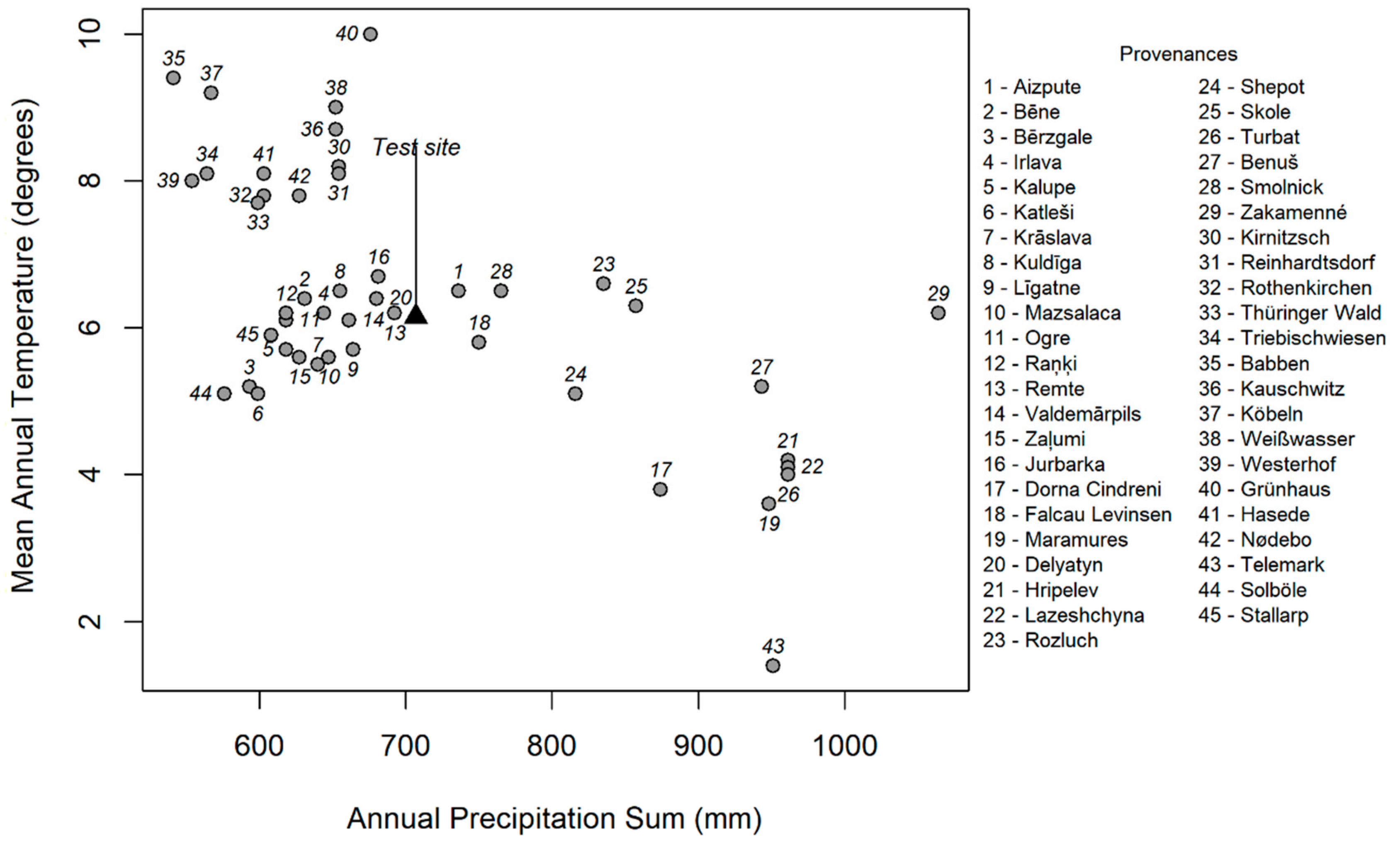
2.1. Experimental Material
2.2. Data Analysis
3. Results
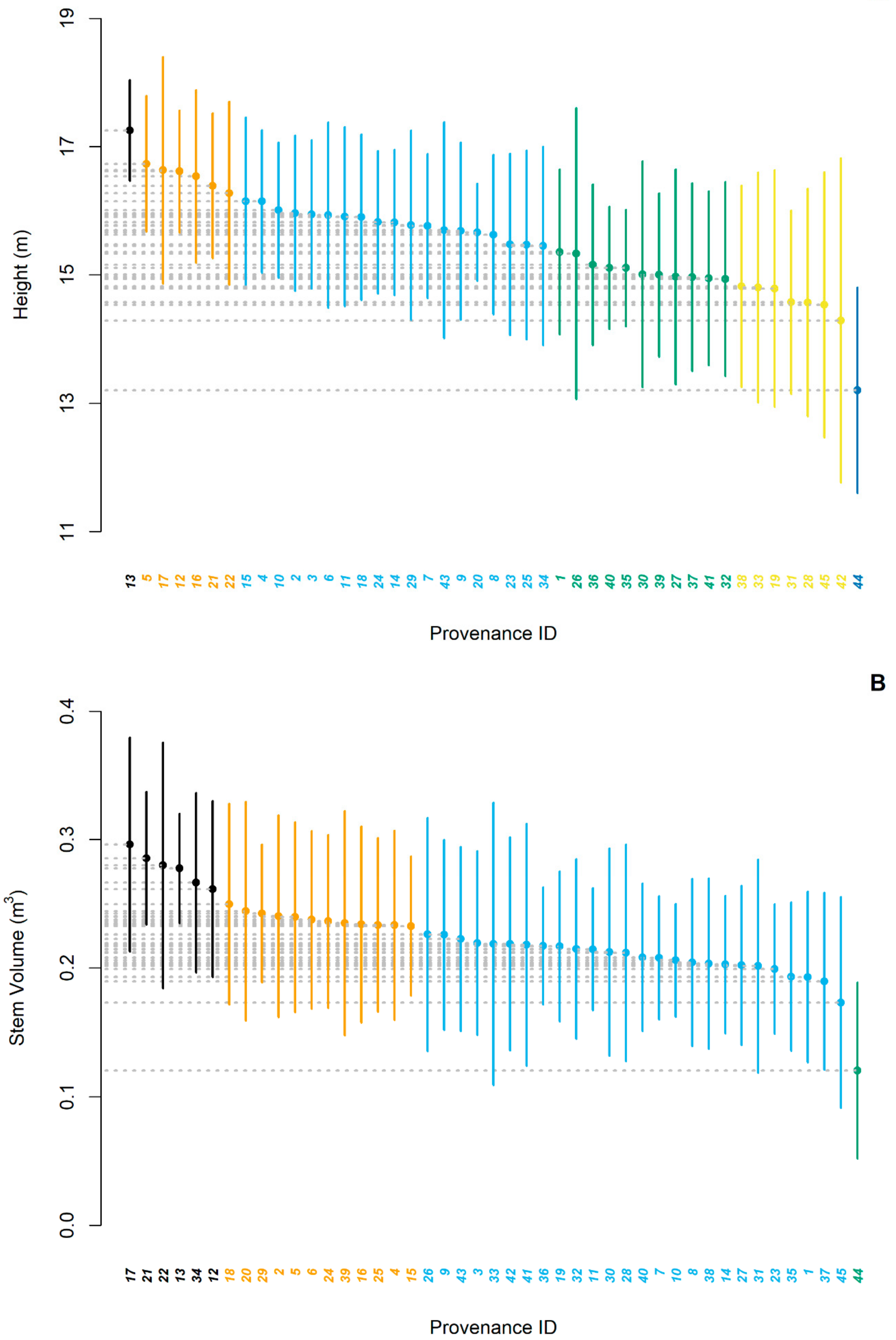
3.1. Provenance Effect
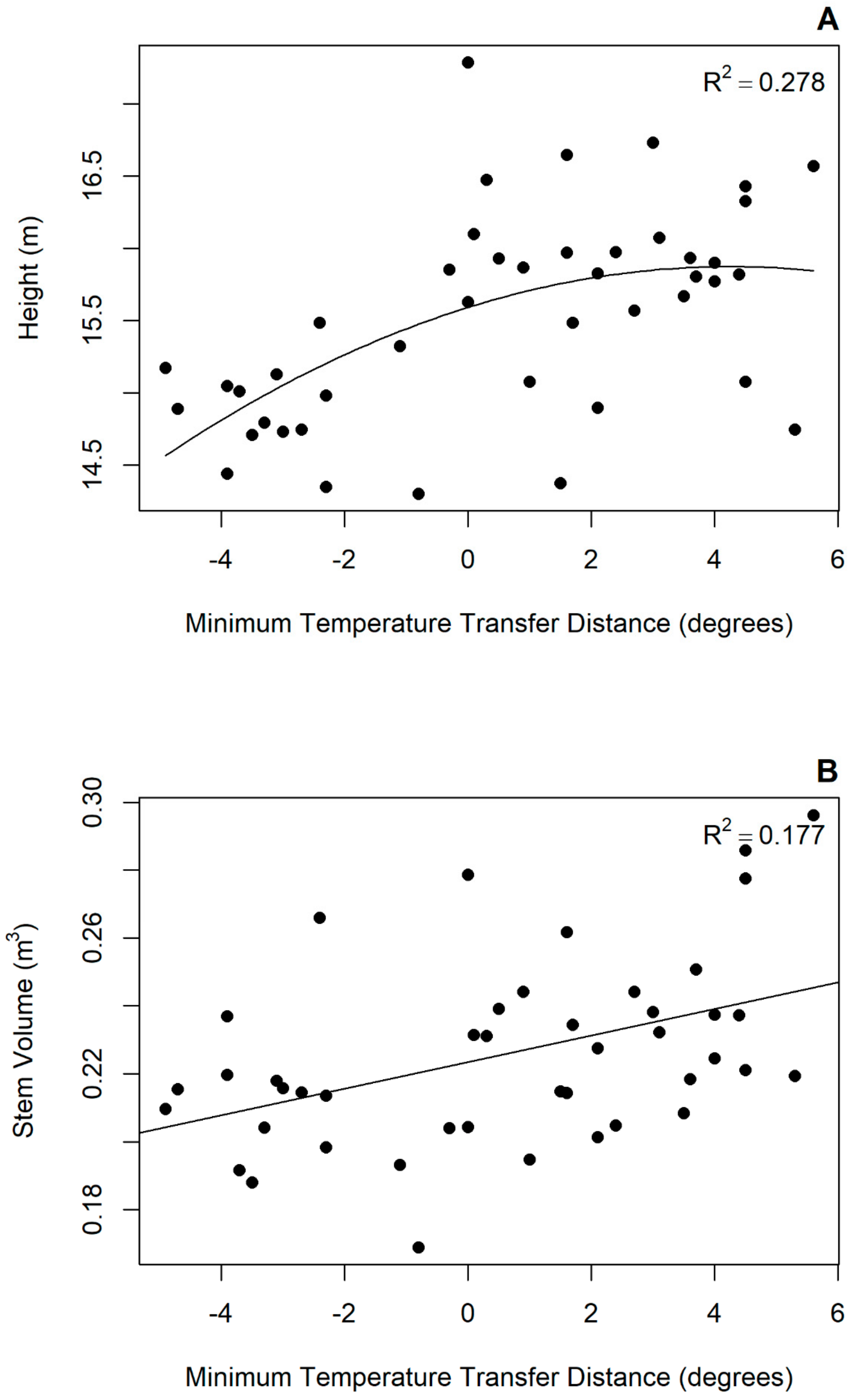
3.2. Transfer Functions
4. Discussion
4.1. Provenance Effect on Growth Performance
4.2. Provenance Response to Tranfer
4.3. Relation between Provenances and the Significant Climate Variables
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pretzsch, H.; Biber, P.; Schütze, G.; Uhl, E.; Rötzer, T. Forest stand growth dynamics in Central Europe have accelerated since 1870. Nat. Commun. 2014, 5, 4967. [Google Scholar] [CrossRef] [PubMed]
- Intergovernmental Panel on Climate Change. Climate Change 2014 Synthesis Report-IPCC; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2014; ISBN 9789291691432.
- Schmidt-Vogt, H. Die Fichte; Verlag Paul Parey: Hamburg/Berlin, Germany, 1977; Volume 1. [Google Scholar]
- Boshier, D.; Broadhurst, L.; Cornelius, J.; Gallo, L.; Koskela, J.; Loo, J.; Petrokofsky, G.; St Clair, B. Is local best? Examining the evidence for local adaptation in trees and its scale. Environ. Evid. 2015, 4, 20. [Google Scholar] [CrossRef]
- Lindner, M.; Garcia-Gonzalo, J.; Kolström, M.; Green, T.; Reguera, R.; Maroschek, M.; Seidl, R.; Lexer, M.J.; Netherer, S.; Schopf, A.; et al. Impacts of Climate Change on European Forests and Options for Adaptation; European Commission: Luxembourg, Belgium, 2008. [Google Scholar]
- Keskitalo, E.C.H.; Bergh, J.; Felton, A.; Björkman, C.; Berlin, M.; Axelsson, P.; Ring, E.; Ågren, A.; Roberge, J.M.; Klapwijk, M.J.; et al. Adaptation to climate change in Swedish forestry. Forests 2016, 7, 28. [Google Scholar] [CrossRef]
- Haappanen, M.; Jansson, G.; Nielsen, U.B.; Steffenrem, A.; Stener, L.-G. The Status of Tree Breeding and Its Potential for Improving Biomass Production—A Review of Breeding Activities and Genetic Gains in Scandinavia and Finland; SkogForsk: Uppsala, Sweden, 2015. [Google Scholar]
- Dzerina, B.; Girdziusas, S.; Lazdina, D.; Lazdins, A.; Jansons, J.; Neimane, U.; Jansons, Ā. Influence of spot mounding on height growth and tending of Norway spruce: Case study in Latvia. For. Stud. 2016, 65, 24–33. [Google Scholar] [CrossRef]
- Jansson, G.; Danusevičius, D.; Grotehusman, H.; Kowalczyk, J.; Krajmerova, D.; Skrøppa, T.; Wolf, H. Norway Spruce (Picea abies (L.) H.Karst.). In Forest Tree Breeding in Europe; Springer: Dordrecht, The Netherlands, 2013; pp. 123–176. [Google Scholar]
- Giertych, M. Genetics. In Biology and Ecology of Norway Spruce; Tjoelker, M.G., Boratyński, A., Bugała, W., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 115–155. ISBN 978-1-4020-4841-8. [Google Scholar]
- Ujvári-Jármay, É.; Nagy, L.; Mátyás, C. The IUFRO 1964/68 Inventory Provenance Trial of Norway Spruce in Nyírjes, Hungary—Results and conclusions of five decades. Lign. Hung 2016, 12, 3–178. [Google Scholar] [CrossRef]
- Kapeller, S.; Dieckmann, U.; Schueler, S. Varying selection differential throughout the climatic range of Norway spruce in Central Europe. Evol. Appl. 2017, 10, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Matyas, C. Modeling climate change effects with provenance test data. Tree Physiol. 1994, 14, 797–804. [Google Scholar] [CrossRef]
- Westin, J.; Haapanen, M. Norway spruce—Picea abies (L.) Karst. In Best Practice for Tree Breeding in Europe; Mullin, T.J., Lee, S.J., Eds.; Skogforsk: Uppsala, Sweden, 2013; pp. 29–47. [Google Scholar]
- Baumanis, I.; Jansons, A.; Neimane, U. Priede: Selekcija, ģenētika un Sēklkopība Latvijā [Scots Pine: Breeding, Genetics and Seed Orchard Management]; Daugavpils Universitātes Akadēmiskais apgāds’’Saule’’: Daugavpils, Latvija, 2014. [Google Scholar]
- Rone, V. Pirmie egļu provenienču vērtēšanas rezultāti Latvijā [First results from Norway spruce provenance trials in Latvia]. Jaun. Mežsaimniecībā 1984, 26, 33–38. [Google Scholar]
- Gailis, A. Norway spruce provenances in Latvia. In Proceedings of the IUFRO (S2.2-11) Symposium, Riga, Latvia, 14–18 September 1993; Rone, V., Ed.; LSFRI Silava: Riga, Latvia, 1993; pp. 44–49. [Google Scholar]
- Krutzsch, P. The IUFRO 1964-68 Provenance Test with Norway Spruce (Picea abies (L.) Karst.). Silvae Genet. 1974, 23, 58–62. [Google Scholar]
- Krutzsch, P. IUFRO’s role in coniferous tree improvement: Norway spruce (Picea abies (L.) Karst.). Silvae Genet. 1992, 41, 143–150. [Google Scholar]
- Ulbrichová, I.; Podrázský, V.; Beran, F.; Zahradník, D.; Fulín, M.; Procházka, J.; Kubeček, J. Picea abies provenance test in the Czech Republic after 36 years—Central European provenances. J. For. Sci. 2015, 61, 465–477. [Google Scholar] [CrossRef]
- Pacalaj, M.; Longauer, R.; Krajmerová, D.; Gömöry, D. Effect of site altitude on the growth and survival of Norway spruce (Picea abies L.) provenances on the Slovak plots of IUFRO experiment 1972. J. For. Sci. 2002, 48, 16–26. [Google Scholar] [CrossRef]
- Budeanu, M.; Şofletea, N.; Pârnuţǎ, G. Testing Romanian seed sources of Norway spruce (Picea abies): Results on growth traits and survival at age 30. Ann. For. Res. 2012, 55, 43–52. [Google Scholar]
- Zubizarreta-Gerendiain, A.; Gort-Oromi, J.; Mehtätalo, L.; Peltola, H.; Venäläinen, A.; Pulkkinen, P. Effects of cambial age, clone and climatic factors on ring width and ring density in Norway spruce (Picea abies) in southeastern Finland. For. Ecol. Manag. 2012, 263, 9–16. [Google Scholar] [CrossRef]
- Krajmerová, D.; Longauer, R.; Pacalaj, M.; Gömöry, D. Influence of provenance transfer on the growth and survival of Picea abies provenances. Dendrobiology 2009, 61, 17–23. [Google Scholar]
- Zolciak, A.; Oszako, T.; Sabor, J. Evaluation of the health status of Picea abies provenances growing on the IUFRO 1964-68 experimental plots. Dendrobiology 2009, 61, 63–68. [Google Scholar]
- Kapeller, S.; Lexer, M.J.; Geburek, T.; Hiebl, J.; Schueler, S. Intraspecific variation in climate response of Norway spruce in the eastern Alpine range: Selecting appropriate provenances for future climate. For. Ecol. Manag. 2012, 271, 46–57. [Google Scholar] [CrossRef]
- Ulbrichová, I.; Podrázský, V.; Olmez, Z.; Beran, F.; Procházka, J.; Fulín, M.; Kubeček, J.; Zahradník, D. Growth performance of norway spruce in the czech-german provenance trial plot Ledeč. Sci. Agric. Bohem. 2013, 44, 223–231. [Google Scholar] [CrossRef]
- Şofletea, N.; Budeanu, M. Response of Norway spruce (Picea abies (L.) H. Karst) seed stand progenies tested under different site conditions. Šumarski List 2015, 139, 47–56. [Google Scholar]
- Nakvasina, E.N.; Volkov, A.G.; Prozherina, N.A. Provenance experiment with spruce (Picea abies (L.) Karst. and Picea obovata (Ledeb.)) in the North of Russia (Arkhangelsk region). Folia For. 2017, 59, 219–230. [Google Scholar] [CrossRef][Green Version]
- Matisons, R.; Šņepsts, G.; Puriņa, L.; Donis, J.; Jansons, Ā. Dominant height growth of European beech at the northeasternmost stands in Europe. Silva Fenn. 2018, 52. article id 7818. [Google Scholar] [CrossRef]
- Matisons, R.; Puriņa, L.; Adamovičs, A.; Robalte, L.; Jansons, Ā. European beech in its northeasternmost stands in Europe: Varying climate-growth relationships among generations and diameter classes. Dendrochronologia 2017, 45, 123–131. [Google Scholar] [CrossRef]
- Johnsen, Ø.; Skrøppa, T. Adaptive properties of Picea abies progenies are influenced by environmental signals during sexual reproduction. Euphytica 1996, 92, 67–71. [Google Scholar] [CrossRef]
- Skrøppa, T.; Kohmann, K.; Johnsen, Ø.; Steffenrem, A.; Edvardsen, Ø.M. Field performance and early test results of offspring from two Norway spruce seed orchards containing clones transferred to warmer climates. Can. J. For. Res. 2007, 37, 515–522. [Google Scholar] [CrossRef]
- Stoehr, M.U.; L’Hirondelle, S.J.; Binder, W.D.; Webber, J.E. Parental environment aftereffects on germination, growth, and adaptive traits in selected white spruce families. Can. J. For. Res. 1998, 28, 418–426. [Google Scholar] [CrossRef]
- Foff, V.; Weiser, F.; Foffova, E.; Gömöry, D. Growth response of European larch (Larix decidua Mill.) populations to climatic transfer A Novel Approach for Controlled Pollination in Casuarina equisetifolia. Silvae Genet. 2014, 63, 67–75. [Google Scholar] [CrossRef]
- Laiviņš, M.; Melecis, V. Bio-geographical interpretation of climate data in Latvia: Multidimensional analysis. Acta Univ. Latv. 2003, 654, 7–22. [Google Scholar]
- Persson, A.; Persson, B. Survival, Growth and Quality of Norway Spruce (Picea abies (L.) Karst) Provenances at the Three Swedish SITEs of the IUFRO 1964/68 Provenance Experiment; Department of Forest Yield Research; Swedish University of Agricultural Sciences: Garpenberg, Sweden, 1992. [Google Scholar]
- Dietrichson, J. Norway spruce provenance trials in Nordic countries. In Proceedings of the IUFRO Joint Meeting of Working Parties on Norway Spruce Provenances and Norway Spruce Breeding, Bucharest, Romania, 24–26 September 1979; Lower Saxony Forest Research Institute: Escherode, Germany, 1979; pp. 3–14. [Google Scholar]
- Fottland, H.; Skrøppa, T. The IUFRO 1964/68 Provenance Experiment with Norway Spruce (Picea abies) in Norway. Variation in Mortality and Height Growth; Norsk institutt for skogforskning: Aas, Norway, 1989; ISBN 8271694588. [Google Scholar]
- Liepa, I. Pieauguma Mācība [Increment Science]; Latvijas Lauksaimniecības universitāte: Jelgava, Latvia, 1996. [Google Scholar]
- Harris, I.; Jones, P.D.; Osborn, T.J.; Lister, D.H. Updated high-resolution grids of monthly climatic observations - the CRU TS3.10 Dataset. Int. J. Climatol. 2014, 34, 623–642. [Google Scholar] [CrossRef]
- Wang, T.; Hamann, A.; Yanchuk, A.; O’neill, G.A.; Aitken, S.N. Use of response functions in selecting lodgepole pine populations for future climates. Glob. Chang. Biol. 2006, 12, 2404–2416. [Google Scholar] [CrossRef]
- R Core Team (2016). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.R-project.org/. (accessed on 31 March 2017).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models using lme4. J. Stat. Softw. 2014, 67, 1–48. [Google Scholar]
- Jelihovschi, E.; Faria, J.C.; Allaman, I.B. ScottKnott: A Package for Performing the Scott-Knott Clustering Algorithm in R. Tema 2014, 15, 003. [Google Scholar] [CrossRef]
- Rehfeldt, G.E.; Tchebakova, N.M.; Barnhardt, L.K. Efficacy of climate transfer functions: Introduction of Eurasian populations of Larix into Alberta. Can. J. For. Res. 1999, 29, 1660–1668. [Google Scholar] [CrossRef]
- Holst, H. Growth of Norway Spruce (Picea abies L. Karst.) Provenances in Eastern North America; Department of Forestry Publication: Ontario, USA, 1963; 15p. [Google Scholar]
- Fowler, D.P.; Coles, J.F. Norway spruce provenance experiments in the Maritimes region of Canada. For. Chron. 1980, 56, 155–160. [Google Scholar] [CrossRef][Green Version]
- Persson, B.; Persson, A. Variation in stem properties in a IUFRO 1964/1968 Picea abies provenance experiment in southern Sweden. Silvae Genet. 1997, 46, 94–101. [Google Scholar]
- Morgenstern, E.K. Geographic variation in forest trees: Genetic Basis and Application of Knowdlege in Silviculture; UBC Press: Vancouver, BC, Canada, 1996; ISBN 077480579X. [Google Scholar]
- Korshikov, I.I.; Privalikhin, S.N. Genetic structure of populations of Norway spruce (Picea abies (L.) Karst. from Ukrainian Carpathians. Russ. J. Genet. 2007, 43, 1364–1372. [Google Scholar] [CrossRef]
- Radu, R.G.; Curtu, L.A.; Spârchez, G.; Şofletea, N. Genetic diversity of Norway spruce [Picea abies (L.) Karst.] in Romanian Carpathians. Ann. For. Res. 2014, 57, 19–29. [Google Scholar] [CrossRef]
- Granhus, A.; Øyen, B.-H. Vekst, Produksjon Og Klimarelaterte Skader i Fem Proveniensforsøk Med Gran (Picea abies L. Karst.) På Østlandet; Norsk institutt for skog og landskap: Aas, Norway, 2009; ISBN 8231100903. [Google Scholar]
- Skroppa, T.; Magnussen, S. Provenance Variation in Shoot Growth Components of Norway Spruce. Silvae Genet. 1993, 42, 111–120. [Google Scholar]
- Gerendiain, A.Z.; Peltola, H.; Pulkkinen, P.; Kellomäki, S. Effects of genetic entry and competition by neighbouring trees on growth and wood properties of cloned Norway spruce (Picea abies). Ann. For. Sci. 2009, 66, 806. [Google Scholar] [CrossRef]
- O’Neill, G.A.; Hamann, A.; Wang, T. Accounting for population variation improves estimates of the impact of climate change on species’ growth and distribution. J. Appl. Ecol. 2008, 45, 1040–1049. [Google Scholar] [CrossRef]
- Kapeller, S.; Schuler, S.; Huber, G.; Boi, G.; Wohlgemuth, T.; Klumpp, R. Provenance Trials in Alpine Range – Review and Perspectives for Applications in Climate Change. In Management Strategies to Adapt Alpine Space Forests to Climate Change Risks; In Tech: Rijeka, Croatia, 2013; pp. 233–256. ISBN 978-953-51-1194-8. [Google Scholar]
- Pretzsch, H. Forest Dynamics, Growth, and Yield. In Forest Dynamics, Growth and Yield; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–39. ISBN 978-3-540-88306-7. [Google Scholar]
- Gömöry, D.; Longauer, R.; Hlásny, T.; Pacalaj, M.; Strmeň, S.; Krajmerová, D. Adaptation to common optimum in different populations of Norway spruce (Picea abies Karst.). Eur. J. For. Res. 2012, 131, 401–411. [Google Scholar]
- Metslaid, M.; Maaten, T.; Kurm, M.; Seemen, H.; Kiviste, A. Comparison of height growth of two picea abies provenances from Estonia and Belarus. Balt. For. 2015, 21, 253–258. [Google Scholar]
- Langvall, O. Impact of climate change, seedling type and provenance on the risk of damage to norway spruce (Picea abies (L.) Karst.) seedlings in Sweden due to early summer frosts. Scand. J. For. Res. 2011, 26, 56–63. [Google Scholar] [CrossRef]
- Howe, G.T.; Aitken, S.N.; Neale, D.B.; Jermstad, K.D.; Wheeler, N.C.; Chen, T.H. From genotype to phenotype: Unraveling the complexities of cold adaptation in forest trees. Can. J. Bot. 2003, 81, 1247–1266. [Google Scholar] [CrossRef]
- Rehfeldt, G.E.; Leites, L.P.; Joyce, D.G.; Weiskittel, A.R. Role of population genetics in guiding ecological responses to climate. Glob. Chang. Biol. 2018, 24, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Leites, L.P.; Robinson, A.P.; Rehfeldt, G.E.; Marshall, J.D.; Crookston, N.L. Height-growth response to climatic changes differs among populations of Douglas-fir: A novel analysis of historic data. Ecol. Appl. 2012, 22, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Danusevicius, D. Growth peculiarities of Norway spruce provenances and families in Lithuania. Norway spruce provenances and breeding. In Proceedings of the IUFRO (S2.2-11) Symposium, Riga, Latvia, 14–18 September; Rone, V., Ed.; LSFRI Silava: Riga, Latvia, 1993; pp. 38–43. [Google Scholar]
- Mäkinen, H.; Nöjd, P.; Kahle, H.-P.; Neumann, U.; Tveite, B.; Mielikäinen, K.; Röhle, H.; Spiecker, H. Radial growth variation of Norway spruce (Picea abies (L.) Karst.) across latitudinal and altitudinal gradients in central and northern Europe. For. Ecol. Manag. 2002, 171, 243–259. [Google Scholar]
- Berlin, M.; Persson, T.; Jansson, G.; Haapanen, M.; Ruotsalainen, S.; Bärring, L.; Andersson Gull, B. Scots pine transfer effect models for growth and survival in Sweden and Finland. Silva Fenn. 2016, 50. [Google Scholar] [CrossRef]
- Sáenz-Romero, C.; Lamy, J.-B.; Ducousso, A.; Musch, B.; Ehrenmann, F.; Delzon, S.; Cavers, S.; Chałupka, W.; Dağdaş, S.; Hansen, J.K.; et al. Adaptive and plastic responses of Quercus petraea populations to climate across Europe. Glob. Chang. Biol. 2017, 23, 2831–2847. [Google Scholar] [CrossRef] [PubMed]
- Bréda, N.; Sanchez, L.; Rozenberg, P.; Sergent, A.-S.; Bastein, J.-C. Coastal and interior Douglas-fir provenances differ in growth performance and response to drought episodes at adult age. Ann. For. Sci. 2014, 71, 709–720. [Google Scholar]
- Berlin, M.; Jansson, G.; Högberg, K.-A. Genotype by environment interaction in the southern Swedish breeding population of Picea abies using new climatic indices. Scand. J. For. Res. 2015, 30, 112–121. [Google Scholar] [CrossRef]
| Model Response Variable | Sum of Squares | Degrees of Freedom | Mean Square | F | p-Value |
|---|---|---|---|---|---|
| Height | 193.630 | 44 | 4.007 | 4.017 | <0.001 |
| Stem volume | 0.539 | 44 | 0.012 | 2.990 | <0.001 |
| Transfer Distance | Response Variable | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tree Height | Stem Volume | |||||||||
| β0 | β1 | β2 | Adj-R2 | F Statistic | β0 | β1 | β2 | Adj-R2 | F Statistic | |
| APS | na 1 | na | na | na | na | 0.223 | −0.0001 | na | 0.078 | 4.644 |
| MAT | 15.523 | 2.145 | −0.9747 | 0.215 | 6.884 | 0.227 | 0.006 | na | 0.127 | 7.254 |
| MAX | na | na | na | na | na | na | na | na | na | na |
| MIN | 15.523 | 2.487 | −0.833 | 0.278 | 9.276 | 0.223 | 0.004 | na | 0.177 | 10.250 |
| AHM | 15.523 | 1.4793 | −1.1611 | 0.119 | 3.909 | 0.228 | 0.002 | na | 0.115 | 6.598 |
| FROST | 15.523 | −1.852 | −1.245 | 0.188 | 5.970 | 0.222 | −0.001 | na | 0.151 | 8.673 |
| APS | MAT | MIN | MAX | AHM | FROST | |
|---|---|---|---|---|---|---|
| APS | −0.61 | −0.56 | −0.57 | −0.92 | 0.69 | |
| MAT | <0.01 | 0.90 | 0.82 | 0.84 | −0.96 | |
| MIN | <0.01 | <0.01 | 0.51 | 0.79 | −0.94 | |
| MAX | <0.01 | <0.01 | <0.01 | 0.71 | −0.71 | |
| AHM | <0.01 | <0.01 | <0.01 | <0.01 | −0.89 | |
| FROST | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeltiņš, P.; Katrevičs, J.; Gailis, A.; Maaten, T.; Desaine, I.; Jansons, Ā. Adaptation Capacity of Norway Spruce Provenances in Western Latvia. Forests 2019, 10, 840. https://doi.org/10.3390/f10100840
Zeltiņš P, Katrevičs J, Gailis A, Maaten T, Desaine I, Jansons Ā. Adaptation Capacity of Norway Spruce Provenances in Western Latvia. Forests. 2019; 10(10):840. https://doi.org/10.3390/f10100840
Chicago/Turabian StyleZeltiņš, Pauls, Juris Katrevičs, Arnis Gailis, Tiit Maaten, Iveta Desaine, and Āris Jansons. 2019. "Adaptation Capacity of Norway Spruce Provenances in Western Latvia" Forests 10, no. 10: 840. https://doi.org/10.3390/f10100840
APA StyleZeltiņš, P., Katrevičs, J., Gailis, A., Maaten, T., Desaine, I., & Jansons, Ā. (2019). Adaptation Capacity of Norway Spruce Provenances in Western Latvia. Forests, 10(10), 840. https://doi.org/10.3390/f10100840






