Comparing Negative Impacts of Prunus serotina, Quercus rubra and Robinia pseudoacacia on Native Forest Ecosystems
Abstract
1. Introduction
- (a).
- To evaluate the possible differences in humus forms and subsequent variations in other soil physical and chemical properties among forest stands;
- (b).
- To determine if variations in biotic components occurred among forest stands, particularly ascertaining possible reductions in biodiversity levels and biological soil quality; and
- (c).
- To recognize the relationships among biotic and abiotic determinants across the different forest stands.
2. Materials and Methods
2.1. Study Area and Sites
2.2. Soil Abiotic Characteristics
2.3. Soil Biotic Characteristics: Microbial Activity and Microarthropod Communities
2.4. Plant Communities
2.5. Data Analysis
3. Results
3.1. Soil Abiotic Characteristics
3.1.1. Humus Forms
3.1.2. Mineral Soil
3.2. Soil Biotic Characteristics
3.3. Plant Community
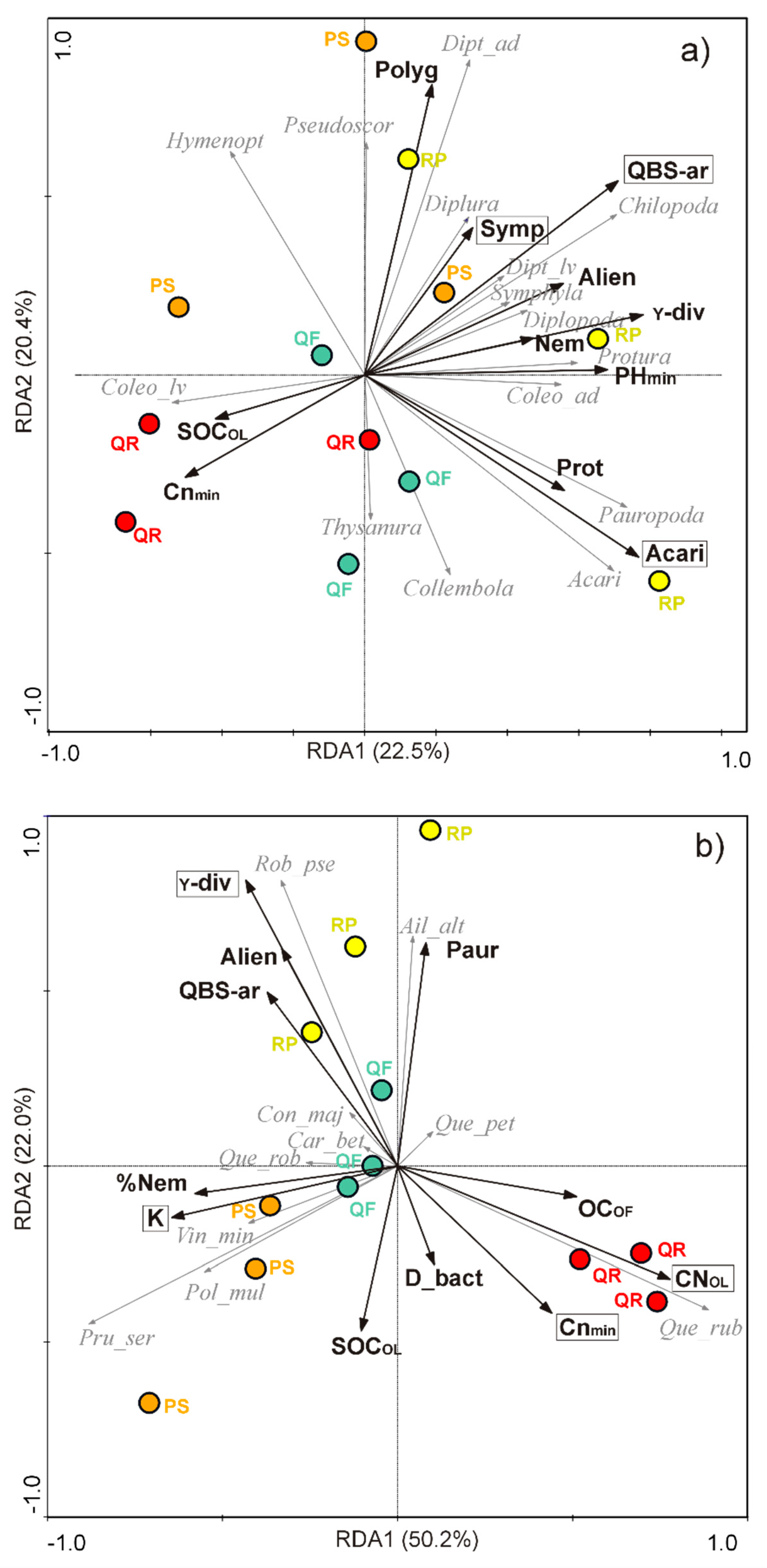
3.4. Relationships between Biotic Communities and Ecological Determinants
4. Discussion
4.1. Soil Abiotic Characteristics: Humus Forms
4.2. Soil Abiotic Factors: Mineral Soil Properties
4.3. Biotic Factors: Microarthropod and Plant Communities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ehrenfeld, J.G. Ecosystem consequences of biological invasions. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 59–80. [Google Scholar] [CrossRef]
- Hejda, M.; Pyšek, P.; Jarošík, V. Impact of invasive plants on the species richness, diversity and composition of invaded communities. J. Ecol. 2009, 97, 393–403. [Google Scholar] [CrossRef]
- Mollot, G.; Pantel, J.H.; Romanuk, T.N. Chapter Two—The effects of invasive species on the decline in species richness: A global meta-analysis. Adv. Ecol. Res. 2017, 56, 61–83. [Google Scholar] [CrossRef]
- Elgersma, J.J.; Ehrenfeld, J.G. Linear and non-linear impacts of a non-native plant invasion on soil microbial community structure and function. Biol. Invasions 2011, 13, 757–768. [Google Scholar] [CrossRef]
- Suding, K.N.; Stanley Harpole, W.; Fukami, T.; Kulmatiski, A.; MacDougall, A.S.; Stein, C.; van der Putten, W.H. Consequences of plant–soil feedbacks in invasion. J. Ecol. 2013, 101, 298–308. [Google Scholar] [CrossRef]
- Vilá, M.; Espinar, J.L.; Hejda, M.; Hulme, P.E.; Jarošík, V.; Maron, J.L.; Pergl, J.; Schaffner, U.; Sun, Y.; Pyšek, P. Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 2011, 14, 702–708. [Google Scholar] [CrossRef]
- Mincheva, T.; Barni, E.; Varese, G.C.; Brusa, G.; Cerabolini, B.; Siniscalco, C. Litter quality, decomposition rates and saprotrophic mycoflora in Fallopia japonica (Houtt.) Ronse Decraene and in adjacent native grassland vegetation. Acta Oecol. 2014, 54, 29–35. [Google Scholar] [CrossRef]
- Dassonville, N.; Vanderhoeven, S.; Vanparys, V.; Hayez, M.; Gruber, W.; Meerts, P. Impacts of alien invasive plants on soil nutrients are correlated with initial site conditions in NW Europe. Oecologia 2008, 157, 131–140. [Google Scholar] [CrossRef]
- Panetta, F.D.; Gooden, B. Managing for biodiversity: Impact and action thresholds for invasive plants in natural ecosystems. NeoBiota 2017, 34, 53–66. [Google Scholar] [CrossRef]
- Callaway, R.M.; Bedmar, E.J.; Reinhart, K.O.; Silvan, C.G.; Klironomos, J. Effects of soil biota from different ranges on Robinia invasion: Acquiring mutualists and escaping pathogens. Ecology 2011, 92, 1027–1035. [Google Scholar] [CrossRef]
- Brundu, G.; Richardson, D.M. Planted forests and invasive alien trees in Europe: A Code for managing existing and future plantings to mitigate the risk of negative impacts from invasions. NeoBiota 2016, 30, 5–47. [Google Scholar] [CrossRef]
- Godefroid, S.; Phartyal, S.S.; Weyembergh, G.; Koedam, N. 2005. Ecological factors controlling the abundance of non-native invasive black cherry (Prunus serotina) in deciduous forest understory in Belgium. For. Ecol. Manag. 2005, 210, 91–105. [Google Scholar] [CrossRef]
- Kawaletz, H.; Mölder, I.; Zerbe, S.; Annighöfer, P.; Terwei, A.; Ammer, C. Exotic tree seedlings are much more competitive than natives but show underyielding when growing together. J. Plant Ecol. 2013, 6, 305–315. [Google Scholar] [CrossRef][Green Version]
- Major, K.C.; Nosko, P.; Kuehne, C.; Campbell, D.; Bauhus, J. Regeneration dynamics of non-native northern red oak (Quercus rubra L.) populations as influenced by environmental factors: A case study in managed hardwood forests of southwestern Germany. For. Ecol. Manag. 2013, 291, 144–153. [Google Scholar] [CrossRef]
- Halarewicz, A.; Żołnierz, L. Changes in the understorey of mixed coniferous forest plant communities dominated by the American black cherry (Prunus serotina Ehrh.). For. Ecol. Manag. 2014, 313, 91–97. [Google Scholar] [CrossRef]
- Sádlo, J.; Vítková, M.; Pergl, J.; Pyšek, P. Towards site-specific management of invasive alien trees based on the assessment of their impacts: The case of Robinia pseudoacacia. NeoBiota 2017, 35, 1–34. [Google Scholar] [CrossRef]
- Woziwoda, B.; Krzyżanowska, A.; Dyderski, M.K.; Jagodziński, A.M.; Stefańska-Krzaczek, E. Propagule pressure, presence of roads, and microsite variability influence dispersal of introduced Quercus rubra in temperate Pinus sylvestris forest. For. Ecol. Manag. 2018, 428, 35–45. [Google Scholar] [CrossRef]
- Aerts, R.; Ewald, M.; Nicolas, M.; Piat, J.; Skowronek, S.; Lenoir, J.; Hattab, T.; Garzón-López, C.X.; Feilhauer, H.; Schmidtlein, S.; et al. Invasion by the alien tree Prunus serotina alters ecosystem functions in a temperate deciduous forest. Front. Plant Sci. 2017, 8, 179. [Google Scholar] [CrossRef]
- Miltner, S.; Kupka, I.; Třeštík, M. Effects of Northern red oak (Quercus rubra L.) and sessile oak (Quercus petraea (Mattusch.) Liebl.) on the forest soil chemical properties. For. J. 2016, 62, 169–172. [Google Scholar] [CrossRef]
- Medina-Villar, S.; Castro-Díez, P.; Alonso, A.; Cabra-Rivas, I.; Parker, I.M.; Pérez-Corona, E. Do the invasive trees, Ailanthus altissima and Robinia pseudoacacia, alter litterfall dynamics and soil properties of riparian ecosystems in Central Spain? Plant Soil 2015, 396, 311–324. [Google Scholar] [CrossRef]
- Woziwoda, B.; Kopeć, D.; Witkowski, J. The negative impact of intentionally introduced Quercus rubra L. on a forest community. Acta Soc. Bot. Pol. 2014, 83, 39–49. [Google Scholar] [CrossRef]
- Vítková, M.; Müllerová, J.; Sádlo, J.; Pergl, J.; Pyšek, P. Black locust (Robinia pseudoacacia) beloved and despised: A story of an invasive tree in Central Europe. For. Ecol. Manag. 2017, 384, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Sitzia, T.; Campagnaro, T.; Dainese, M.; Cierjacks, A. Plant species diversity in alien black locust stands: A paired comparison with native stands across a north-Mediterranean range expansion. For. Ecol. Manag. 2012, 285, 85–91. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Jagodziński, A.M. Drivers of invasive tree and shrub natural regeneration in temperate forests. Biol. Invasions 2018, 20, 2363–2379. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Jagodziński, A.M. Low impact of disturbance on ecological success of invasive tree and shrub species in temperate forests. Plant Ecol. 2018, 219, 1369–1380. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Jagodziński, A.M. Functional traits of acquisitive invasive woody species differ from conservative invasive and native species. NeoBiota 2019, 41, 91–113. [Google Scholar] [CrossRef]
- Blasi, C. (Ed.) La Vegetazione D’Italia Con Carta Delle Serie di Vegetazione in Scala 1:500000; Palombi & Partner S.r.L.: Roma, Italy, 2010. [Google Scholar]
- Ciancio, O.; Mercurio, R.; Nocentini, S. La sperimentazione di specie forestali esotiche in Italia, Risultati dopo un sessantennio. Ann. Ist. Sper. Selvic. 1982, 12–13, 105–713. [Google Scholar]
- ERSAF. Carta Dei Suoli Della Lombardia, Scala 1:250000; Regione Lombardia: Milano, Italy, 2004. [Google Scholar]
- D’Alessio, D.; Febelli, C. I Suoli Della Pianura Milanese Settentrionale; ERSAL: Milano, Italy, 1999; p. 147. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, Update 2015; FAO: Roma, Italy, 2015. [Google Scholar]
- Burt, R. Soil Survey Laboratory Methods Manual, NSS Laboratory Soil Survey Investigations; Report. No. 42; US Department of Agriculture, Natural Resource Conservation Service: Washington, DC, USA, 2012. Available online: https://www.nrcs.usda.gov/ (accessed on 12 August 2019).
- Bray, R.H.; Kurtz, L.T. Determination of total organic and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–45. [Google Scholar] [CrossRef]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; USDA: Washington, DC, USA, 1954.
- Zanella, A.; Ponge, J.F.; Jabiol, B.; Sartori, G.; Kolb, E.; Le Bayon, R.C.; Gobat, J.M.; Aubert, M.; De Waal, R.; Van Delft, B.; et al. Humusica 1, article 5: Terrestrial humus systems and forms—Keys of classification of humus systems and forms. Appl. Soil Ecol. 2018, 122, 75–86. [Google Scholar] [CrossRef]
- Ponge, J.F.; Chevalier, R.; Loussot, P. Humus index: An integrated tool for the assessment of forest floor and topsoil properties. Soil Sci. Soc. Am. J. 2002, 66, 1996–2001. [Google Scholar] [CrossRef]
- Korkina, I.N.; Vorobeichik, E.L. The humus index: A promising tool for environmental monitoring. Russ. J. Ecol. 2016, 47, 526–531. [Google Scholar] [CrossRef]
- Isermeyer, H. Eine einfache Methode zur Bestimmung der Bodenatmung und der Carbonate im Boden. Z. Pflanzenernähr. Düng. Bodenkd. 1952, 56, 26–38. [Google Scholar] [CrossRef]
- Jäggi, W. Die Bestimmung der CO2-Bildung als Maß der bodenbiologischen Aktivität. Schw. Landw. Forsch. 1976, 15, 371–380. [Google Scholar]
- Parisi, V.; Menta, C.; Gardi, C.; Jacomini, C.; Mozzanica, E. Microarthropod communities as a tool to assess soil quality and biodiversity: A new approach in Italy. Agric. Ecosyst. Environ. 2005, 105, 323–333. [Google Scholar] [CrossRef]
- Menta, C.; Contia, F.D.; Pinto, S.; Leoni, A.; Lozano-Fondón, C. Monitoring soil restoration in an open-pit mine in northern Italy. Appl. Soil Ecol. 2014, 83, 22–29. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Perez-Riverol, Y.; Kuhn, M.; Vizcaíno, J.A.; Hitz, M.P.; Audain, E. Accurate and fast feature selection workflow for high-dimensional omics data. PLoS ONE 2017, 12, e0189875. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2016. Available online: https://www.R-project.org/ (accessed on 12 August 2019).
- Fox, J.; Friendly, M.; Weisberg, S. Hypothesis tests for multivariate linear models using the car package. R J. 2013, 5, 39–52. [Google Scholar] [CrossRef]
- Wei, T.; Simko, V. R Package “Corrplot”: Visualization of a Correlation Matrix (Version 0.84). Available online: https://github.com/taiyun/corrplot/ (accessed on 28 August 2019).
- Zink, J.A. The Pedologic Landscape: Organization of the Soil Material. In Geopedology; Zinck, J.A., Metternicht, G., Bocco, G., Del Valle, H.F., Eds.; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Jonczak, J.; Parzych, A.; Sobisz, Z. Decomposition of four tree species leaf litters in headwater riparian forest. Balt. For. 2015, 21, 133–143. [Google Scholar]
- Steffen, K.T.; Cajthaml, T.; Snajdr, J.; Baldrian, P. Differential degradation of oak (Quercus petraea) leaf litter by litter-decomposing basidiomycetes. Res. Microbiol. 2007, 158, 447–455. [Google Scholar] [CrossRef]
- Bonifacio, E.; Petrillo, M.; Petrella, F.; Tambone, F.; Celi, L. Alien red oak affects soil organic matter cycling and nutrient availability in low-fertility well-developed soils. Plant Soil 2015, 395, 215–229. [Google Scholar] [CrossRef]
- Lorenz, K.; Preston, C.M.; Krumrei, S.; Feger, K.H. Decomposition of needle/leaf litter from Scots pine, black cherry, common oak and European beech at a conurbation forest site. Eur. J. For. Res. 2004, 123, 177–188. [Google Scholar] [CrossRef]
- Talbot, J.M.; Finzi, A.C. Differential effects of sugar maple, red oak, and hemlock tannins on carbon and nitrogen cycling in temperate forest soils. Oecologia 2008, 155, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Regina, S.I.; Tarazona, T. Nutrient cycling in a natural beech forest and adjacent planted pine in northern Spain. Forestry 2001, 74, 11–28. [Google Scholar] [CrossRef]
- Graça, M.A.S.; Poquet, J.M. Do climate and soil influence phenotypic variability in leaf litter, microbial decomposition and shredder consumption? Oecologia 2014, 174, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Aragón, R.; Montti, L.; Ayup, M.M.; Fernández, R. Exotic species as modifiers of ecosystem processes: Litter decomposition in native and invaded secondary forests of NW Argentina. Acta Oecol. 2014, 54, 21–28. [Google Scholar] [CrossRef]
- Lazzaro, L.; Mazza, G.; d’Errico, G.; Fabiani, A.; Giuliani, C.; Inghilesi, A.F.; Lagomarsino, A.; Lastrucci, L.; Pastorelli, R.; Roversi, P.F.; et al. How ecosystems change following invasion by Robinia pseudoacacia: Insights from soil chemical properties and soil microbial, nematode, microarthropod and plant communities. Sci. Total Environ. 2018, 622, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Rice, S.K.; Westerman, B.; Federici, R. Impacts of the exotic, nitrogen-fixing black locust (Robinia pseudoacacia) on nitrogen-cycling in a pine–oak ecosystem. Plant Ecol. 2004, 174, 97–107. [Google Scholar] [CrossRef]
- Barber, S.A. Soil Nutrient Bioavailability: A Mechanistic Approach; John Wiley and Sons: New York, NY, USA, 1995. [Google Scholar]
- Kohyt, J.; Skubała, P. Communities of mites (Acari) in litter and soil under the invasive red oak (Quercus rubra L.) and native pedunculate oak (Q. robur L.). Biol. Lett. 2013, 50, 111–124. [Google Scholar] [CrossRef]
- Riepšas, E.; Straigytė, L. Invasiveness and ecological effects of red oak (Quercus rubra L.) in Lithuanian Forests. Balt. For. 2008, 14, 122–130. [Google Scholar]
- Chmura, D. Impact of alien tree species Quercus rubra L. on understorey environment and flora: A study of the Silesian upland (Southern Poland). Pol. J. Ecol. 2013, 61, 431–442. [Google Scholar]
- Bedano, J.C.; Sacchi, L.; Natale, E.; Reinoso, H. Saltcedar (Tamarix ramosissima) Invasion Alters Decomposer Fauna and Plant Litter Decomposition in a Temperate Xerophytic Deciduous Forest. Adv. Ecol. 2014. [Google Scholar] [CrossRef]
- Gergócs, V.; Hufnagel, L. The effect of microarthropods on litter decomposition depends on litter quality. Eur. J. Soil Biol. 2016, 75, 24–30. [Google Scholar] [CrossRef]
- Litt, A.R.; Cord, E.E.; Fulbright, T.E.; Schuster, G.L. Effects of invasive plants on arthropods. Conserv. Biol. 2014, 28, 1532–1549. [Google Scholar] [CrossRef] [PubMed]
- Spafford, R.D.; Lortie, C.J.; Butterfield, B.J. A systematic review of arthropod community diversity in association with invasive plants. NeoBiota 2013, 16, 81–102. [Google Scholar] [CrossRef]
- Levin, L.A.; Neira, C.; Grosholz, E.D. Invasive cordgrass modified wetland trophic function. Ecology 2006, 87, 419–432. [Google Scholar] [CrossRef]
- Wolkovich, E.M.; Bolger, D.T.; Holway, D.A. Complex responses to invasive grass litter by ground arthropods in a Mediterranean scrub ecosystem. Oecologia 2009, 161, 697–708. [Google Scholar] [CrossRef]
- Terwei, A.; Zerbe, S.; Mölder, I.; Annigöhfer, P.; Kawaletz, H.; Ammer, C. Response of floodplain understorey species to environmental gradients and tree invasion: A functional trait perspective. Biol. Invasions 2016, 18, 2951–2973. [Google Scholar] [CrossRef]
- Slabejová, D.; Bacigál, T.; Hegedüšová, K.; Májeková, J.; Medvecka, J.P.; Mikulová, K.; Šibíková, M.; Škodová, I.; Zaliberová, M.; Jarolímek, I. Comparison of the understory vegetation of native forests and adjacent Robinia pseudoacacia plantations in the Carpathian-Pannonian region. For. Ecol. Manag. 2019, 439, 28–40. [Google Scholar] [CrossRef]
- Benesperi, R.; Giuliani, C.; Zanetti, S.; Gennai, M.; Mariotti-Lippi, M.; Guidi, T.; Nascimbene, J.; Foggi, B. Forest plant diversity is threatened by Robinia pseudoacacia L. (black-locust) invasion. Biodivers. Conserv. 2012, 21, 3555–3568. [Google Scholar] [CrossRef]
- Stefanowicz, A.M.; Stanek, M.; Nobis, M.; Zubek, S. Species-specific effects of plant invasions on activity, biomass, and composition of soil microbial communities. Biol. Fertil. Soils 2016, 52, 841–852. [Google Scholar] [CrossRef]
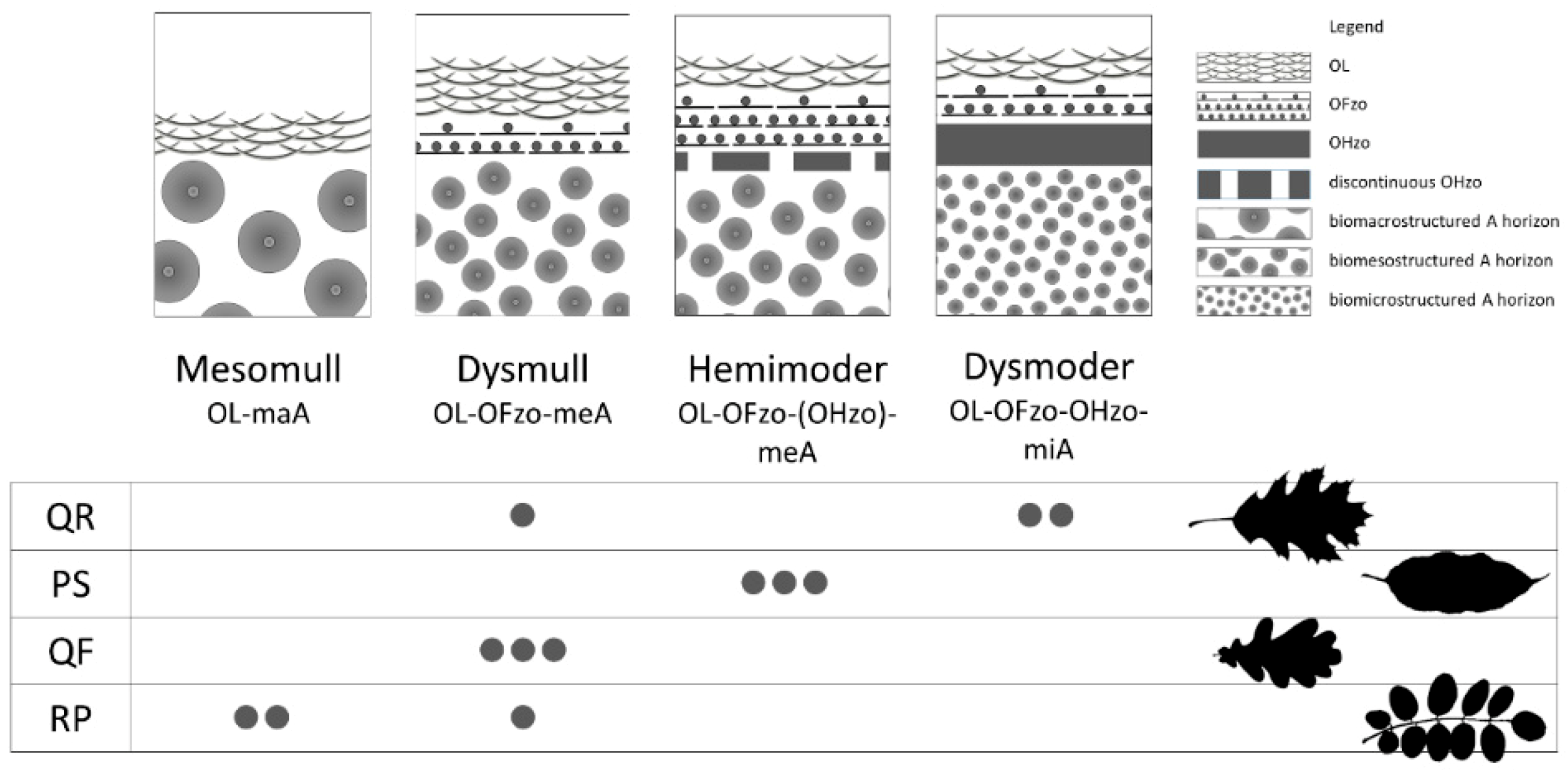
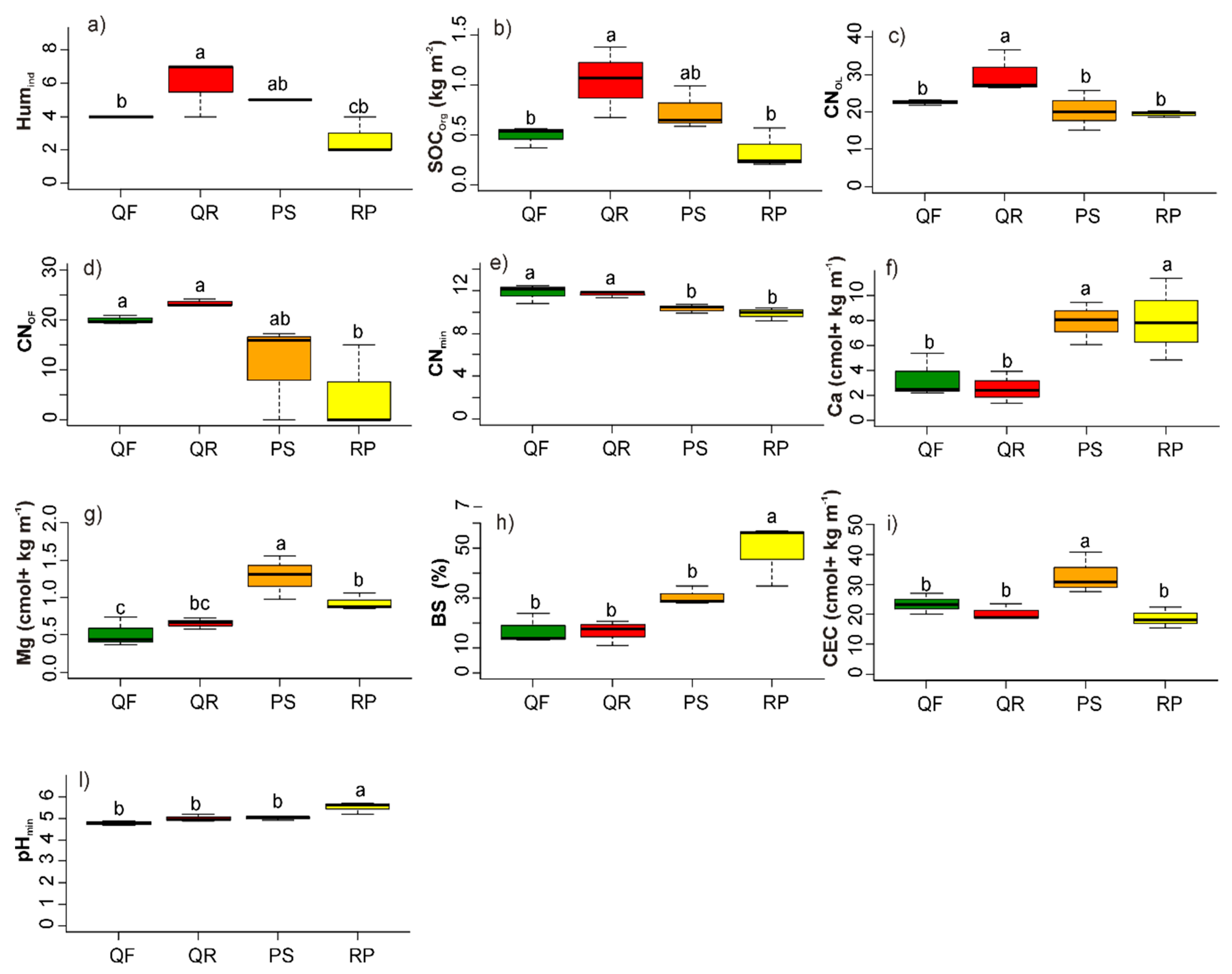
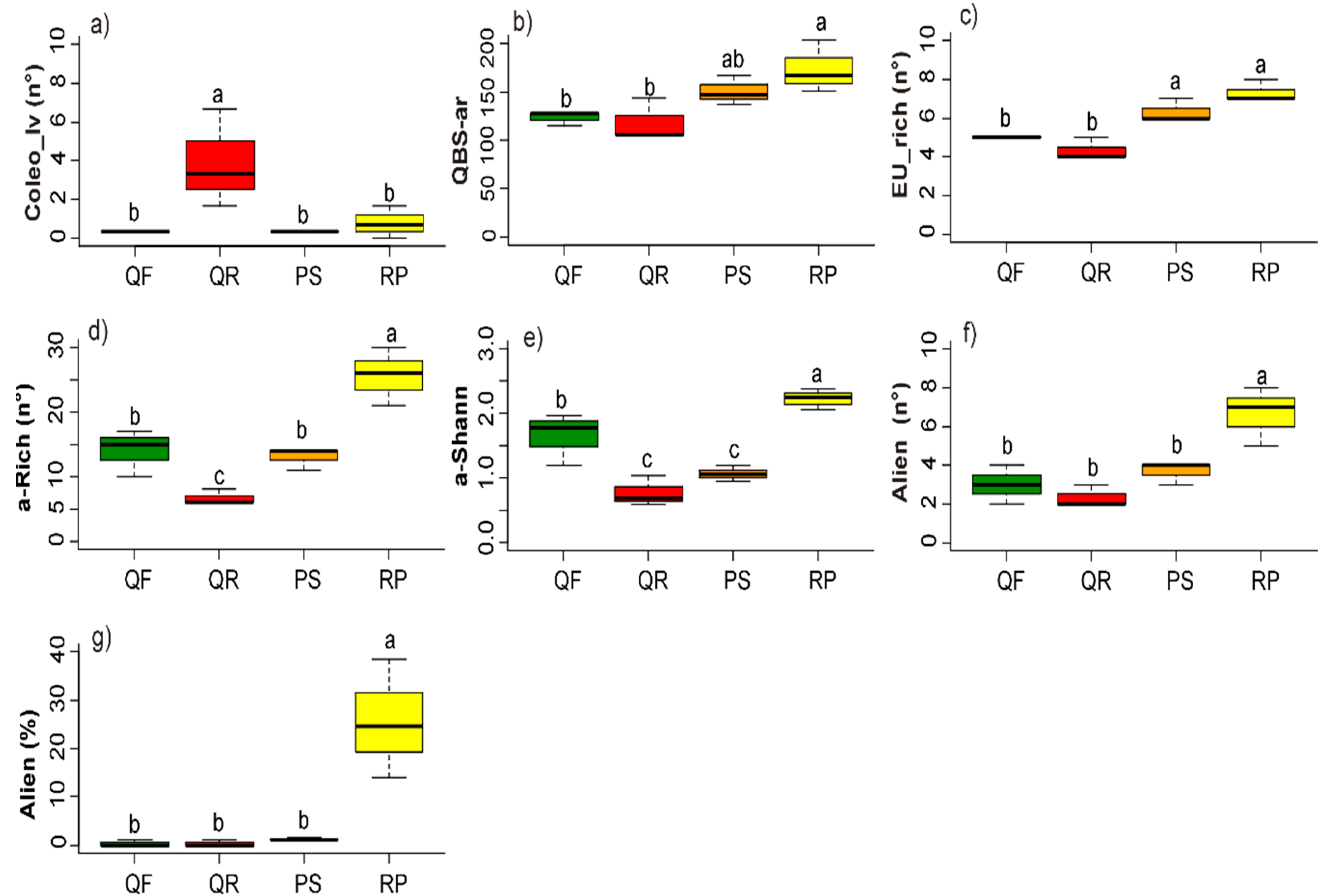
| Ecosystem Component | Factor | Unit | Abbreviation |
|---|---|---|---|
| Humus forms | Dysmull | presence/absence | / |
| Dysmoder | presence/absence | / | |
| Hemimoder | presence/absence | / | |
| Mesomull | presence/absence | / | |
| Properties of organic horizons | Soil organic carbon stock of OL organic horizon | kg m−2 | SOCOL |
| Soil organic carbon stock of OF organic horizon | kg m−2 | SOCOF | |
| Soil organic carbon stock of OH organic horizon | kg m−2 | SOCOH | |
| Soil organic carbon stock of | kg m−2 | SOCOrg | |
| C:N ratio of OL organic horizon | ratio | CNOL | |
| C:N ratio of OF organic horizon | ratio | CNOF | |
| C:N ratio of OH organic horizon | ratio | CNOH | |
| Properties of mineral layer | Base saturation of the 0–10 cm mineral layer | % | BS |
| Cation exchange capacity of the 0–10 cm mineral layer | cmol(+) kg−1 | CEC | |
| Exchangeable calcium of the 0–10 cm mineral layer | cmol(+) kg−1 | Ca | |
| Exchangeable magnesium of the 0–10 cm mineral layer | cmol(+) kg−1 | Mg | |
| Exchangeable sodium of the 0–10 cm mineral layer | cmol(+) kg−1 | Na | |
| Exchangeable potassium of the 0–10 cm mineral layer | cmol(+) kg−1 | K | |
| Potential Respiration | mgCO2 g−1 24 h−1 | Resp | |
| Soil organic carbon stock of the 0–60 cm mineral layer | kg m−2 | SOCmin | |
| C:N ratio of the 0–60 cm mineral layer | ratio | CNmin | |
| Soil pH in water of the 0–60 cm mineral layer | / | pHmin | |
| Available phosphorus of 0–60 cm mineral layer | mg kg−1 | Pav | |
| Ratio of OCOrg and OC of the 0–10 cm mineral layer | ratio | Littind | |
| Humus index | / | Humind | |
| Bacteria | Bacteria richness | n/g of soil | Bact |
| Live bacteria richness | n/g of soil | L_bact | |
| Dead bacteria richness | n/g of soil | D_bact | |
| Microarthropod community | Acari abundance | n/sample | Acari |
| Coleoptera larvae abundance | n/sample | Coleo_lv | |
| Collembola abundance | n/sample | Coll | |
| Pauropoda abundance | n/sample | Paur | |
| Protura abundance | n/sample | Prot | |
| Symphyla abundance | n/sample | Symp | |
| Biological Quality Soil index | index | QBS-ar | |
| Euedaphic arthropod taxa α-diversity | n/plot | Eu_rich | |
| Plant community | Plant species richness expressed as number of plant species within the plot | n/plant | α-rich |
| Plant species diversity expressed as Shannon H’ | Index | α-Shann | |
| Plant species diversity expressed as species turnover according Whittaker | index | β-Whit | |
| Plant species diversity expressed as species turnover according the Jaccard index | index | β-Jacc | |
| Plant species diversity expressed as total number of species within a woodland type | n/plot | γ-div | |
| Number of nemoral species | n/plot | Nem | |
| Cover of nemoral species | % | %Nem | |
| Number of alien species | n/plot | Alien | |
| Cover of aliens species | %/plot | %Alien | |
| Number of individuals of the indicator species Polygonatum multiflorum | n/plot | Polyg |
| (a) Microarthropod Community | RDA Axes | Total Variance | |||
| Axis 1 | Axis 2 | Axis 3 | Axis 4 | ||
| Eigenvalues: | 0.225 | 0.203 | 0.148 | 0.104 | 1.000 |
| Cumulative percentage variance of species data and species-environment relation: | 22.5 | 42.9 | 57.7 | 68.1 | |
| Variable | Lambda1 | LambdaA | P | F | |
| QBS-ar | 0.19 | 0.19 | *** | 2.62 | |
| Acari | 0.19 | 0.19 | ** | 2.41 | |
| Symp | 0.14 | 0.11 | * | 1.87 | |
| Polyg | 0.17 | 0.09 | ns | 1.48 | |
| Nem | 0.11 | 0.08 | ns | 1.45 | |
| Prot | 0.14 | 0.08 | ns | 1.49 | |
| Alien | 0.12 | 0.08 | ns | 1.69 | |
| γ-div | 0.17 | 0.06 | ns | 1.73 | |
| pHtot | 0.14 | 0.07 | ns | 2.56 | |
| CNtot | 0.10 | 0.03 | ns | 1.99 | |
| OCOL | 0.08 | 0.02 | ns | 0 | |
| (b) Plant Community | |||||
| Axis 1 | Axis 2 | Axis 3 | Axis 4 | Total variance | |
| Eigenvalues: | 0.502 | 0.220 | 0.103 | 0.086 | 1.000 |
| Cumulative percentage variance of species data and species-environment relation: | 50.2 | 72.2 | 82.6 | 91.2 | |
| Variable | Lambda1 | LambdaA | P | F | |
| CNOL | 0.33 | 0.33 | *** | 4.89 | |
| γ-div | 0.25 | 0.16 | *** | 3.56 | |
| K | 0.22 | 0.16 | * | 2.95 | |
| CNtot | 0.19 | 0.09 | * | 2.38 | |
| %Nem | 0.18 | 0.06 | ns | 1.93 | |
| D_bact | 0.04 | 0.07 | ns | 2.33 | |
| QBS-ar | 0.16 | 0.05 | ns | 2.69 | |
| Paur | 0.11 | 0.03 | ns | 1.7 | |
| Alien | 0.16 | 0.03 | ns | 2.31 | |
| OCOL | 0.07 | 0.01 | ns | 1.62 | |
| OCOF | 0.16 | 0.01 | ns | 0 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gentili, R.; Ferrè, C.; Cardarelli, E.; Montagnani, C.; Bogliani, G.; Citterio, S.; Comolli, R. Comparing Negative Impacts of Prunus serotina, Quercus rubra and Robinia pseudoacacia on Native Forest Ecosystems. Forests 2019, 10, 842. https://doi.org/10.3390/f10100842
Gentili R, Ferrè C, Cardarelli E, Montagnani C, Bogliani G, Citterio S, Comolli R. Comparing Negative Impacts of Prunus serotina, Quercus rubra and Robinia pseudoacacia on Native Forest Ecosystems. Forests. 2019; 10(10):842. https://doi.org/10.3390/f10100842
Chicago/Turabian StyleGentili, Rodolfo, Chiara Ferrè, Elisa Cardarelli, Chiara Montagnani, Giuseppe Bogliani, Sandra Citterio, and Roberto Comolli. 2019. "Comparing Negative Impacts of Prunus serotina, Quercus rubra and Robinia pseudoacacia on Native Forest Ecosystems" Forests 10, no. 10: 842. https://doi.org/10.3390/f10100842
APA StyleGentili, R., Ferrè, C., Cardarelli, E., Montagnani, C., Bogliani, G., Citterio, S., & Comolli, R. (2019). Comparing Negative Impacts of Prunus serotina, Quercus rubra and Robinia pseudoacacia on Native Forest Ecosystems. Forests, 10(10), 842. https://doi.org/10.3390/f10100842






