Changes in the Profiles of Yield, Yield Component, Oil Content, and Citral Content in Litsea cubeba (Lour.) Persoon Following Foliar Fertilization with Zinc and Boron
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Plant Material and Treatment
2.3. Plant Measurements
2.3.1. Preparation of Fruit Sampling
2.3.2. Determination of the Fruit Essential Oil Percentage
2.3.3. Volatile Compounds Using GC-MS Analysis
2.4. Statistical Analysis
3. Results
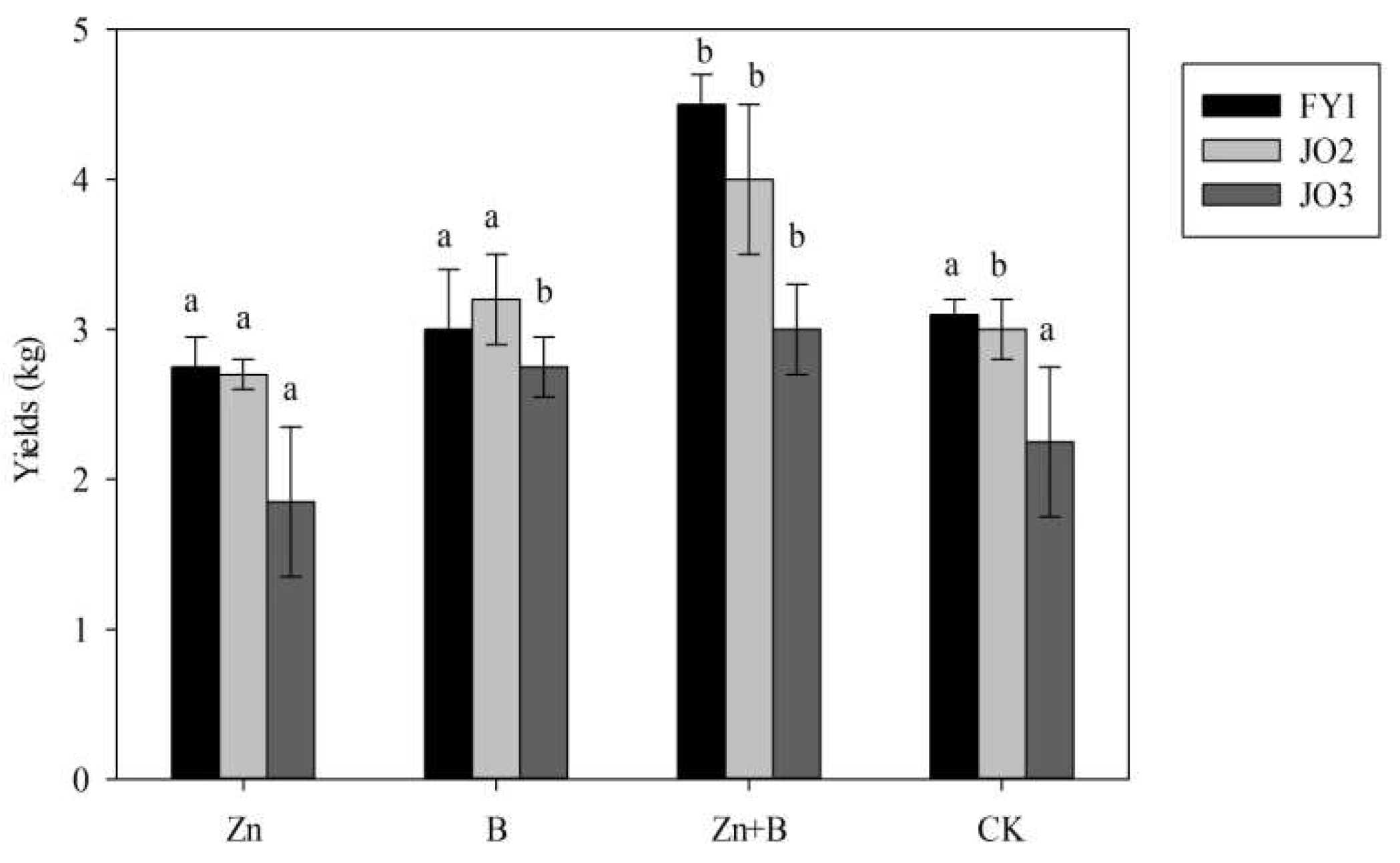
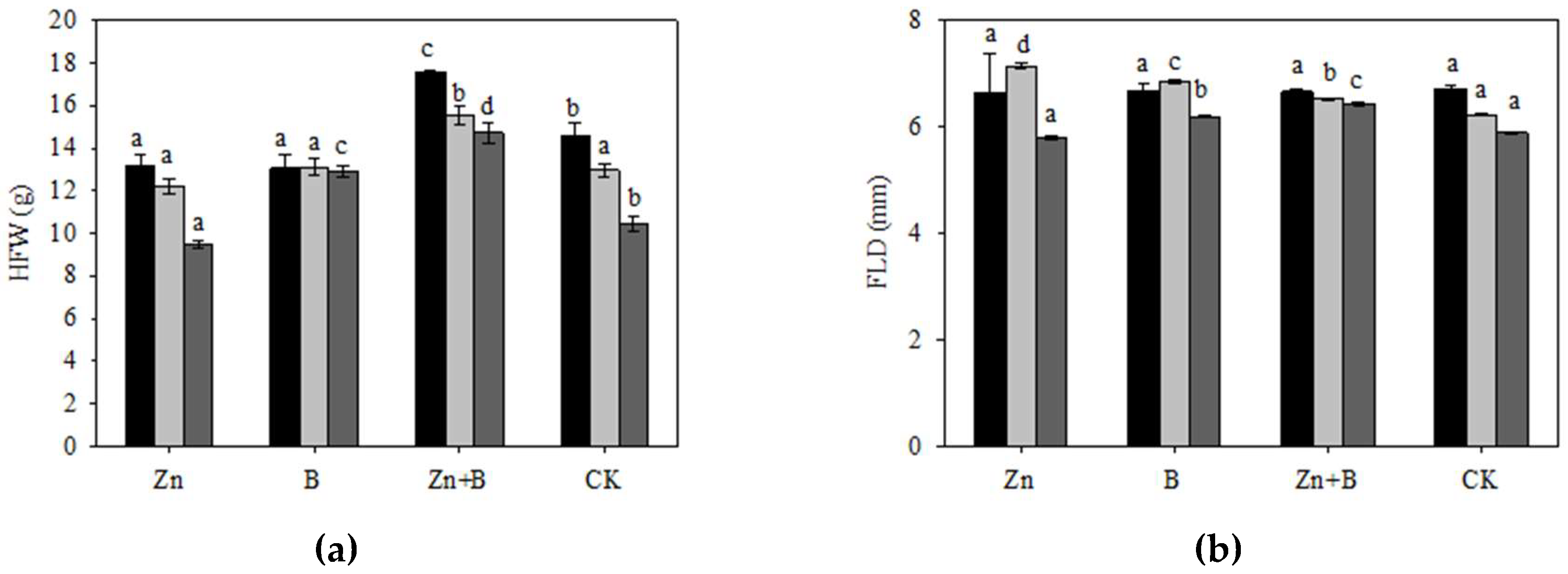
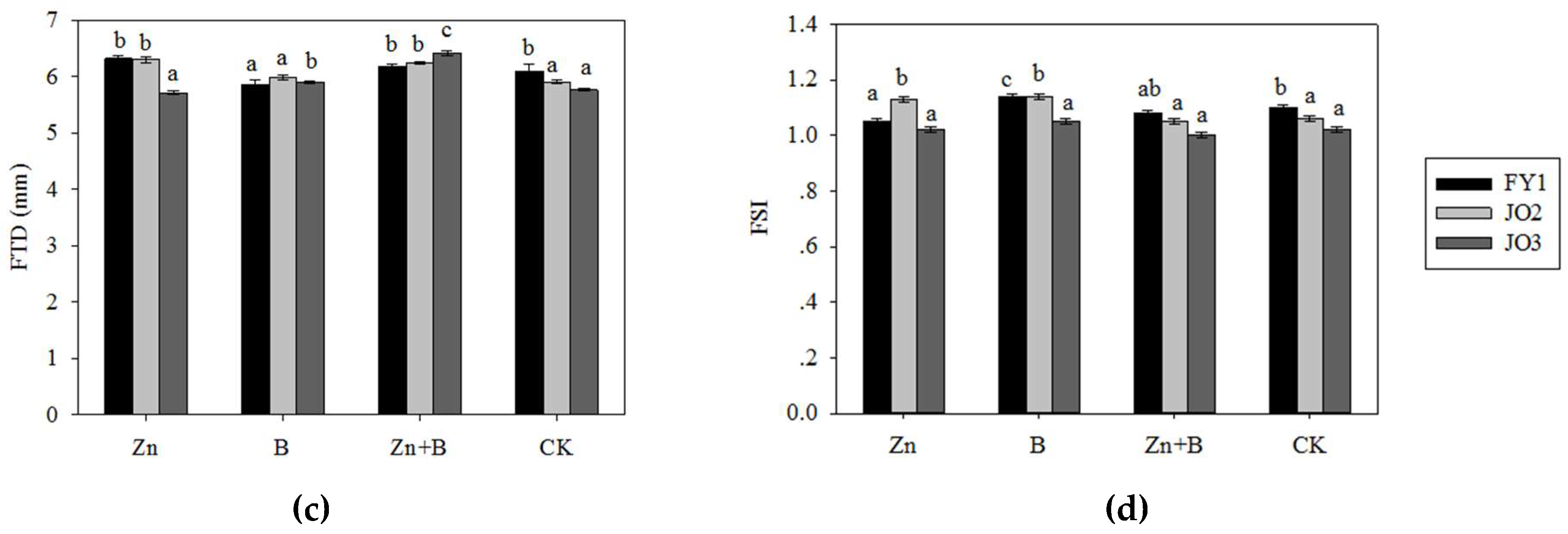
3.1. The Change in Yield and Yield Components during the Different Foliar Applications of Micronutrient Solutions
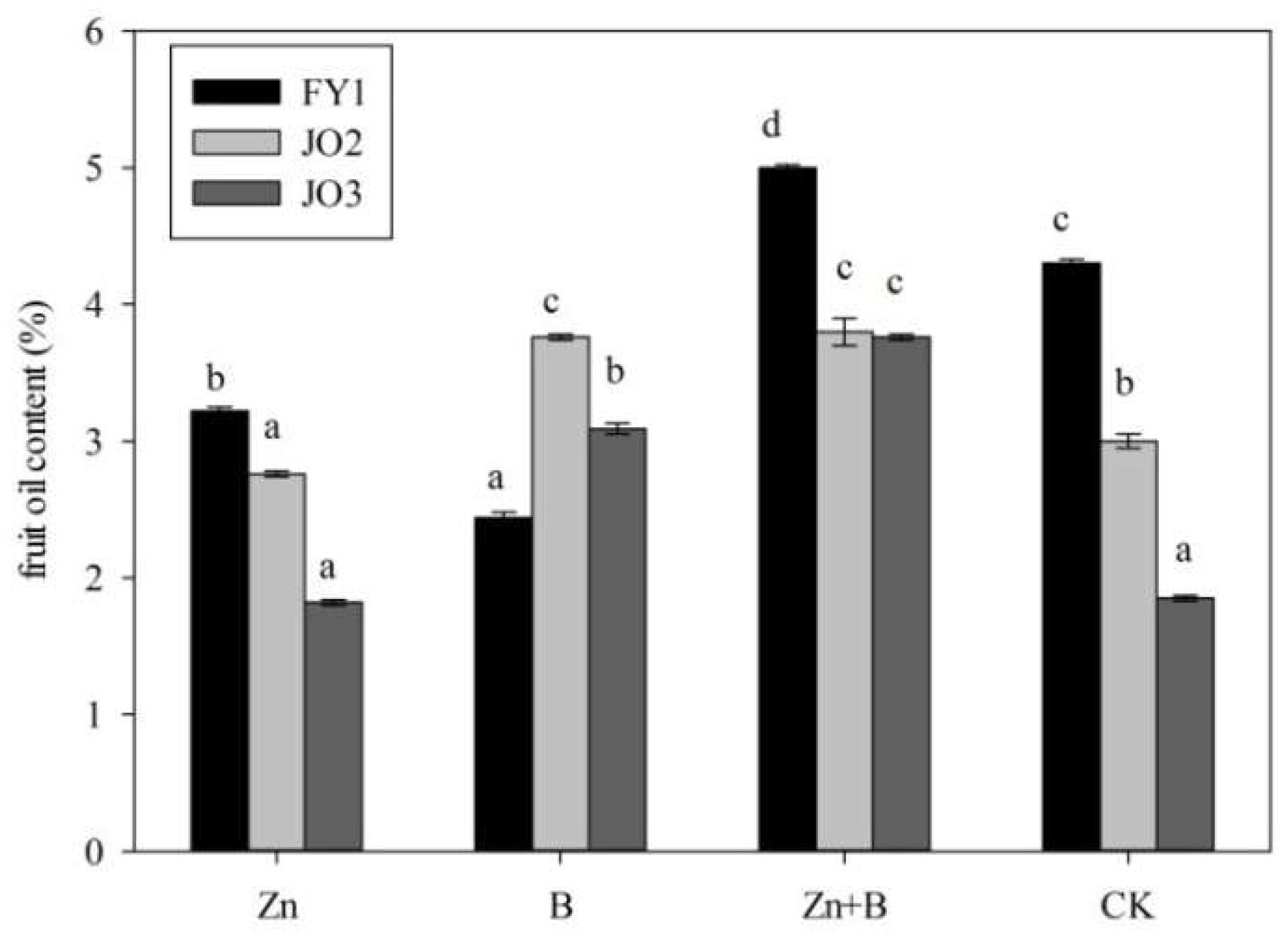
3.2. The Change in Fruit Oil Content during the Different Foliar Applications of Micronutrient Solutions
3.3. The Change in Citral Contents in the Oil during the Different Foliar Applications of Micronutrient Solutions
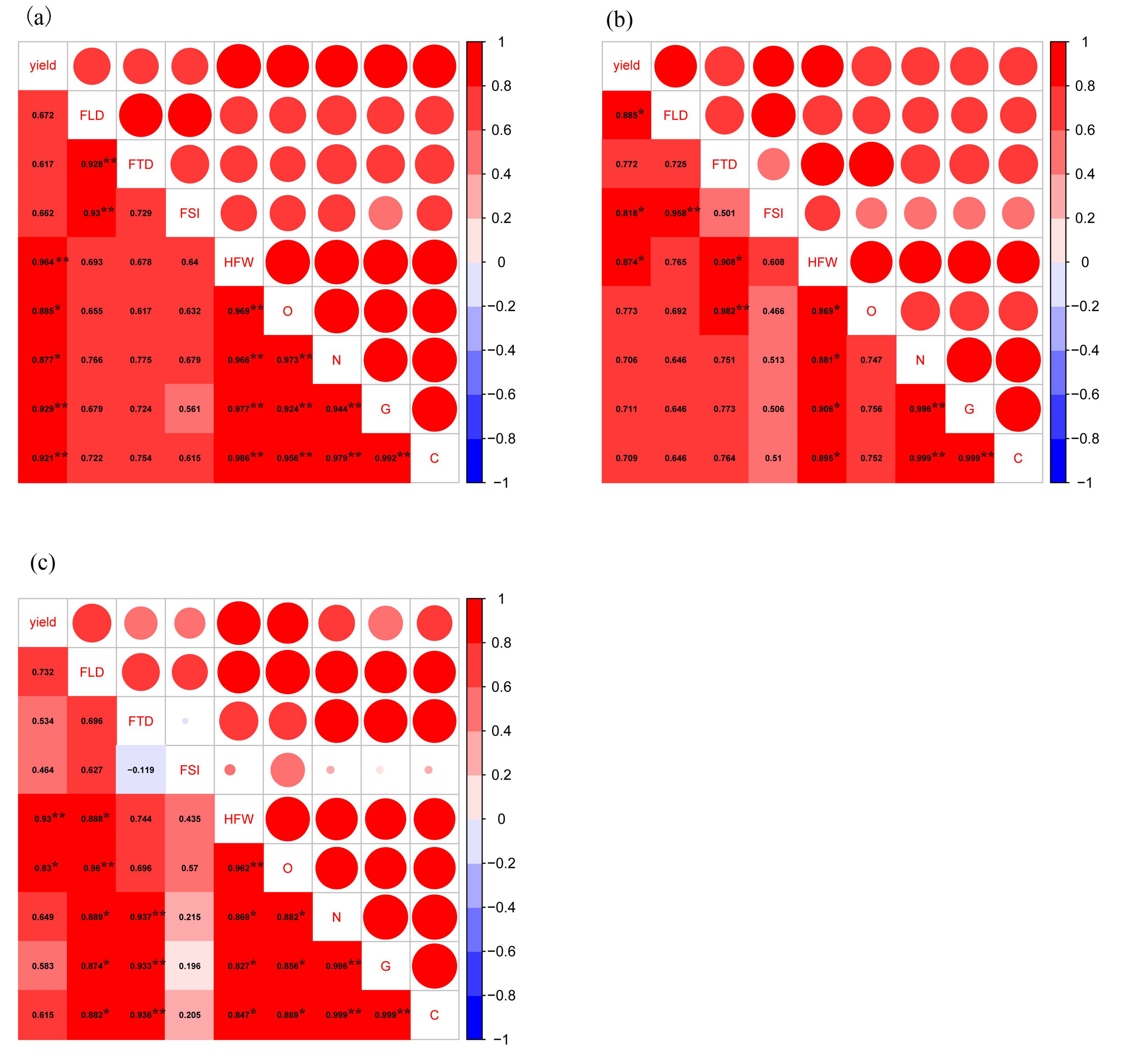
3.4. Correlations
4. Discussion
4.1. The Effect of Zn and B Foliar Applications on the Yield and Yield Components in L. cubeba
4.2. The Effect of Zn and B Foliar Applications on the Oil Content and Citral Contents in L. cubeba
4.3. The Tool to Select New Cultivars with High Yield, High Oil Content, and High Citral Content under Zn and B Foliar Applications
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, X.W. Angiospermae, Dicotyledoneae, Lauraceae, Hernandiaceae. In Florae Reipublicae Popularis Sinicae; Wu, Z.Y., Ed.; Science Press: Beijing, China, 2004; pp. 271–272. [Google Scholar]
- Si, L.L.; Chen, Y.C.; Han, X.J.; Zhan, Z.Y.; Tian, S.P.; Cui, Q.Q.; Wang, Y.D. Chemical composition of essential oils of Litsea cubeba harvested from its distribution areas in China. Molecules 2012, 17, 7057–7066. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.K.; Choi, E.M.; Lee, J.H. Antioxidant activity of Litsea cubeba. Fitoterapia 2005, 76, 684–686. [Google Scholar] [CrossRef] [PubMed]
- Pumnuan, J.; Chandrapatya, A.; Insung, A. Acaricidal activities of plant essential oils from three plants on the mushroom mite, Luciaphorus perniciosus Rack (Acari: Pygmephoridae). Pak. J. Zool. 2010, 42, 247–252. [Google Scholar]
- Liao, P.C.; Yang, T.S.; Chou, J.C.; Chen, J.; Lee, S.C.; Kuo, Y.H.; Ho, C.L.; Chao, L.K.P. Anti-inflammatory activity of neral and geranial isolated from fruits of Litsea cubeba Lour. J. Funct. Foods 2015, 19, 248–258. [Google Scholar] [CrossRef]
- Ho, C.L.; Jie-Pinge, O.; Liu, Y.C.; Hung, C.P.; Tsai, M.C.; Liao, P.C.; Wang, E.I.; Chen, Y.L.; Su, Y.C. Compositions and in vitro anticancer activities of the leaf and fruit oils of Litsea cubeba from Taiwan. Nat. Prod. 2010, 5, 617–620. [Google Scholar]
- Seal, S.; Chatterjee, P.; Bhattacharya, S.; Pal, D.; Dasgupta, S.; Kundu, R.; Mukherjee, S.; Bhattacharya, S.; Bhuyan, M.; Bhattacharyya, P.R. Vapor of volatile oils from Litsea cubeba seed induces apoptosis and causes cell cycle arrest in lung cancer cells. PLoS ONE 2012, 7, e47014. [Google Scholar] [CrossRef]
- Chen, H.C.; Chang, W.T.; Hseu, Y.C.; Chen, H.Y.; Chuang, C.; Lin, C.C.; Lee, M.S.; Lin, M.K. Immunosuppressive effect of Litsea cubeba L. essential oil on dendritic cell and contact hypersensitivity responses. Int. J. Mol. Sci. 2016, 17, 1319. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, J.; Qimei, L.; Yan, X.; Zhao, J.; Yuan, H.; Qin, Z.; Wang, M. The fungicidal terpenoids and essential oil from Litsea cubeba in Tibet. Molecules 2010, 15, 7075–7082. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, J.; Lv, W.; Zhao, Q.; Shi, G. Antibacterial, antifungal and cytotoxic isoquinoline alkaloids from Litsea cubeba. Molecules 2012, 17, 12950–12960. [Google Scholar] [CrossRef]
- Amer, A.; Mehlhorn, H. Repellency effect of forty-one essential oils against Aedes, Anopheles, and Culex mosquitoes. Parasitol. Res. 2006, 99, 478–490. [Google Scholar] [CrossRef]
- Seo, S.M.; Kim, J.; Lee, S.G.; Shin, C.H.; Shin, S.C.; Park, I.K. Fumigant antitermitic activity of plant essential oils and components from Ajowan (Trachyspermum ammi), Allspice (Pimenta dioica), caraway (Carum carvi), dill (Anethum graveolens), Geranium (Pelargonium graveolens), and Litsea (Litsea cubeba) oils against Japanese termite (Reticulitermes speratus Kolbe). J. Agric. Food Chem. 2009, 57, 6596–6602. [Google Scholar] [PubMed]
- Chen, Y.C.; Wang, Y.D.; Han, X.J.; Si, L.L.; Wu, Q.K.; Lin, L.L. Biology and chemistry of Litsea cubeba, a promising industrial tree in China. J. Essent. Oil Res. 2013, 25, 103–111. [Google Scholar] [CrossRef]
- Dordas, C.A.; Sioulas, C. Safflower yield, chlorophyll content, photosynthesis, and water use efficiency response to nitrogen fertilization under rainfed conditions. Ind. Crops Prod. 2008, 27, 75–85. [Google Scholar] [CrossRef]
- Santoni, F.; Paolini, J.; Barboni, T.; Costa, J. Relationships between the leaf and fruit mineral compositions of Actinidia deliciosa var. Hayward according to nitrogen and potassium fertilization. Food Chem. 2014, 147, 269–271. [Google Scholar] [CrossRef]
- Tekaya, M.; Mechri, B.; Cheheb, H.; Attia, F.; Chraief, I.; Ayachi, M.; Boujneh, D.; Hammami, M. Changes in the profiles of mineral elements, phenols, tocopherols and soluble carbohydrates of olive fruit following foliar nutrient fertilization. LWT Food Sci. Technol. 2014, 59, 1047–1053. [Google Scholar] [CrossRef]
- Ahmad, W.; Zia, M.H.; Malhi, S.S.; Niaz, A.; Saifullah. Boron deficiency in soils and crops: A review. In Crop Plant; Aakash, G., Ed.; InTech: Rijeka, Croatia, 2012; pp. 77–114. [Google Scholar]
- Chen, X.; Ludewig, U. Biomass increase under zinc deficiency caused by delay of early flowering in Arabidopsis. J. Exp. Bot. 2018, 69, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Jose Brandao-Neto, M.D.; Vivian Stefan, M.D.; Berenice, B.; Mendonca, M.D.; Walter Bloise, M.D.; Ana Valeria, B.; Castro, M.D. The essential role of zinc in growth. Nutr. Res. 1995, 15, 335–358. [Google Scholar] [CrossRef]
- Yoshida, S.; Tanaka, A. Zinc deficiency of the rice plant in calcareous soils. Soil Sci. Plant Nutr. 1969, 15, 75–80. [Google Scholar] [CrossRef]
- Han, S.; Chen, L.S.; Jiang, H.X.; Smith, B.R.; Yang, L.T.; Xie, C.Y. Boron deficiency decreases growth and photosynthesis, and increases starch and hexoses in leaves of citrus seedlings. J. Plant Physiol. 2008, 165, 1331–1341. [Google Scholar] [CrossRef]
- Widodo; Broadley, M.R.; Rose, T.; Frei, M.; Pariasca-Tanaka, J.; Yoshihashi, T.; Thomson, M.; Hammond, J.P.; Aprile, A.; Close, T.J.; et al. Response to zinc deficiency of two rice lines with contrasting tolerance is determined by root growth maintenance and organic acid exudation rates, and not by zinc-transporter activity. New Phytol. 2010, 186, 400–414. [Google Scholar] [CrossRef]
- Rosolem, C.A.; Bogiani, J.C. Physiology of boron stress in cotton. In Stress Physiology in Cotton; Oosterhuis, D.M., Ed.; The Cotton Foundation: Cordova, TN, USA, 2011; pp. 113–124. [Google Scholar]
- Bogiani, J.C.; Sampaio, T.F.; Abreu-Junior, C.H.; Rosolem, C.A. Boron uptake and translocation in some cotton cultivars. Plant Soil 2014, 375, 241–253. [Google Scholar] [CrossRef]
- Nanda, A.K.; Pujol, V.; Wissuwa, M. Patterns of stress response and tolerance based on transcriptome profiling of rice crown tissue under zinc deficiency. J. Exp. Bot. 2017, 68, 1715–1729. [Google Scholar] [CrossRef] [PubMed]
- Hamza, S.; Leela, N.K.; Srinivasan, V.; Nileena, C.R.; Dinesh, R. Influence of zinc on yield and quality profile of ginger (Zingiber officinale Rosc.). J. Spices Aromat. Crops 2013, 22, 91–94. [Google Scholar]
- Desouky, I.M.; Haggag, L.F.; Elmigeed, M.M.M.A.; Kishk, Y.F.M.; Elhady, E.S. Effect of boron and calcium nutrients sprays on fruit set, oil content and oil quality of some olive oil cultivars. World J. Agric. Sci. 2009, 5, 180–185. [Google Scholar]
- Wang, J.W.; Hui, M.; Zhao, H.B.; Huang, D.L.; Wang, Z.H. Different increases in maize and wheat grain zinc concentrations caused by soil and foliar applications of zinc in Loess Plateau, China. Field Crops Res. 2012, 135, 89–96. [Google Scholar] [CrossRef]
- Saadati, S.; Moallemi, N.; Mortazavi, S.M.H.; Seyyednejad, S.M. Effects of zinc and boron foliar application on soluble carbohydrate and oil contents of three olive cultivars during fruit ripening. Sci. Hortic. 2013, 164, 30–34. [Google Scholar] [CrossRef]
- Mei, L.; Li, Q.H.; Wang, H.; Sheng, O.; Peng, S.A. Boron deficiency affects root vessel anatomy and mineral nutrient allocation of Poncirus trifoliata (L.) Raf. Acta Physiol. Plant. 2016, 38, 86. [Google Scholar] [CrossRef]
- Kutman, U.B.; Kutman, B.Y.; Ceylan, Y.; Ova, E.A.; Cakmak, I. Contributions of root uptake and remobilization to grain zinc accumulation in wheat depending on post-anthesis zinc availability and nitrogen nutrition. Plant Soil 2012, 361, 177–187. [Google Scholar] [CrossRef]
- Wang, S.; Meng, L.; Tian, X.; Jin, L.; Li, H.; Ni, Y.; Zhao, J.; Chen, Y.; Guo, C.; Zhao, A. Foliar Zinc, nitrogen, and phosphorus application effects on micronutrient concentrations in winter wheat. Agron. J. 2015, 107, 61. [Google Scholar] [CrossRef]
- Gao, M.; Chen, Y.; Wang, Y. Evaluation of the yields and chemical compositions of the essential oils of different Litsea cubeba varieties. J. Essent. Oil Bear. Plants 2016, 19, 1888–1902. [Google Scholar] [CrossRef]
- Gao, M.; Lin, L.Y.; Chen, Y.C.; Wang, Y.D. Digital gene expression profiling to explore differentially expressed genes associated with terpenoid biosynthesis during fruit development in Litsea cubeba. Molecules 2016, 21, 1251. [Google Scholar] [CrossRef] [PubMed]
- Han, X.J.; Wang, Y.D.; Chen, Y.C.; Lin, L.Y.; Wu, Q.K. Transcriptome Sequencing and Expression Analysis of Terpenoid Biosynthesis Genes in Litsea cubeba. PLoS ONE 2013, 8, e76890. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shi, R.; Rezaul, K.M.; Zhang, F.; Zou, C. Iron and zinc concentrations in grain and flour of winter wheat as affected by foliar application. J. Agric. Food Chem. 2010, 58, 12268–12274. [Google Scholar] [CrossRef] [PubMed]
- Penfold, A.R.; Morrison, F.R.; Willis, J.L.; McKern, H.H.G.; Spies, M.C. The essential oil of a physiological form of Eucalyptus citriodora Hook. J. Proc. R. Soc. New South Wales 1951, 85, 120–122. [Google Scholar]
- Zhang, Y.Q.; Sun, Y.X.; Ye, Y.L.; Karim, M.R.; Xue, Y.F.; Yan, P.; Meng, Q.F.; Cui, Z.L.; Cakmak, I.; Zhang, F.S. Zinc biofortification of wheat through fertilizer applications in different locations of China. Field Crops Res. 2012, 125, 1–7. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, Q.; Tang, L. Geographical distribution of trace elements-deficient soils in China. Acta Pedol. Sin. 1982, 19, 209–223. [Google Scholar]
- Cakmak, I.M.; Kalayci, Y.; Kaya, A.A.; Torun, N.; Aydin, Y.; Wang, Z.; Arisoy, H.; Erdem, A.; Yazici, O.L.O.; et al. Biofortification and localization of zinc in wheat grain. J. Agric. Food Chem. 2010, 58, 9092–9102. [Google Scholar] [CrossRef]
- Cakmak, I.; Pfeiffer, W.H.; McClafferty, B. Biofortification of durum wheat with zinc and iron. Cereal Chem. 2010, 87, 10–20. [Google Scholar] [CrossRef]
- Rengel, Z.; Graham, R.D. Importance of seed Zn content for wheat growth on Zn-deficient soil. Plant Soil 1995, 173, 259–266. [Google Scholar] [CrossRef]
- Yilmaz, A.; Ekiz, H.; Gültekin, I.; Torun, B.; Barut, H.; Karanlik, S.; Cakmak, I. Effect of seed zinc content on grain yield and zinc concentration of wheat grown in zinc-deficient calcareous soils. J. Plant Nutr. 1998, 21, 2257–2264. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, Q.Q.; Tong, L.H. Boron-deficient soils and their distribution in China. Acta Pedol. Sin. 1980, 17, 228–239. [Google Scholar]
- Pallotta, M.; Schnurbusch, T.; Hayes, J.; Hay, A.; Baumann, U.; Paull, J.; Langridge, P.; Sutton, T. Molecular basis of adaptation to high soil boron in wheat landraces and elite cultivars. Nature 2014, 514, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, S.; Shekafandeh, A.; Taslimpour, M.R. Effect of GA3 and zinc sulfate on fruit yield and oil percentage of ‘Shengeh’ olive trees. Int. J. Fruit Sci. 2010, 10, 228–234. [Google Scholar] [CrossRef]
- Connor, D.J.; Fereres, E. The physiology of adaptation and yield expression in olive. Hortic. Rev. 2005, 31, 155–229. [Google Scholar]

| Characteristics | Soil Depth | |
|---|---|---|
| 0–30 cm | 30–60 cm | |
| pH (1:1 H2O) | 5.69 | 5.82 |
| Organic matter (g/kg) | 7.74 | 5.75 |
| Total N (g/kg) | 0.60 | 0.44 |
| Total P (g/kg) | 0.35 | 0.34 |
| Total K (g/kg) | 9.01 | 6.84 |
| POlsen (mg/kg) | 13.43 | 19.50 |
| K (exchangeable mg/kg) | 44.63 | 40.00 |
| Ca (g/kg) | 2.49 | 2.47 |
| Mg (g/kg) | 6.15 | 5.57 |
| S (mg/kg) | 398.67 | 213.33 |
| Cu (mg/kg) | 17.20 | 12.73 |
| Fe (g/kg) | 25.87 | 28.63 |
| Zn (mg/kg) | 92.83 | 74.07 |
| Available Zn(mg/kg) | 6.10 | 2.61 |
| B (mg/kg) | 22.10 | 21.40 |
| Available B (mg/kg) | 0.08 | 0.04 |
| Mn (g/kg) | 0.18 | 0.35 |
| Nutrition | FY1 | JO2 | JO3 |
|---|---|---|---|
| N (g/kg) | 24.6 | 27.2 | 26.5 |
| P (g/kg) | 5.05 | 2.55 | 2.46 |
| K (g/kg) | 10.7 | 16.9 | 15.8 |
| Ca (g/kg) | 7.86 | 8.94 | 8.47 |
| Mg (g/kg) | 2.29 | 2.25 | 2.27 |
| S (g/kg) | 1.85 | 2.13 | 2.01 |
| Cu (mg/kg) | 7.75 | 8.34 | 8.16 |
| Fe (g/kg) | 0.155 | 0.188 | 0.16 |
| Zn (mg/kg) | 32.9 | 42.6 | 39.4 |
| B (mg/kg) | 17.9 | 28.4 | 27.9 |
| Variation | Yield | HFW | Oil Content | Citral | Neral | Geranial | FLD | FTD | FSI |
|---|---|---|---|---|---|---|---|---|---|
| variety (V) | 38.63 ** | 42.60 ** | 416.19 ** | 1783.70 ** | 260.74 ** | 680.65 ** | 39.09 ** | 28.38 | 35.08 ** |
| fertilization (F) | 31.09 ** | 32.07 ** | 449.92 ** | 3253.98 ** | 602.73 ** | 1086.04 ** | 21.57 ** | 25.43 | 30.05 ** |
| V × F | 1.35 ns | 1.86 ns | 185.37 ** | 1482.95 ** | 249.10 ** | 538.89 ** | 1.05 ns | 1.15 ns | 1.34 ns |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, M.; Chen, Y.; Wu, L.; Wang, Y. Changes in the Profiles of Yield, Yield Component, Oil Content, and Citral Content in Litsea cubeba (Lour.) Persoon Following Foliar Fertilization with Zinc and Boron. Forests 2019, 10, 59. https://doi.org/10.3390/f10010059
Gao M, Chen Y, Wu L, Wang Y. Changes in the Profiles of Yield, Yield Component, Oil Content, and Citral Content in Litsea cubeba (Lour.) Persoon Following Foliar Fertilization with Zinc and Boron. Forests. 2019; 10(1):59. https://doi.org/10.3390/f10010059
Chicago/Turabian StyleGao, Ming, Yicun Chen, Liwen Wu, and Yangdong Wang. 2019. "Changes in the Profiles of Yield, Yield Component, Oil Content, and Citral Content in Litsea cubeba (Lour.) Persoon Following Foliar Fertilization with Zinc and Boron" Forests 10, no. 1: 59. https://doi.org/10.3390/f10010059
APA StyleGao, M., Chen, Y., Wu, L., & Wang, Y. (2019). Changes in the Profiles of Yield, Yield Component, Oil Content, and Citral Content in Litsea cubeba (Lour.) Persoon Following Foliar Fertilization with Zinc and Boron. Forests, 10(1), 59. https://doi.org/10.3390/f10010059




