Diagnosis and Management of Sexually Transmitted Infections Using Artificial Intelligence Applications Among Key and General Populations in Sub-Saharan Africa: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Questions
- What evidence exists regarding the effectiveness of AI technologies in the diagnosis and management of STIs among key and general populations in SSA, based on available studies and curated databases?
- What AI technologies are currently documented in the literature for use in diagnosing and managing STIs among key and general populations in SSA, and what are their reported applications and limitations?
- What barriers and opportunities, including infrastructure, computational support, and database requirements, are identified in the implementation of AI technologies for STI diagnosis and management among key and general populations in SSA?
2.2. The Methods and Approach
2.3. Search Strategy and Database Searches
2.4. Eligibility Criteria
2.5. Selection and Screening of Documents
2.6. Assessment of Quality
2.7. Data Extraction
2.8. Risk of Bias Assessments
2.9. Search Results
2.10. Quality and Bias Assessment
3. Results
3.1. Study Characteristics
3.2. The Artificial Intelligence Approaches Used in the Diagnosis and Management of Sexually Transmitted Infections
Classification of Artificial Intelligence Approaches Based on Implementation: Practical Use Versus Methodological
3.3. Study Comparators
3.4. Successes, Opportunities, and Challenges Related to AI Approaches in STI Diagnosis and Management
3.5. Best-Performing AI Approaches
3.6. Reported Challenges, Evaluations, and Future Directions for AI Technologies in Healthcare
3.6.1. Limitations Cited by the Reviewed Studies
3.6.2. Evaluation of Artificial Intelligence Approaches by the Reviewed Studies
3.6.3. Summary of Future Research Directions by the Reviewed Studies
3.7. Meta-Analysis
3.7.1. Descriptive Analysis
Uses of Artificial Intelligence and Machine Learning Approaches
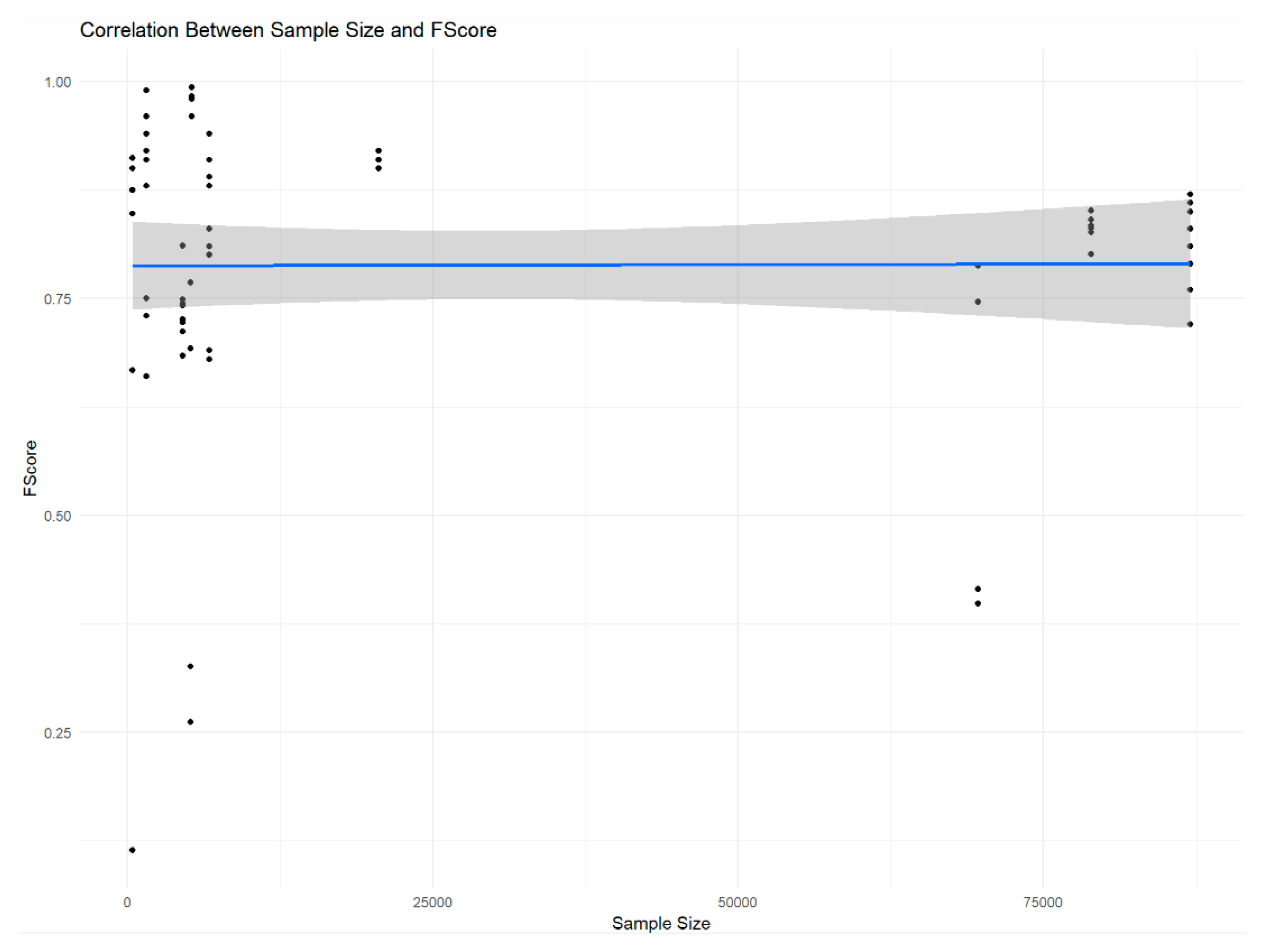
Sample Sizes and F1-Scores
Effects Modelling
Standardized Mean Differences
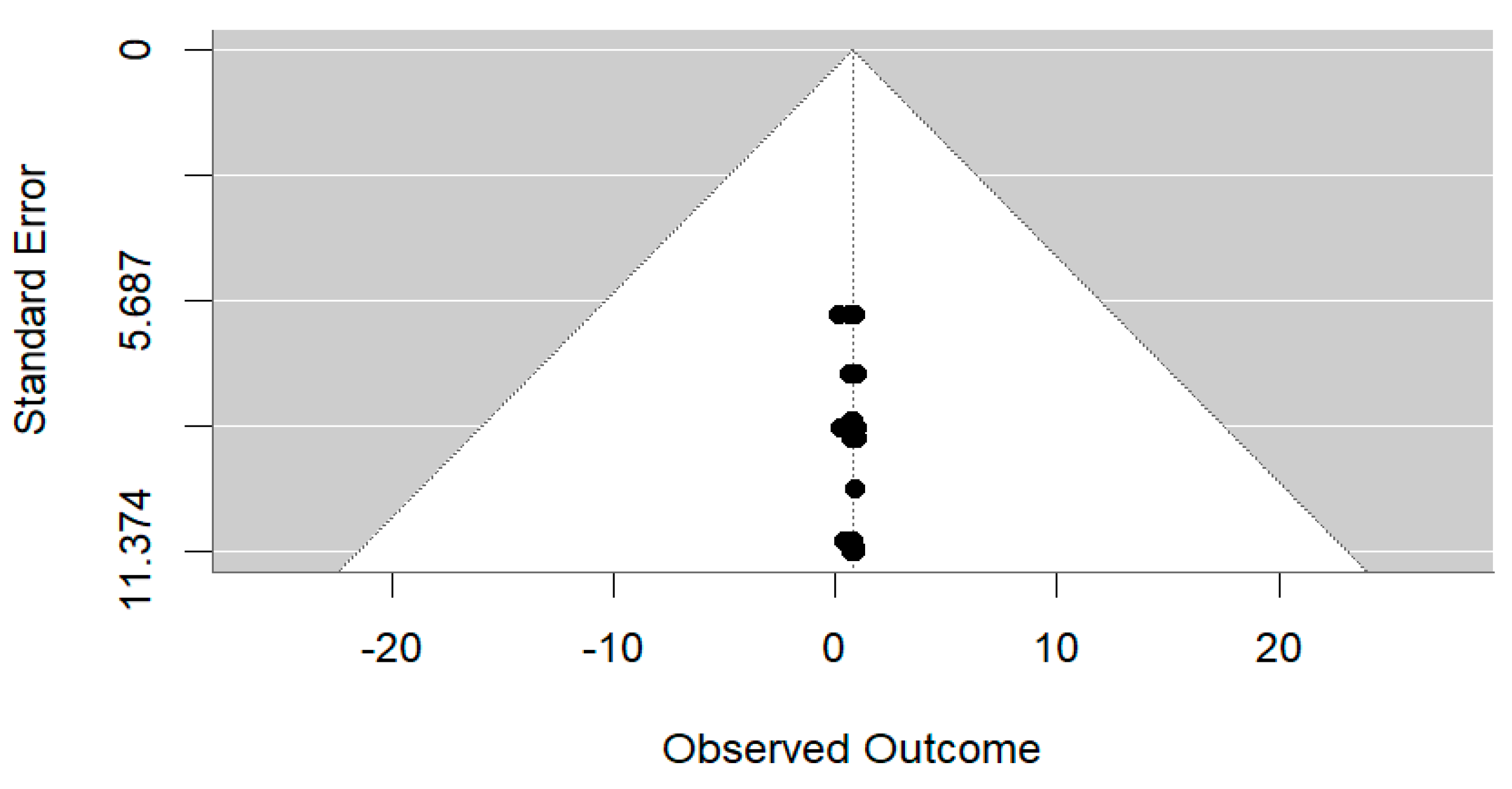
Forest Plot
3.7.2. Subgroup Analyses
4. Discussion
5. The Implications of AI Approaches in STI Diagnosis and Management
6. Strengths and Limitations
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Regional Action Plans for Ending AIDS and the Epidemics of Viral Hepatitis and Sexually Transmitted Infections 2022–2030; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Moyo, E.; Moyo, P.; Murewanhema, G.; Mhango, M.; Chitungo, I.; Dzinamarira, T. Key populations and Sub-Saharan Africa’s HIV response. Front. Public Health 2023, 11, 1079990. [Google Scholar] [CrossRef] [PubMed]
- Marcus, R.; Gill, K.; Smith, P.; Rouhani, S.; Mendelsohn, A.; Mendel, E.; Lince-Deroche, N.; Naidoo, K.; Ahmed, N.; Stirrup, O.; et al. Acceptability, feasibility, and cost of point of care testing for sexually transmitted infections among South African adolescents where syndromic management is standard of care. BMC Health Serv. Res. 2023, 23, 1078. [Google Scholar] [CrossRef] [PubMed]
- Moseley, P.; Bamford, A.; Eisen, S.; Lyall, H.; Kingston, M.; Thorne, C.; Piñera, C.; Rabie, H.; Prendergast, A.J.; Kadambari, S. Resurgence of congenital syphilis: New strategies against an old foe. Lancet Infect. Dis. 2024, 24, e24–e35. [Google Scholar] [CrossRef]
- Azeez, Z.F.; Al-Saadi, Z.H.A.; Masood, Q.K.A. Diagnostic Approaches for Sexually Transmitted Infections in Clinical Microbiology: A Comprehensive Review. Int. J. Med. Sci. Dent. Health 2024, 10, 1–16. [Google Scholar] [CrossRef]
- Vargas, S.; Calvo, G.; Qquellon, J.; Vasquez, F.; Blondeel, K.; Ballard, R.; Toskin, I. Point-of-care testing for sexually transmitted infections in low-resource settings. Clin. Microbiol. Infect. 2022, 28, 946–951. [Google Scholar] [CrossRef]
- Jarolimova, J.; Platt, L.R.; Curtis, M.R.; Philpotts, L.L.; Bekker, L.G.; Morroni, C.; Shahmanesh, M.; Mussa, A.; Barracks, K.; Ciaranello, A.L.; et al. Curable sexually transmitted infections among women with HIV in sub-Saharan Africa. AIDS 2022, 36, 697–709. [Google Scholar] [CrossRef]
- Coskun, A.F.; Topkaya, S.N.; Yetisen, A.K.; Cetin, A.E. Portable Multiplex Optical Assays. Adv. Opt. Mater. 2019, 7, 1801109. [Google Scholar] [CrossRef]
- Ahmadi, M.; Pahlavani, M.; Karimi, A.; Moradi, M.; Lawrence, J. The Impact of the Fourth Industrial Revolution on the Transitory Stage of the Automotive Industry. In Sustainable Manufacturing in Industry 40; Gholami, H., Abdul-Nour, G., Sharif, S., Streimikiene, D., Eds.; Springer Nature: Singapore, 2023; pp. 79–96. [Google Scholar] [CrossRef]
- Kaur, K.; Singh, C.; Kumar, Y. Diagnosis and Detection of Congenital Diseases in New-Borns or Fetuses Using Artificial Intelligence Techniques: A Systematic Review. Arch. Comput. Methods Eng. 2023, 30, 3031–3058. [Google Scholar] [CrossRef]
- Mbunge, E.; Batani, J. Application of deep learning and machine learning models to improve healthcare in sub-Saharan Africa: Emerging opportunities, trends and implications. Telemat. Inform. Rep. 2023, 11, 100097. [Google Scholar] [CrossRef]
- Oguamanam, C. Transition to the Fourth Industrial Revolution: Africa’s Science, Technology and Innovation Framework and Indigenous Knowledge Systems. Afr. J. Leg. Stud. 2022, 15, 1–37. [Google Scholar] [CrossRef]
- Mazibuko-Makena, Z. The potential of Fourth Industrial Revolution technologies to transform healthcare: The question of access for the marginalized. In Leap 4.0.: African Perspectives on the Fourth Industrial Revolution; Mapungubwe Institute for Strategic Reflection (MISTRA): Sandton, South Africa, 2021. [Google Scholar]
- Muehlematter, U.J.; Bluethgen, C.; Vokinger, K.N. FDA-cleared artificial intelligence and machine learning-based medical devices and their 510(k) predicate networks. Lancet Digit. Health 2023, 5, e618–e626. [Google Scholar] [CrossRef]
- Ahmed, Z.; Mohamed, K.; Zeeshan, S.; Dong, X. Artificial intelligence with multi-functional machine learning platform development for better healthcare and precision medicine. Database 2020, 2020, baaa010. [Google Scholar] [CrossRef] [PubMed]
- Bamrah, J.; Kumar, R.; Kapur, S. Artificial Intelligence in Medicine: Friend or Foe. Sushruta J. Health Policy Opin. 2023, 15. [Google Scholar] [CrossRef]
- Spours, K. Transitioning vocational education and training in Africa: A social skills ecosystem perspective, VET Africa 4.0 Collective, Bristol University Press, ISBN: 978-1-5292-2463-4 paperback. J. Vocat. Educ. Train. 2024, 76, 747–755. [Google Scholar] [CrossRef]
- Prinsloo, P.; Kaliisa, R. Data privacy on the African continent: Opportunities, challenges and implications for learning analytics. Br. J. Educ. Technol. 2022, 53, 894–913. [Google Scholar] [CrossRef]
- Owoyemi, A.; Owoyemi, J.; Osiyemi, A.; Boyd, A. Artificial Intelligence for Healthcare in Africa. Front. Digit Health 2020, 2, 6. [Google Scholar] [CrossRef]
- Ahmad, H.F.; Rafique, W.; Rasool, R.U.; Alhumam, A.; Anwar, Z.; Qadir, J. Leveraging 6G, extended reality, and IoT big data analytics for healthcare: A review. Comput. Sci Rev. 2023, 48, 100558. [Google Scholar] [CrossRef]
- Donoghue, D.; Higgins, E.O. What Are the Sustainable Development Goals? In Value Creation for a Sustainable World: Innovating for Ecological Regeneration and Human Flourishing; Palgrave Macmillan: London, UK, 2023. [Google Scholar] [CrossRef]
- Muigua, K. Ensuring Healthy Lives and Well-being for All Kenyans. J. Confl. Manag. Sustain. Dev. 2021, 6, 142–183. [Google Scholar]
- Albuquerque, G.; Fernandes, F.; Barbalho, I.M.P.; Barros, D.M.S.; Morais, P.S.G.; Morais, A.H.F.; Santos, M.M.; Galvão-Lima, L.J.; Sales-Moioli, A.I.L.; Santos, J.P.Q.; et al. Computational methods applied to syphilis: Where are we, and where are we going? Front. Public Health 2023, 11, 1201725. [Google Scholar] [CrossRef]
- Mhlanga, D. Exploring the Evolution of Artificial Intelligence and the Fourth Industrial Revolution an Overview. In FinTech and Artificial Intelligence for Sustainable Development; Springer Nature: Cham, Switzerland, 2023; pp. 15–39. (Sustainable Development Goals Series). [Google Scholar] [CrossRef]
- Hossain, K.A. Analysis of present and future use of artificial intelligence (AI) in line with the Fourth Industrial Revolution (4IR). Sci. Res. J. 2023, 11, 1–50. [Google Scholar]
- Wang, D.W. Reaching Your New Digital Heights: 32 Pivotal Mindset Leaps of Digital Transformation, 1st ed.; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar] [CrossRef]
- Sarkis-Onofre, R.; Catalá-López, F.; Aromataris, E.; Lockwood, C. How to properly use the PRISMA Statement. Syst. Rev. 2021, 10, 117. [Google Scholar] [CrossRef]
- World Health Organization. Consolidated Guidelines on HIV Prevention, Testing, Treatment, Service Delivery, and Monitoring: Recommendations for a Public Health Approach; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- National Department of Health. South Africa’s National Strategic Plan for HIV, TB and STIs 2017–2022; National Department of Health: Pretoria, South Africa, 2017. [Google Scholar]
- Ahmad, K.; Abdelrazek, M.; Arora, C.; Bano, M.; Grundy, J. Requirements engineering for artificial intelligence systems: A systematic mapping study. Inf. Softw. Technol. 2023, 158, 107176. [Google Scholar] [CrossRef]
- Darraj, A.; Hudays, A.; Hazazi, A.; Hobani, A.; Alghamdi, A. The Association between Emergency Department Overcrowding and Delay in Treatment: A Systematic Review. Healthcare 2023, 11, 385. [Google Scholar] [CrossRef]
- Uddin, H.; Hasan, M.K.; Castro-Delgado, R. Effects of mass casualty incidents on anxiety, depression and PTSD among doctors and nurses: A systematic review protocol. BMJ Open 2023, 13, e075478. [Google Scholar] [CrossRef]
- Long, H.A.; French, D.P.; Brooks, J.M. Optimising the value of the critical appraisal skills programme (CASP) tool for quality appraisal in qualitative evidence synthesis. Res. Methods Med. Health Sci. 2020, 1, 31–42. [Google Scholar] [CrossRef]
- Andaur Navarro, C.L.; Damen, J.A.A.; Takada, T.; Nijman, S.W.J.; Dhiman, P.; Ma, J.; Collins, G.S.; Bajpai, R.; Riley, R.D.; Moons, K.G.; et al. Completeness of reporting of clinical prediction models developed using supervised machine learning: A systematic review. BMC Med. Res. Methodol. 2022, 22, 12. [Google Scholar] [CrossRef]
- Farrah, K.; Young, K.; Tunis, M.C.; Zhao, L. Risk of bias tools in systematic reviews of health interventions: An analysis of PROSPERO-registered protocols. Syst. Rev. 2019, 8, 280. [Google Scholar] [CrossRef]
- Schmidt, L.; Sinyor, M.; Webb, R.T.; Marshall, C.; Knipe, D.; Eyles, E.C.; John, A.; Gunnell, D.; Higgins, J.P.T. A narrative review of recent tools and innovations toward automating living systematic reviews and evidence syntheses. Z. Für Evidenz Fortbild. Und Qual. Im Gesundheitswesen 2023, 181, 65–75. [Google Scholar] [CrossRef]
- Adeboye, N.O.; Bashiru, K.A.; Afolabi, H.A.; Ojurongbe, T. Diagnosing Sexually Transmitted Disease from Some Symptoms Using Machine Learning Models. J. Stat. Model Anal. 2023, 5, 65–80. [Google Scholar] [CrossRef]
- Balzer, L.B.; Havlir, D.V.; Kamya, M.R.; Chamie, G.; Charlebois, E.D.; Clark, T.D.; Koss, C.A.; Kwarisiima, D.; Ayieko, J.; Sang, N.; et al. Machine Learning to Identify Persons at High-Risk of Human Immunodeficiency Virus Acquisition in Rural Kenya and Uganda. Clin. Infect. Dis. 2020, 71, 2326–2333. [Google Scholar] [CrossRef] [PubMed]
- Alie, M.S.; Negesse, Y. Machine learning prediction of adolescent HIV testing services in Ethiopia. Front. Public Health 2024, 12, 1341279. [Google Scholar] [CrossRef]
- Alzubaidi, L.; Al-Sabaawi, A.; Bai, J.; Dukhan, A.; Alkenani, A.H.; Al-Asadi, A.; Alwzwazy, H.A.; Manoufali, M.; Fadhel, M.A.; Albahri, A.S.; et al. Towards risk-free trustworthy artificial intelligence: Significance and requirements. Int. J. Intell. Syst. 2023, 2023, 4459198. [Google Scholar] [CrossRef]
- Belete, D.M.; Huchaiah, M.D. A Deep Learning Approaches for Modeling and Predicting of HIV Test Results Using EDHS Dataset. In Future Opportunities and Tools for Emerging Challenges for HIV/AIDS Control; Okware, S., Ed.; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Birri Makota, R.B.; Musenge, E. Predicting HIV infection in the decade (2005–2015) pre-COVID-19 in Zimbabwe: A supervised classification-based machine learning approach. PLoS Digit Health 2023, 2, e0000260. [Google Scholar] [CrossRef]
- Chikusi, H. Machine Learning Model for Prediction and Visualization of HIV Index Testing in Northern Tanzania. Master’s Thesis, Nelson Mandela African Institution of Science and Technology, Arusha, Tanzania, 2022. [Google Scholar] [CrossRef]
- Chingombe, I.; Musuka, G.; Mbunge, E.; Chemhaka, G.; Cuadros, D.F.; Murewanhema, G.; Chaputsira, S.; Batani, J.; Muchemwa, B.; Mapingure, M.P.; et al. Predicting HIV Status Using Machine Learning Techniques and Bio-Behavioural Data from the Zimbabwe Population-Based HIV Impact Assessment (ZIMPHIA15-16). In Artificial Intelligence Trends in Systems; Silhavy, R., Ed.; Lecture Notes in Networks and Systems; Springer International Publishing: Cham, Switzerland, 2022; Volume 502, pp. 247–258. [Google Scholar] [CrossRef]
- Chingombe, I.; Dzinamarira, T.; Cuadros, D.; Mapingure, M.P.; Mbunge, E.; Chaputsira, S.; Madziva, R.; Chiurunge, P.; Samba, C.; Herrera, H.; et al. Predicting HIV Status among Men Who Have Sex with Men in Bulawayo & Harare, Zimbabwe Using Bio-Behavioural Data, Recurrent Neural Networks, and Machine Learning Techniques. Trop. Med. Infect. Dis. 2022, 7, 231. [Google Scholar] [CrossRef] [PubMed]
- Esra, R.; Carstens, J.; Le Roux, S.; Mabuto, T.; Eisenstein, M.; Keiser, O.; Orel, E.; Merzouki, A.; De Voux, L.; Maskew, M.; et al. Validation and Improvement of a Machine Learning Model to Predict Interruptions in Antiretroviral Treatment in South Africa. JAIDS J. Acquir. Immune Defic. Syndr. 2023, 92, 42–49. [Google Scholar] [CrossRef]
- Laybohr Kamara, I.; Wang, L.; Guo, Y.; Huo, S.; Guo, Y.; Xu, C.; Liao, Y.; Liu, W.J.; Ma, W.; Gao, G.F. Spatial–temporal heterogeneity and determinants of HIV prevalence in the Mano River Union countries. Infect. Dis. Poverty 2022, 11, 116. [Google Scholar] [CrossRef] [PubMed]
- Mamo, D.N.; Yilma, T.M.; Fekadie, M.; Sebastian, Y.; Bizuayehu, T.; Melaku, M.S.; Walle, A.D. Machine learning to predict virological failure among HIV patients on antiretroviral therapy in the University of Gondar Comprehensive and Specialized Hospital, in Amhara Region, Ethiopia, 2022. BMC Med. Inform. Decis. Mak. 2023, 23, 75. [Google Scholar] [CrossRef]
- Maskew, M.; Sharpey-Schafer, K.; De Voux, L.; Crompton, T.; Bor, J.; Rennick, M.; Chirowodza, A.; Miot, J.; Molefi, S.; Onaga, C.; et al. Applying machine learning and predictive modeling to retention and viral suppression in South African HIV treatment cohorts. Sci. Rep. 2022, 12, 12715. [Google Scholar] [CrossRef]
- Mitiku, Z.T. Developing Predictive Model for HIV Status in Index Case Testing Among Contacts Using Machine Learning. Doctoral Dissertation, Faculty of Computing, School of Research and Postgraduate Studies, Bahir Dar Institute of Technology, Bahir Dar University, Bahir Dar, Ethiopia, 2023. [Google Scholar]
- Mutai, C.K.; McSharry, P.E.; Ngaruye, I.; Musabanganji, E. Use of unsupervised machine learning to characterise HIV predictors in sub-Saharan Africa. BMC Infect Dis. 2023, 23, 482. [Google Scholar] [CrossRef]
- Mutai, C.K.; McSharry, P.E.; Ngaruye, I.; Musabanganji, E. Use of machine learning techniques to identify HIV predictors for screening in sub-Saharan Africa. BMC Med. Res. Methodol. 2021, 21, 159. [Google Scholar] [CrossRef] [PubMed]
- Oladokun, O.O. Predicting HIV Status Among Women in South Africa Using Machine Learning: Comparing Decision Tree Model and Logistic Regression. Master Thesis, Faculty of Humanities, University of the Witwatersrand, Johannesburg, South Africa, 2019. [Google Scholar]
- Orel, E.; Esra, R.; Estill, J.; Marchand-Maillet, S.; Merzouki, A.; Keiser, O. Machine Learning to Identify Socio-Behavioural Predictors of HIV Positivity in East and Southern Africa. medRxiv 2020. Available online: http://medrxiv.org/lookup/doi/10.1101/2020.01.27.20018242 (accessed on 2 July 2024).
- Turbé, V.; Herbst, C.; Mngomezulu, T.; Meshkinfamfard, S.; Dlamini, N.; Mhlongo, T.; Smit, T.; Cherepanova, V.; Shimada, K.; Budd, J.; et al. Deep learning of HIV field-based rapid tests. Nat. Med. 2021, 27, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Van Heerden, A.; Ntinga, X.; Vilakazi, K. The potential of conversational agents to provide a rapid HIV counseling and testing services. In Proceedings of the 2017 International Conference on the Frontiers and Advances in Data Science (FADS), Xi’an, China, 23–25 October 2017; pp. 80–85. [Google Scholar] [CrossRef]
- Adedimeji, A.; Sinayobye, J.d.; Asiimwe-Kateera, B.; Chaudhry, J.; Buzinge, L.; Gitembagara, A.; Murenzi, G.; Mugenzi, P.; Patel, V.V.; Castle, P.E.; et al. Social contexts as mediator of risk behaviors in Rwandan men who have sex with men (MSM): Implications for HIV and STI transmission. PLoS ONE 2019, 14, e0211099. [Google Scholar] [CrossRef]
- Hicks, S.A.; Strümke, I.; Thambawita, V.; Hammou, M.; Riegler, M.A.; Halvorsen, P.; Parasa, S. On evaluation metrics for medical applications of artificial intelligence. Sci. Rep. 2022, 12, 5979. [Google Scholar] [CrossRef]
- Chen, A.; Chan, Y.K.; Mocumbi, A.O.; Ojji, D.B.; Waite, L.; Beilby, J.; Codde, J.; Dobe, I.; Nkeh-Chungag, B.N.; Damasceno, A.; et al. Hypertension among people living with human immunodeficiency virus in sub-Saharan Africa: A systematic review and meta-analysis. Sci. Rep. 2024, 14, 16858. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, F. Innovation in Africa: Levelling the Playing Field to Promote Technology Transfer; Oxford University Press: Oxford, UK, 2024. [Google Scholar]
- Kraemer-Mbula, E.; Hanlin, R.; Byrne, R.; Daniels, C.; Kingiri, A. Transformative Innovation in Times of Change: Lessons for Africa from the 2020 Global Pandemic; African Books Collective: Oxford, UK, 2024. [Google Scholar]
- Adetokunboh, O.O.; Are, E.B. Spatial distribution and determinants of HIV high burden in the Southern African sub-region. PLoS ONE 2024, 19, e0301850. [Google Scholar] [CrossRef]
- Rosenberg, N.E.; Shook-Sa, B.E.; Liu, M.; Stranix-Chibanda, L.; Yotebieng, M.; Sam-Agudu, N.A.; Hudgens, M.G.; Phiri, S.J.; Mutale, W.; Bekker, L.G.; et al. Adult HIV-1 incidence across 15 high-burden countries in sub-Saharan Africa from 2015 to 2019: A pooled analysis of nationally representative data. Lancet HIV 2023, 10, e175–e185. [Google Scholar] [CrossRef]
- McGlonn, K.L. HIV Outcome Relationships Among Gender Inequality, Poverty, and Population Growth in Africa. Doctoral Dissertation, Florida Agricultural and Mechanical University, Tallahassee, FL, USA, 2023. [Google Scholar]
- Bullock, E.L.; Healey, S.P.; Yang, Z.; Oduor, P.; Gorelick, N.; Omondi, S.; Ouko, E.; Cohen, W.B. Three Decades of Land Cover Change in East Africa. Land 2021, 10, 150. [Google Scholar] [CrossRef]
- Adebowale, A.; Salawu, A.; Fagbamigbe, A.; Khasakhala, A.; Palamuleni, M.; Fawole, O. Demographic and epidemiological transitions and burden of adolescent healthcare in sub-Saharan Africa: A review. Afr. J. Reprod. Health 2023, 27, 109–126. [Google Scholar] [CrossRef]
- Johnson, K. HIV and Aids in Africa. In Oxford Research Encyclopedia of African History; Oxford University Press: Oxford, UK, 2021. [Google Scholar]
- Obeagu, E.I.; Alum, E.U.; Obeagu, G.U. Factors Associated with Prevalence of HIV Among Youths: A Review of Africa Perspective. Madonna Univ. J. Med. Health Sci. 2023, 3, 13–18. [Google Scholar]
- Signé, L. Strategies for Effective Health Care for Africa in the Fourth Industrial Revolution: Bridging the Gap Between the Promise and Delivery; Africa Growth Initiative at Brookings, 2021; Available online: https://www.brookings.edu/articles/strategies-for-effective-health-care-for-africa-in-the-fourth-industrial-revolution/ (accessed on 5 October 2021).
- Yah, C.S.; Ndlovu, S.; Kutywayo, A.; Naidoo, N.; Mahuma, T.; Mullick, S. The prevalence of pregnancy among adolescent girls and young women across the Southern African development community economic hub: A systematic review and meta-analysis. Health Promot. Perspect. 2020, 10, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.Y.; Skirbekk, V.F.; Tyrovolas, S.; Kassebaum, N.J.; Dieleman, J.L. Measuring population ageing: An analysis of the Global Burden of Disease Study 2017. Lancet Public Health 2019, 4, e159–e167. [Google Scholar] [CrossRef]
- Prudden, H. Determinants of Population Variability in HIV Across West Africa: Ecological and Mathematical Modelling Analyses. Doctoral Dissertation, London School of Hygiene & Tropical Medicine, London, UK, 2016. Available online: http://researchonline.lshtm.ac.uk/id/eprint/2634790 (accessed on 28 October 2024).
- Acharya, A.; Kumar, N.; Singh, K.; Byrareddy, S.N. Mpox in MSM: Tackling Stigma, Minimizing Risk Factors, Exploring Pathogenesis, and Treatment Approaches. Biomed. J. 2024, 48, 100746. [Google Scholar] [CrossRef]
- Tong, L.; Shi, W.; Isgut, M.; Zhong, Y.; Lais, P.; Gloster, L.; Sun, J.; Swain, A.; Giuste, F.; Wang, M.D. Integrating multi-omics data with EHR for precision medicine using advanced artificial intelligence. IEEE Rev. Biomed. Eng. 2023, 17, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Alderman, J.E.; Palmer, J.; Ganapathi, S.; Laws, E.; McCradden, M.D.; Oakden-Rayner, L.; Pfohl, S.R.; Ghassemi, M.; McKay, F.; et al. The value of standards for health datasets in artificial intelligence-based applications. Nat Med. 2023, 29, 2929–2938. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ali, H.; Nikberg, N. Bias in AI-Driven Healthcare: Navigating Ethical Challenges at an Early Stage. Master Dissertation, Linnaeus University, Växjö, Sweden, 2024. Available online: https://www.diva-portal.org/smash/record.jsf?dswid=5737&pid=diva2%3A1901624 (accessed on 28 October 2024).
- Murphy, A.; Bowen, K.; Naqa, I.M.E.; Yoga, B.; Green, B.L. Bridging Health Disparities in the Data-Driven World of Artificial Intelligence: A Narrative Review. J. Racial Ethn. Health Disparities 2024, 1–13. [Google Scholar] [CrossRef]
- Drokow, E.K.; Baffour, A.A.; Effah, C.Y.; Agboyibor, C.; Akpabla, G.S.; Sun, K. Building a Predictive Model to Assist in the Diagnosis of Cervical Cancer. Future Oncol. 2022, 18, 67–84. [Google Scholar] [CrossRef]
- Hashemi, S.; Yousefzadeh, Z.; Abin, A.A.; Ejmalian, A.; Nabavi, S.; Dabbagh, A. Machine Learning-Guided Anesthesiology: A Review of Recent Advances and Clinical Applications. J. Cell. Mol. Anesth. 2024, 9, e145369. Available online: https://brieflands.com/articles/jcma-145369 (accessed on 28 October 2024). [CrossRef]
- Houssein, E.H.; Sayed, A. Dynamic Candidate Solution Boosted Beluga Whale Optimization Algorithm for Biomedical Classification. Mathematics 2023, 11, 707. [Google Scholar] [CrossRef]
- He, S.; Leanse, L.G.; Feng, Y. Artificial intelligence and machine learning assisted drug delivery for effective treatment of infectious diseases. Adv. Drug Deliv. Rev. 2021, 178, 113922. [Google Scholar] [CrossRef]
- Mahameed, A.I.; Mahmood, R.K. Prediction of Sexually Transmitted Diseases Using Deep Convolutional Neural Networks. In Forthcoming Networks and Sustainability in the AIoT Era: Second International Conference FoNeS-AIoT 2024-Volume 1; Springer Nature: Berlin/Heidelberg, Germany, 2024; Volume 1, p. 401. [Google Scholar] [CrossRef]
- Latt, P.M.; Soe, N.N.; Xu, X.; Ong, J.J.; Chow, E.P.; Fairley, C.K.; Zhang, L. Identifying Individuals at High Risk for HIV and Sexually Transmitted Infections with an Artificial Intelligence–Based Risk Assessment Tool. In Open Forum Infectious Diseases; Oxford University Press: Oxford, UK, 2024; Volume 11, p. ofae011. [Google Scholar] [CrossRef]
- Nadarzynski, T.; Knights, N.; Husbands, D.; Graham, C.A.; Llewellyn, C.D.; Buchanan, T.; Montgomery, I.; Khlafa, N.; Tichackova, J.; Odeyemi, R.; et al. The impact of Chatbot-Assisted Self Assessment (CASA) on intentions for sexual health screening in people from minoritised ethnic groups at risk of sexually transmitted infections. Sex Health 2024, 21, SH24058. [Google Scholar] [CrossRef] [PubMed]
- Roche, S.D.; Ekwunife, O.I.; Mendonca, R.; Kwach, B.; Omollo, V.; Zhang, S.; Ongwen, P.; Hattery, D.; Smedinghoff, S.; Morris, S.; et al. Measuring the performance of computer vision artificial intelligence to interpret images of HIV self-testing results. Front. Public Health 2024, 12, 1334881. [Google Scholar] [CrossRef] [PubMed]
- Haug, C.J.; Drazen, J.M. Artificial Intelligence and Machine Learning in Clinical Medicine, 2023. N. Engl. J. Med. 2023, 388, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.; Potdevin, Y.; Mohammadi, E.; Zidowitz, S.; Breyer, S.; Nowotka, D.; Henn, S.; Pechmann, L.; Leucker, M.; Rostalski, P.; et al. Responsible and Regulatory Conform Machine Learning for Medicine: A Survey of Challenges and Solutions. IEEE Access 2022, 10, 58375–58418. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Pratap Singh, R.; Suman, R. Medical 4.0 technologies for healthcare: Features, capabilities, and applications. Internet Things Cyber-Phys. Syst. 2022, 2, 12–30. [Google Scholar] [CrossRef]
- Horgan, D.; Romao, M.; Morré, S.A.; Kalra, D. Artificial Intelligence: Power for Civilisation—And for Better Healthcare. Public Health Genom. 2019, 22, 145–161. [Google Scholar] [CrossRef]
- Nestor, B. Satisfying Model Validity for Robust Clinical Machine Learning for Time-Series. Doctoral Dissertation, University of Toronto, Toronto, ON, Canada, 2023. [Google Scholar]
- Okeibunor, J.C.; Jaca, A.; Iwu-Jaja, C.J.; Idemili-Aronu, N.; Ba, H.; Zantsi, Z.P.; Ndlambe, A.M.; Mavundza, E.; Muneene, D.; Wiysonge, C.S.; et al. The use of artificial intelligence for delivery of essential health services across WHO regions: A scoping review. Front. Public Health 2023, 11, 1102185. [Google Scholar] [CrossRef]
- Jongen, V.W.; Zimmermann, H.M.L.; Goedhart, M.; Bogaards, J.A.; Davidovich, U.; Coyer, L.; de Vries, H.J.; Prins, M.; Hoornenborg, E.; van der Loeff, M.F.S. Can we screen less frequently for STI among PrEP users? Assessing the effect of biannual STI screening on timing of diagnosis and transmission risk in the AMPrEP Study. Sex Transm. Infect. 2022, 99, 149–155. [Google Scholar] [CrossRef]
- Mandreoli, F.; Ferrari, D.; Guidetti, V.; Motta, F.; Missier, P. Real-world data mining meets clinical practice: Research challenges and perspective. Front. Big Data 2022, 5, 1021621. [Google Scholar] [CrossRef]
- Gerke, S. Health AI for good rather than evil? The need for a new regulatory framework for AI-based medical devices. Yale J. Health Pol’y L. Ethics 2021, 20, 432. [Google Scholar]
- Angehrn, Z.; Haldna, L.; Zandvliet, A.S.; Gil Berglund, E.; Zeeuw, J.; Amzal, B.; Cheung, S.A.; Polasek, T.M.; Pfister, M.; Kerbusch, T.; et al. Artificial Intelligence and Machine Learning Applied at the Point of Care. Front. Pharmacol. 2020, 11, 759. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Lu, Z.; Weng, W.; Yang, L.; Yang, L.; Leng, X.; Wang, J.; Lin, Y.F.; Wu, J.; Fu, L.; et al. Diagnosis of neurosyphilis in HIV-negative patients with syphilis: Development, validation, and clinical utility of a suite of machine learning models. eClinicalMedicine 2023, 62, 102080. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.; Behl, T.; Aleya, L.; Rahman, H.; Kumar, A.; Arora, S.; and Bulbul, I. Artificial intelligence as a fundamental tool in management of infectious diseases and its current implementation in COVID-19 pandemic. Environ. Sci. Pollut. Res. 2021, 28, 40515–40532. [Google Scholar] [CrossRef]
- Grace, D.; Gaspar, M.; Wells, A.; Sinno, J.; Daroya, E.; Montess, M.; Hull, M.; Lachowsky, N.J. and Tan, D.H. Injectable Pre-Exposure Prophylaxis for HIV Prevention: Perspectives on the Benefits and Barriers from Gay, Bisexual, and Queer Men and Health System Stakeholders in Ontario, Canada. AIDS Patient Care STDs 2023, 37, 306–315. [Google Scholar] [CrossRef]
- Skosana, G.M. The Psychosocial Effects of Unemployment on Young Adults in Ekurhuleni Metropolitan Area, Gauteng. Doctoral Dissertation, University of South Africa, Pretoria, South Africa, 2021. [Google Scholar]
- Agrebi, S.; Larbi, A. Use of artificial intelligence in infectious diseases. In Artificial Intelligence in Precision Health; Elsevier: Amsterdam, The Netherlands, 2020; pp. 415–438. [Google Scholar] [CrossRef]
- Halminen, O.; Tenhunen, H.; Heliste, A.; Seppälä, T. Artificial Intelligence Applications and Venture Funding in Healthcare; ETLA Working Papers No. 68; The Research Institute of the Finnish Economy (ETLA): Helsinki, Finland, 2019. [Google Scholar]
- Hollan, M. Aidoc AI Software Used to Reduce Missed Follow-Up Appointments. Aidoc AI Software Report. Available online: https://www.pharmexec.com/view/aidoc-ai-reduce-missed-follow-up-appointments (accessed on 21 October 2024).
- Hughes, Y.; Sawleshwarkar, S. Global Epidemiology of Selected Sexually Transmitted Infections: An Overview. In Sexually Transmissible Oral Diseases; Prabhu, S.R., Wagoner, N., Hill, J., Sawleshwarkar, S., Eds.; Wiley: Hoboken, NJ, USA, 2023; pp. 25–35. [Google Scholar] [CrossRef]
- Kaur, J. FutureCare: AI Robots Revolutionizing Health and Healing. In Advances in Medical Technologies and Clinical Practice; Singla, B., Shalender, K., Stamer, K., Eds.; IGI Global: Hershey, PA, USA, 2024; pp. 311–340. [Google Scholar] [CrossRef]
- Marban, A.; Srinivasan, V.; Samek, W.; Fernández, J.; Casals, A. A Recurrent Convolutional Neural Network Approach for Sensorless Force Estimation in Robotic Surgery. Biomed. Signal Process. Control 2019, 50, 134–150. [Google Scholar] [CrossRef]
- Kibe, L.; Kwanya, T.; Nyagowa, H. Harnessing fourth industrial revolution (4IR) technologies for sustainable development in Africa: A meta-analysis. Technol. Sustain. 2023, 2, 244–258. [Google Scholar] [CrossRef]
- Mamphiswana, R.; Bekele, M. The Fourth Industrial Revolution: Prospects and Challenges for Africa. In Proceedings of the International Association for Management of Technology, IAMOT 2020 Conference Proceedings, Cairo, Egypt, 13–17 September 2020. [Google Scholar]
- Zhang, Y.; Bao, R.; Leuba, S.I.; Li, J.; Wang, H.; Zhang, J.; Chu, Z.; Geng, W.; Jiang, Y. and Xu, J. Association of nitrite inhalants use and unprotected anal intercourse and HIV/syphilis infection among MSM in China: A systematic review and meta-analysis. BMC Public Health 2020, 20, 1378. [Google Scholar] [CrossRef]
- Pai, N.P.; Karellis, A.; Kim, J.; Peter, T. Modern diagnostic technologies for HIV. Lancet HIV 2020, 7, e574–e581. [Google Scholar] [CrossRef]
- Khan, A.R.; Hussain, W.L.; Shum, H.C.; Hassan, S.U. Point-of-care testing: A critical analysis of the market and future trends. Front. Lab Chip Technol. 2024, 3, 1394752. [Google Scholar] [CrossRef]
- Mehta, N.; Gupta, S.; Kularathne, Y. The Role and Impact of Artificial Intelligence in Addressing Sexually Transmitted Infections, Nonvenereal Genital Diseases, Sexual Health, and Wellness. Indian Dermatol. Online J. 2023, 14, 793–798. [Google Scholar] [CrossRef] [PubMed]
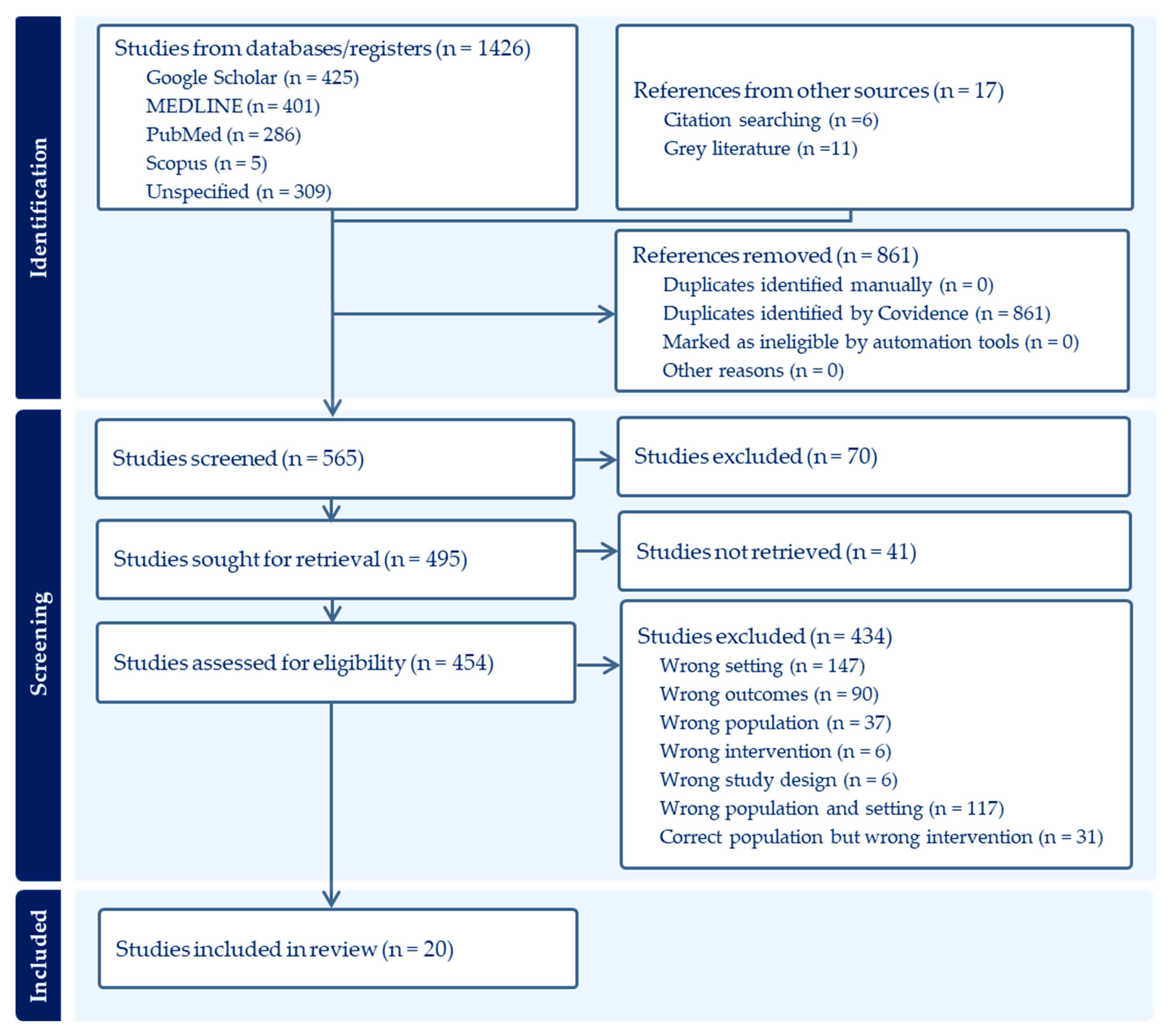
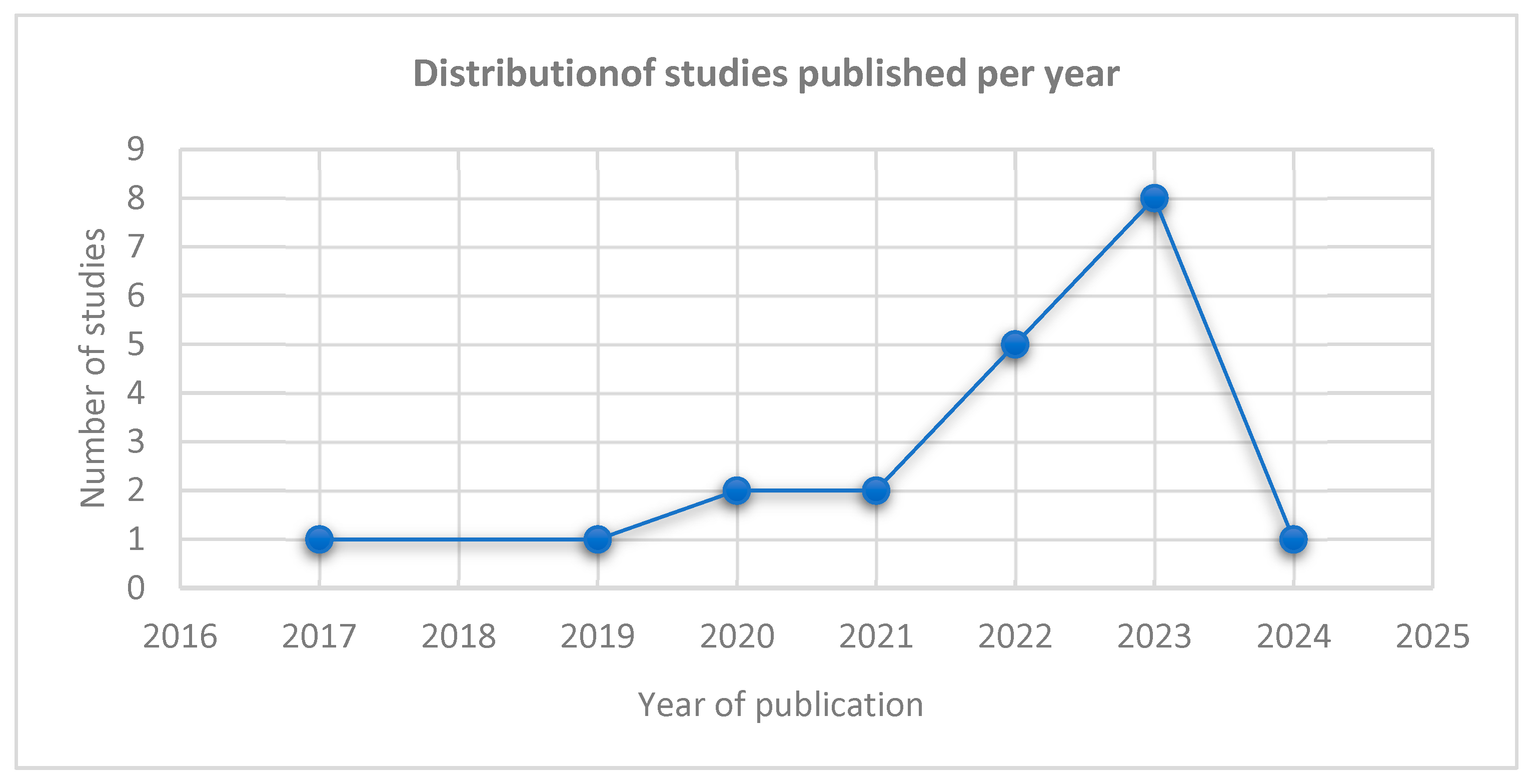
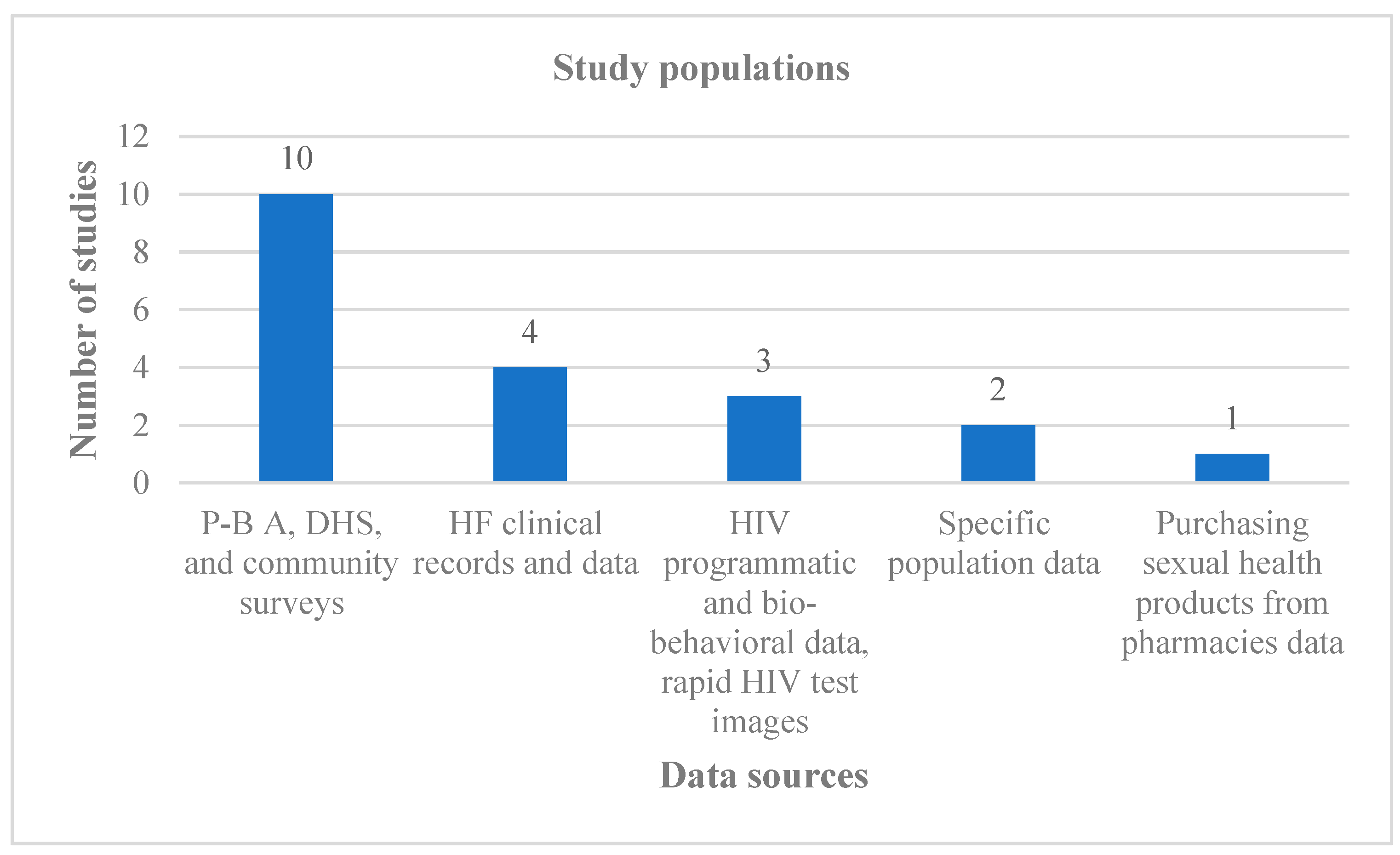
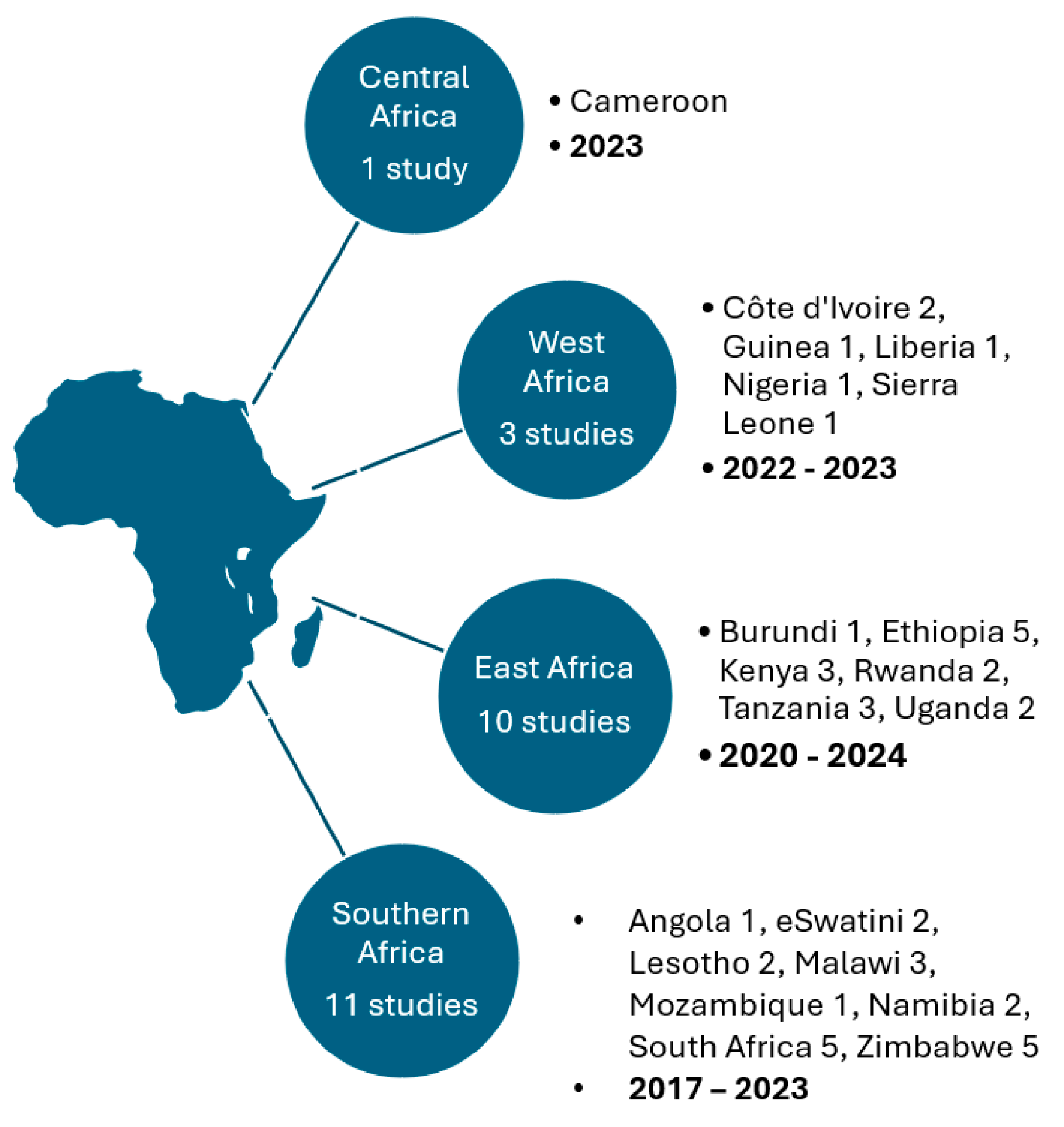
| Component | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| P (population) | Studies involving adults—15 years and above for prospective primary studies and all age groups for retrospective studies; the general population in SSA and KPs at higher risk of STIs in SSA, such as men who have sex with men, transgender people, sex workers, people who inject drugs, people in prisons and detention/incarcerated people, adolescent girls, young women, and PLHIV; Sub-Saharan African countries, the region, and its subregions. | Studies involving children—14 years and below for primary prospective studies; general populations or KPs outside of SSA; countries outside of Sub-Saharan Africa. |
| I (intervention) | Studies examining the applications of AI technologies in diagnosing, treating, and managing STIs. Examples of AI technologies include ML, DL, Natural language processing (NLP), Neural networks (NN), linear regression, logistic regression, decision tree, Support vector machine (SVM) algorithm, naive Bayes algorithm, K-nearest neighbors (KNN) algorithm, K-means, random forest algorithm, dimensionality reduction algorithms, gradient boosting algorithm, AdaBoost algorithm, Graph Neural Networks (GNNs), Federated Learning, BERT, Explainable AI and others. | Other studies not examining the applications of AI technologies in the diagnosis, treatment, and management of STIs. |
| C (comparison) | Studies with or without a comparator group comparing different AI approaches; comparing AI approaches and traditional diagnostic methods and comparing AI approaches and standard of care. | |
| O (outcome) | Studies reporting outcomes related to diagnosing, treating, and managing STIs using AI technologies. | Studies reporting outcomes without using AI approaches. |
| S (Study Design/Characteristics) | Studies that use AI approaches in randomized controlled trials; cohort studies; prospective cohorts; retrospective studies; time series studies; case-control studies; descriptive, analytical, and quasi-experimental studies were conducted in English from 2015 onwards and available in full text. | Studies with unclear methodologies; lacking trustworthiness, reliability, and validity of the research designs; clear biases; conducted using non-English and before 2015; not available in full text (abstract only), incomplete articles, and unpublished materials. |
| Subject matter | Studies on the diagnosis, treatment, and management of STIs using AI technologies. | Studies not addressing the use or applications of AI in the diagnosis, treatment, and/or management of STIs. |
| Literature type | Primary studies—journal articles, book chapters, theses and dissertations, conference presentations, grey literature, and unpublished studies. | All secondary studies—reviews, meta-analyses, editorials, and opinion discussions. |
| Author, Year, Region | Study Design | Population and Sample Size | AI Approaches | Implementation Classification and Comparator | Main Findings | Outcomes |
|---|---|---|---|---|---|---|
| Adeboye et al., 2023, West Africa [37] | Retrospective observational study. | Students, staff, and non-staff (males and females, aged 9–60 years, n = 400). | Logistic regression, KNN, decision tree, naive Bayes, random forest, and AdaBoost. | Methodological, no comparator specified. | Logistic regression was the best-performing model for diagnosing STIs, achieving ~95% accuracy. | Classification accuracy, AUC, recall, and F-score of the logistic regression model. |
| Alie and Negesse 2024, East Africa [39] | Retrospective study. | Adolescent respondents (n = 4502). | J48 decision tree, random forest, SVM, multi-layer perceptron, naïve Bayes, logistic gradient boosting, and logistic regression. | Methodological, no comparator specified. | J48 decision tree algorithm demonstrated high accuracy in detecting HIV positivity and predicting testing behaviors. | Knowledge of HIV testing probability and predictors based on awareness of AIDS and STIs. |
| Alzubaidi et al., 2023, East Africa [40] | Observational study. | Both genders aged ≥18 years (n = 854). | AI algorithms using computer vision technology. | Methodological, client self-interpretation, pharmacy provider interpretation, and expert panel interpretation. | AI vision technology reduced false negatives, identified positives correctly, and avoided missed infections. | Sensitivity, specificity, positive predictive value, negative predictive value, and detection of missed infections. |
| Balzer et al., 2020, East Africa [38] | Retrospective observational study. | Males and females aged ≥15 years (n = 75,558). | Logistic regression, penalized logistic regression, generalized additive models, stepwise logistic regression, and ML algorithms. | Methodological, risk group, and model-based approaches | ML models were most effective in identifying individuals at risk for HIV and improving prevention strategies. | Efficiency and sensitivity of different strategies in predicting 1-year risk of HIV seroconversion. |
| Belete and Huchaiah 2023, East Africa [41] | Retrospective observational study. | Age and gender not specified (n = 78,877). | Deep learning models. | Methodological, no comparator specified. | Developed an accurate prognostic tool for predicting HIV/AIDS test results. | Model accuracy in prognostic prediction for HIV/AIDS outcomes. |
| Birri Makota and Musenge 2023, Southern Africa [42] | Retrospective cross-sectional study. | Males and females living with and without HIV (Aged 15–49 females, 15–54 males, n = 6672). | Supervised classification-based machine learning approach. | Methodological, no comparator specified. | Predicted HIV infection using classification-based ML models. | Predictive performance for HIV infection diagnosis. |
| Chikusi 2022, East Africa [43] | Retrospective observational study. | Males and females (n = 6346). | Random forest, XGBoost, and artificial neural networks. | Methodological, feature engineering: home-based community VCT, mobile testing, outreach testing, and VCT. | Identified HIV knowledge as the most significant element contributing to HIV index testing or assessment. | Mean absolute error (MAE) for random forest, XGBoost, and artificial neural networks. |
| Chingombe et al., 2022, Southern Africa [44] | Retrospective observational study. | Males and females (n = 20,577). | Random forest, SVM, and logistic regression. | Methodological, no comparator specified. | ML models identified high-risk individuals for targeted HIV prevention and screening strategies. | Targeted HIV prevention and screening strategies. |
| Chingombe et al., 2022, Southern Africa [45] | Retrospective observational study. | MSM (n = 1538). | DL and ML algorithms: RNN, bagging classifier, gradient boosting classifier, SVM, and Gaussian naïve Bayes classifier. | Methodological, traditional HIV testing approaches. | RNNs predicted HIV status with high precision, recall, and F1-scores, significantly improving early screening. | Precision, recall, accuracy, F1-score, and AUC for RNN and ML models. |
| Esra et al., 2023, Southern Africa [46] | Observational retrospective study. | ART patients (median age 33 years; n = 264,877). | Adaptive boosting, categorical boosting, logistic regression, and gradient boosting. | Methodological, no comparator specified. | ML model for patient retention on ART successfully validated and extended. | Interruptions in ART prediction, sensitivity, positive predictive value, and F1-scores. |
| Laybohr Kamara et al., 2022, West Africa [47] | Observational retrospective ecological study. | Males and females (aged 15–49 years; n = 158,408). | Geodetector and LASSO regression. | Methodological, no comparator specified. | LASSO model correctly predicted HIV prevalence, highlighting regional hotspots over time. | HIV prevalence patterns and spatial–temporal heterogeneity. |
| Mamo et al., 2023, East Africa [48] | Observational retrospective institution-based cross-sectional study. | HIV-positive adults receiving treatment (aged ≥18 years; n = 5264). | KNN, random forest, decision tree, gradient boosting, XGBoost, logistic regression, and SVM. | Methodological, no comparator specified. | Random forest classifier best predicted virological failure with high sensitivity and AUC. | Virological failure prediction based on viral load tests. |
| Maskew et al., 2022, Southern Africa [49] | Observational longitudinal study. | Males and females (median age 39 years; n = 809,977). | Logistic regression, random forest, and AdaBoost. | Methodological, no comparator specified. | ML models correctly identified HIV patients at risk for disengagement and unsuppressed viral load. | Patient retention and viral load suppression. |
| Mitiku 2023, East Africa [50] | Retrospective study. | Males and females (aged 20–39; n = 14,922). | Random forest, XGBoost, and artificial neural networks. | Methodological, manual HIV index case testing. | Random forest algorithm outperformed others in precision, recall, and F1-scores. | Development of HIV status predictive models for effective case testing. |
| Mutai et al., 2023, SSA Regions [51] | Observational study. | Males and females (n = 302,355). | Unsupervised machine learning. | Methodological, no comparator specified. | Identified clustered countries and predictors of HIV positivity using unsupervised ML. | Identification of HIV predictors and high-risk clusters. |
| Mutai et al., 2021, East and Southern Africa [52] | Observational study. | Males and females (n = 87,044). | Elastic net, KNN, random forest, SVM, XGBoost, and light gradient boosting. | Methodological, no comparator specified. | XGBoost significantly improved HIV positivity identification and screening for high-risk individuals. | Improved predictive accuracy for HIV positivity. |
| Oladokun et al., 2019, Southern Africa [53] | Retrospective cross-sectional study. | Women aged 15–49 years (n = 7808). | Decision tree and logistic regression. | Methodological, no comparator specified. | Decision tree showed higher sensitivity in HIV status classification than logistic regression. | Sensitivity, specificity, and overall predictive accuracy. |
| Orel et al., 2020, SSA Regions [54] | Retrospective study. | Males and females (n = 124,777). | Penalized logistic regression, generalized additive model, SVM, and XGBoost. | Methodological, no comparator specified | XGBoost algorithm showed high accuracy in predicting HIV status. | Classification of HIV rapid diagnostic test images. |
| Turbé et al., 2021, Southern Africa [55] | Objective research design. | Image library (n = 11,374). | Deep learning algorithms: ResNet50, MobileNetV2, and MobileNetV3. | Methodological, traditional visual interpretation. | DL algorithms achieved 98.9% accuracy, outperforming traditional visual methods. | High accuracy in RDT image classification. |
| van Heerden et al., 2017, Southern Africa [56] | Infodemiology approach. | Males and females (Avg age 30.2 years, s.d. 2.3; n = 10). | HIV counseling and testing using conversational agents. | Methodological, human counseling, and testing. | Conversational agents were natural and equivalent to human counseling, fostering user openness. | Acceptability and feasibility of conversational agents for HIV counseling and testing. |
| AI Approach | Use of AI Approaches and Metrics |
|---|---|
| 1. Random forest | (a) Predicting virological failure—sensitivity: 1.00, precision: 0.987, F1-score: 0.993, AUC: 0.9989 (b) Predicting clinic visits and viral load suppression: (i) Clinic visits—accuracy: 66–79%, sensitivity: 60.6%, specificity: 67%, negative predictive value: 94%. (ii) Viral load suppression—accuracy: 76%, sensitivity: 65.6%, negative predictive value: 95%. (c) Accuracy of ML algorithms—accuracy: 85%. (d) Predicting and visualizing HIV index testing—no specific metrics provided. |
| 2. XGBoost | (a) Predicting likelihood of HIV infection—F1-score: 91.4% for males, 90.1% for females. (b) Enhancing HIV positivity identification—F1-score: 90% for males, 92% for females. (c) Predicting HIV status—mean F1-score: 76.8% for males, 78.8% for females. (d) Accuracy of ML algorithms—accuracy: 83.89%. (e) Predicting and visualizing HIV index testing—no metrics provided. |
| 3. Logistic regression | Diagnosis and prediction of STD infection accuracy—classification accuracy: 95%, AUC: 94.6%, recall: 93.9%, F-score: 91.1%. Predicting HIV status—accuracy of 85%, recall of 98%, and F1-score of 92%. |
| 4. ML models | HIV acquisition risk identification, predicting interruptions in treatment—no metrics specified. |
| 5. AI agent | HIV counseling and testing—user acceptance: more than 60% found it natural, 70% felt comfortable, 60% guided to complete the test, |
| 6. AI algorithm | HIV testing—sensitivity: 100%, negative predictive value: 100%, specificity: 99%, positive predictive value: 81.5%. |
| 7. DL models | HIV status prediction: (a) RNN accuracy: 87%, precision: 87%, recall: 87%, F1-score: 87%, AUC: 94%. (b) ANN accuracy: 85.5%, precision: 84.4%, recall: 85.7%, F1-score: 85.1%, AUC: 89.72%. |
| 8. J48 decision tree | HIV detection: accuracy: 81.29%, ROC curve: 86.3%. |
| 9. LASSO model | Identifying HIV testing uptake factors—no metrics specified. |
| 10. Unsupervised ML approaches | Identifying male and female HIV clusters—no metrics specified. |
| Metric | Frequency | Rank |
|---|---|---|
| F1-Score | 9 | 1 |
| Accuracy | 9 | 1 |
| Sensitivity | 8 | 3 |
| AUC (Area Under the Curve) | 7 | 4 |
| Recall | 6 | 5 |
| Specificity | 5 | 6 |
| PPV (Positive Predictive Value) | 4 | 7 |
| NPV (Negative Predictive Value) | 4 | 7 |
| Precision | 4 | 7 |
| Log-Loss | 3 | 10 |
| Misclassification Rate | 1 | 11 |
| Feature Importance | 1 | 11 |
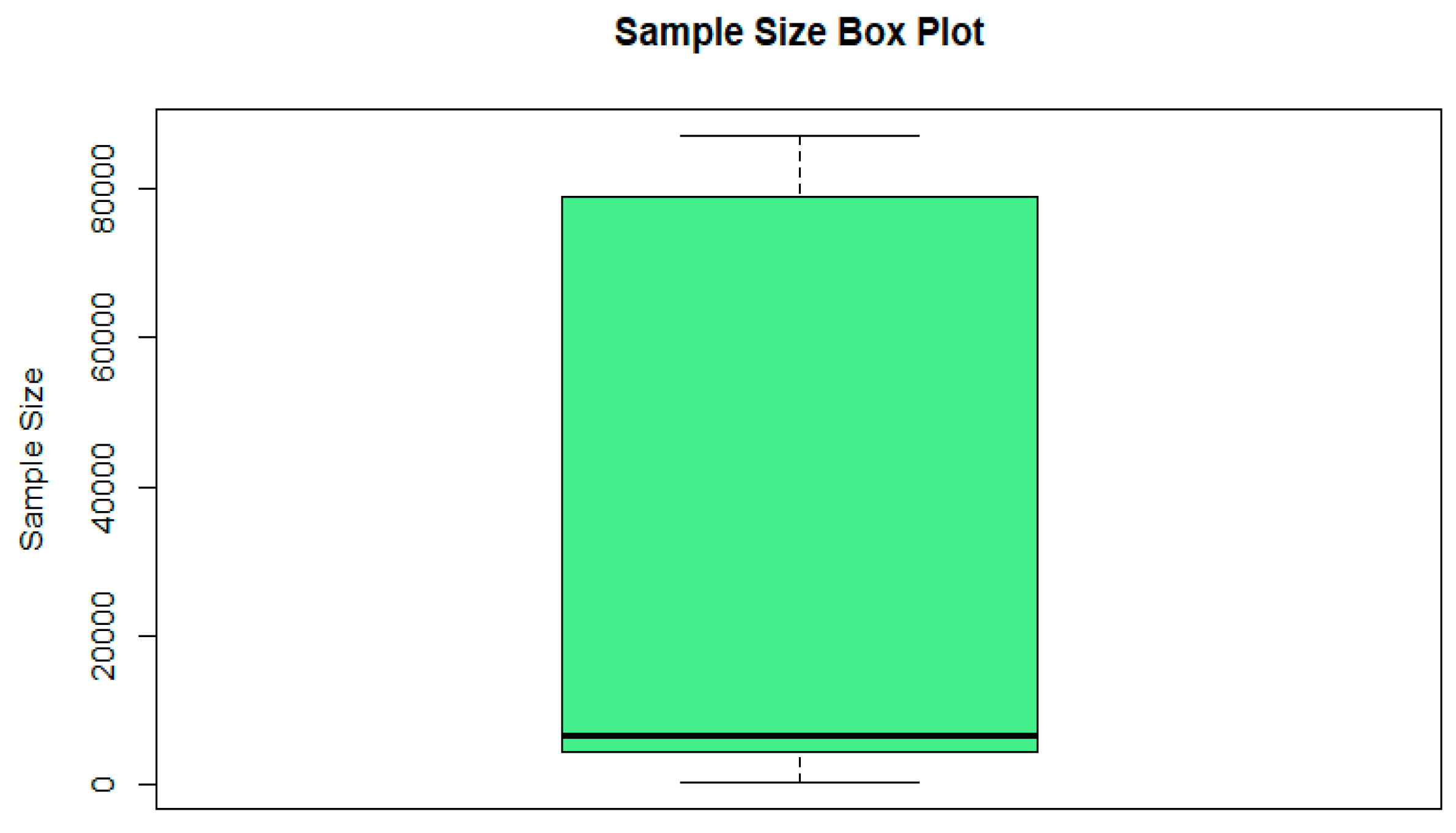
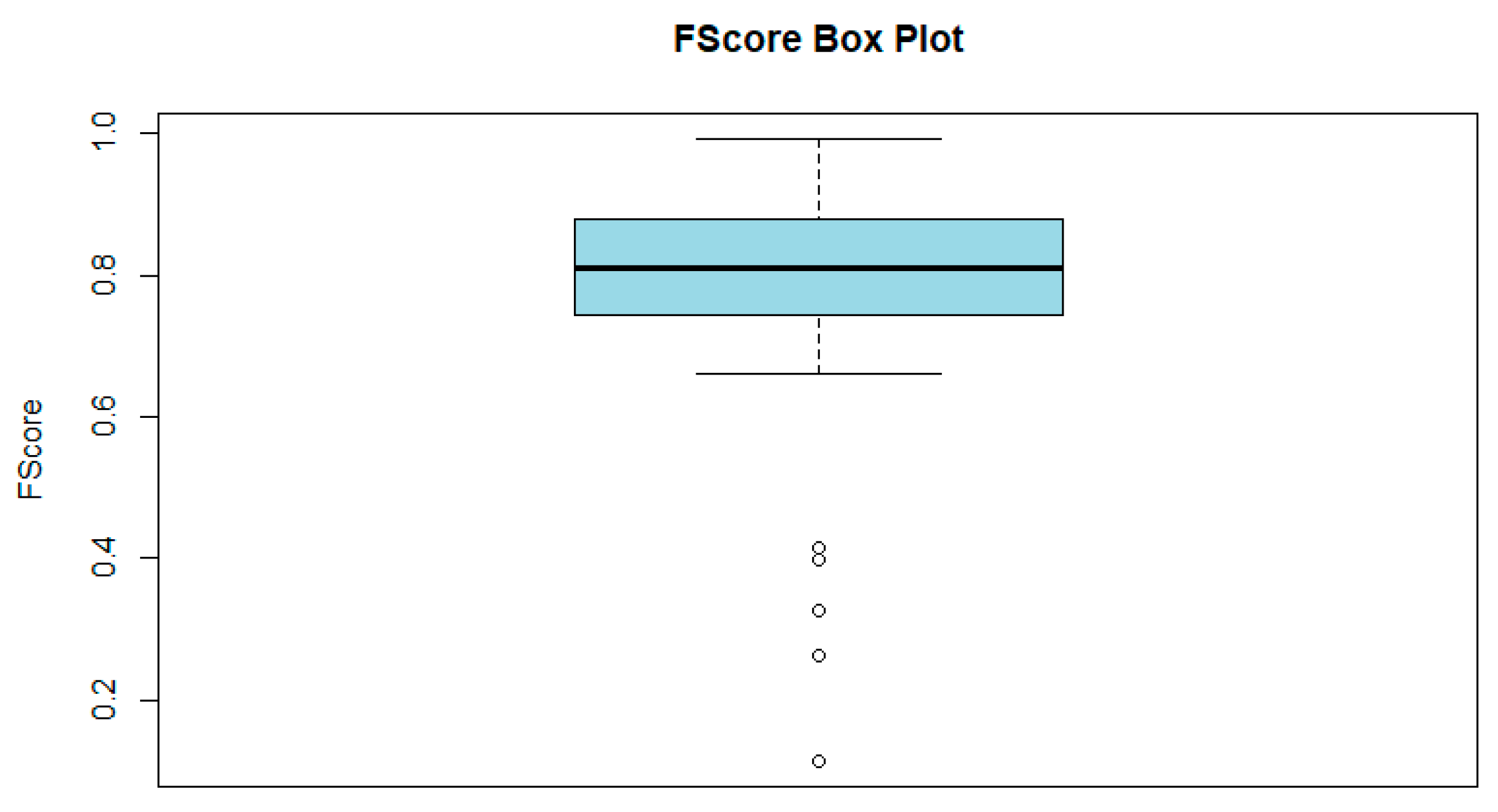
| Statistic | Sample Size | F1-Score |
|---|---|---|
| Mean | 29,471.67 | 0.7886 |
| Median | 6672 | 0.811 |
| Minimum | 400 | 0.114 |
| Maximum | 87,044 | 0.993 |
| Standard Deviation | 36,363.5 | 0.163 |
| Model | SMD | 95% CI | z | p-Value |
|---|---|---|---|---|
| Common effects model | 0.7897 | [0.7883, 0.7910] | 1126.07 | 0 |
| Random effects model | 0.7889 | [0.7511, 0.8268] | 40.82 | 0 |
| Quantifying Heterogeneity | Tau-squared () = 0.0254 | 95% CI [0.0187, 0.0376] |
| Tau () = 0.1594 | [0.1368; 0.1939] | |
| = 99.8% | ||
| Test of Heterogeneity | Q = 29,682.28 | df = 68 |
| p-value = 0 |
| Test | Statistic | p-Value | Conclusion |
|---|---|---|---|
| Kruskal–Wallis test for F1-scores | H = 6.5895 | 0.0862 | Fail to reject the null hypothesis |
| Kruskal–Wallis test for sample sizes | H = 34.6 | 0.000 | Reject the null hypothesis |
| ANOVA for F1-scores | - | 0.0228 | Reject the null hypothesis |
| ANOVA for sample sizes | F = 23.68 | 0.000 | Reject the null hypothesis |
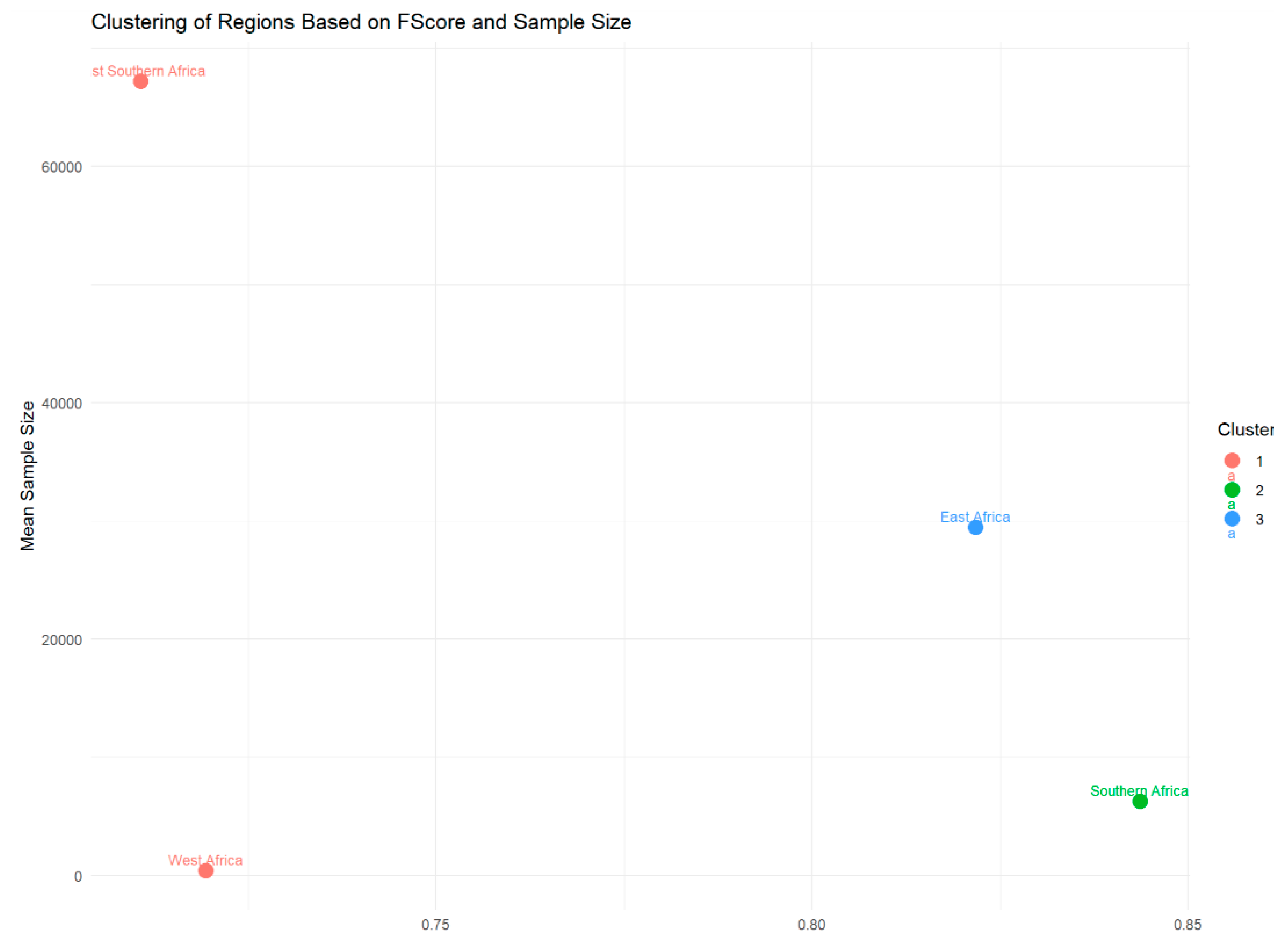
| Dunn’s test for F1-scores | 0.0314 (Southern Africa vs. East Southern Africa | Reject the null hypothesis | |
| Dunn’s test for F1-sample sizes | p-value < 0.05 (East Southern Africa vs. Southern Africa; East Africa vs. West Africa; East Southern Africa vs. West Africa; Southern Africa vs. West Africa; East Africa vs. East Southern Africa; East Africa vs. Southern Africa) | Reject the null hypothesis | |
| Dunn’s test for F1-sample sizes | 1 East Africa vs. Southern Africa | Fail to reject the null hypothesis | |
| Correlation between F1-scores and sample sizes | r = 0.0055 | 0.9643 | Fail to reject the null hypothesis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siyamayambo, C.; Phalane, E.; Phaswana-Mafuya, R.N. Diagnosis and Management of Sexually Transmitted Infections Using Artificial Intelligence Applications Among Key and General Populations in Sub-Saharan Africa: A Systematic Review and Meta-Analysis. Algorithms 2025, 18, 151. https://doi.org/10.3390/a18030151
Siyamayambo C, Phalane E, Phaswana-Mafuya RN. Diagnosis and Management of Sexually Transmitted Infections Using Artificial Intelligence Applications Among Key and General Populations in Sub-Saharan Africa: A Systematic Review and Meta-Analysis. Algorithms. 2025; 18(3):151. https://doi.org/10.3390/a18030151
Chicago/Turabian StyleSiyamayambo, Claris, Edith Phalane, and Refilwe Nancy Phaswana-Mafuya. 2025. "Diagnosis and Management of Sexually Transmitted Infections Using Artificial Intelligence Applications Among Key and General Populations in Sub-Saharan Africa: A Systematic Review and Meta-Analysis" Algorithms 18, no. 3: 151. https://doi.org/10.3390/a18030151
APA StyleSiyamayambo, C., Phalane, E., & Phaswana-Mafuya, R. N. (2025). Diagnosis and Management of Sexually Transmitted Infections Using Artificial Intelligence Applications Among Key and General Populations in Sub-Saharan Africa: A Systematic Review and Meta-Analysis. Algorithms, 18(3), 151. https://doi.org/10.3390/a18030151







