Non-Invasive Systems and Methods Patents Review Based on Electrocardiogram for Diagnosis of Cardiovascular Diseases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Keywords
2.2. Inclusion and Exclusion Criteria
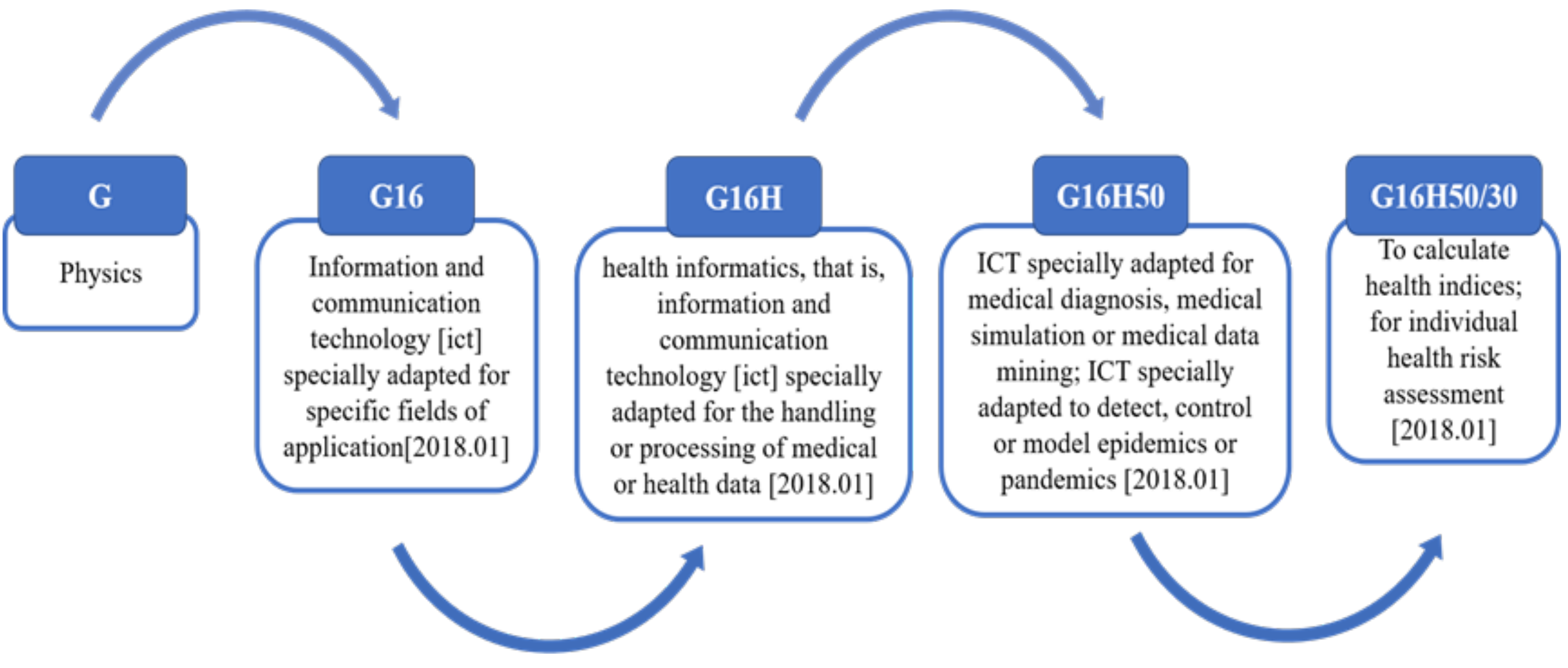
2.3. Classification
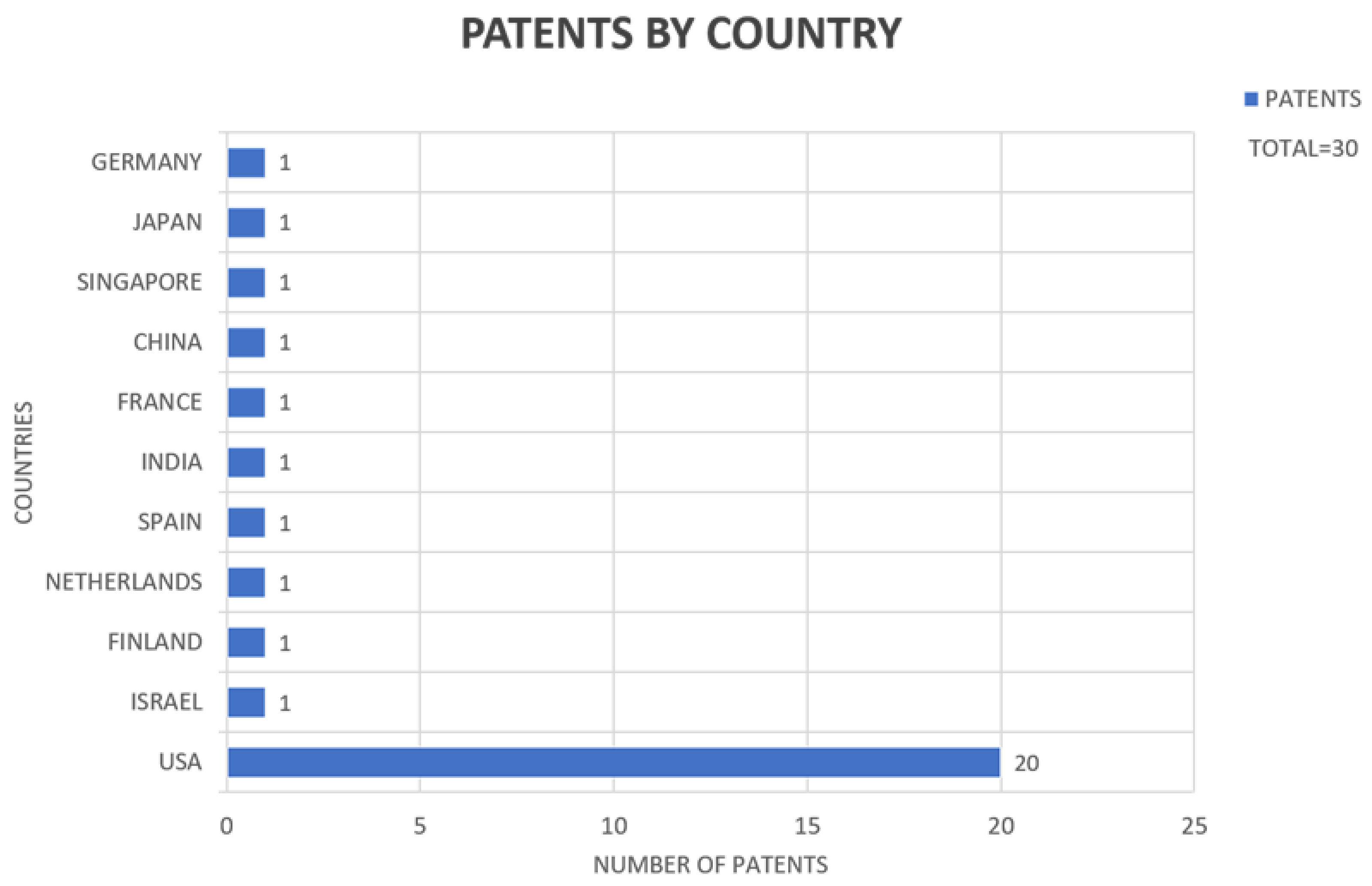
3. Results
3.1. Machine and Process for the Automatic Localization of Sources of Biological Rhythm Disorders
3.2. Method and System for Cardiovascular System Diagnosis
3.3. Method and Software for Cardiovascular Assessment and Risk Detection
3.4. System and Method for Separating Cardiac Signals
3.5. System and Method for Mapping Complex Fractionated Electrocardiogram Information
3.6. ECG Data Monitor
3.7. System for Managing Cardiovascular Health Status
3.8. Electrocardiographic Assessment of Arrhythmia Risk
3.9. Programmable-ECG Sensor Patch
3.10. Cardiac Performance Monitoring System for Use with Mobile Communication Devices
3.11. Method and Device for Early Detection of Heart Attack
3.12. System, Computer-Implemented Method and Computer Program Product for Individualized Multiple-Disease Quantitative Risk Assessment
3.13. Method and Device for Assessing the Risk of Cardiovascular Complications
3.14. QRS Complex Identification in Electrocardiogram Signals
3.15. Method and Device to Predict Adverse Cardiovascular Events and Mortality from an Electrocardiogram-Based Validated Risk Score
3.16. Systems and Methods for Monitoring and Controlling a Cardiovascular State of a Subject
3.17. System and Methods for Cardiovascular Blood Flow and Musculoskeletal Modeling for Predicting Device Failure or Clinical Events
3.18. Automatic Method to Delineate or Categorize an Electrocardiogram
3.19. Printed ECG Electrode and Method
3.20. Analysis of Electrocardiogram Signals
3.21. Cardiovascular Assist System That Quantifies Heart Function and Facilitates Heart Recovery
3.22. Monitor Recorder-Implemented Method for Electrocardiography Value Encoding and Compression
3.23. System and Method for Assessing Clinical Event Risk Based on Heart Rate Complexity
3.24. Continuous Detection and Monitoring of Heart Arrhythmia Using Both Wearable Sensors and Cloud-Resident Analyses
3.25. Electrocardiograph with Extended Lead Function and Extended Lead Electrocardiogram Deriving Method
3.26. ECG Analysis for Diagnosis of Heart Failure and Cardiovascular Disease Using Signals Obtained from an Implantable Monitor
3.27. Multi-Channel Real-Time Cardiovascular Performance Evaluation System and Method
3.28. Systems, Devices, and Methods for Non-Invasive Cardiac Monitoring
3.29. Systems and Methods for Predicting Atrial Arrhythmia
3.30. Compact Mobile Three-Lead Cardiac Monitoring Device
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jameson, J.L.; Fauci, A.S.; Kasper, D.L.; Hauser, S.L.; Longo, D.L.; Loscalzo, J. Harrison’s Principles of Internal Medicine, 20th ed.; McGraw-Hill: Guaynabo, Spain, 2018. [Google Scholar]
- World Health Organization. 2018. Available online: http://www.who.int/en/ (accessed on 20 January 2022).
- WHO. World Health Statistics Monitoring Health for the SDGs, Sustainable Development Goals. 2020. Available online: https://apps.who.int/iris/handle/10665/332070 (accessed on 20 January 2022).
- Bays, H.E.; Taub, P.R.; Epstein, E.; Michos, E.D.; Ferraro, R.A.; Bailey, A.L.; Kelli, H.M.; Ferdinand, K.C.; Echols, M.R.; Weintraub, H.; et al. Ten things to know about ten cardiovascular disease risk factors. Am. J. Prev. Cardiol. 2021, 5, 100149. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino Sr, R.B.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General cardiovascular risk profile for use in primary care: The Framingham heart study. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roger, V.L.; Go, A.S.; Lloyd-Jones, D.M.; Adams, R.J.; Berry, J.D.; Brown, T.M.; Turner, M.B. Heart disease and Stroke Statistics—2011 Update A Report from the American Heart Association. Circulation 2013, 123, e18–e209. [Google Scholar]
- Prabakaran, S.; Vitter, S.; Lundberg, G. Cardiovascular Disease in Women Update: Ischemia, Diagnostic Testing, and Menopause Hormone Therapy. Endocr. Pract. 2022, 28, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Humphries, K.H.; Izadnegadar, M.; Sedlak, T.; Saw, J.; Johnston, N.; Schenck-Gustafsson, K.; Merz, C.B. Sex differences in cardiovascular disease—Impact on care and outcomes. Front. Neuroendocrinol. 2017, 46, 46–70. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.K.; Banerjee, I.; Pal, K. 24-Electrocardiogram signal processing-based diagnostics: Applications of wavelet transform. In Woodhead Publishing Series in Electronic and Optical Materials, Bioelectronics and Medical Devices; Pal, K., Kraatz, H., Khasnobish, A., Bag, S., Banerjee, I., Kuruganti, U., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 591–614. ISBN 9780081024201. [Google Scholar] [CrossRef]
- Xiong, P.; Xue, Y.; Zhang, J.; Liu, M.; Du, H.; Zhang, H.; Hou, Z.; Wang, H.; Liu, X. Localization of myocardial infarction with multi-lead ECG based on DenseNet. Comput. Methods Programs Biomed. 2021, 203, 106024. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, H.; Li, Z.; Qin, L. Deep learning in ECG diagnosis: A review. Knowl.-Based Syst. 2021, 227, 107187. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Al-Kindi, S.G.; Brook, R.D. Air Pollution and Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 2054–2070. [Google Scholar] [CrossRef] [PubMed]
- Bourdrel, T.; Bind, M.-A.; Béjot, Y.; Morel, O.; Argacha, J.-F. Cardiovascular effects of air pollution. Arch. Cardiovasc. Dis. 2017, 110, 634–642. [Google Scholar] [CrossRef]
- Watkins, A.; Danilewitz, M.; Kusha, M.; Massé, S.; Urch, B.; Quadros, K.; Nanthakumar, K. Air Pollution and Arrhythmic Risk: The Smog Is Yet to Clear. Can. J. Cardiol. 2013, 29, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Balachandar, V.; Mahalaxmi, I.; Subramaniam, M.; Kaavya, J.; Kumar, N.S.; Laldinmawii, G.; Cho, S.G. Follow-up studies in COVID-19 recovered patients—Is it mandatory? Sci. Total Environ. 2020, 729, 10. [Google Scholar] [CrossRef] [PubMed]
- Mahfouz, R.A.; Arab, M.; Abdelhamid, M.; Elzayat, A. Fragmented QRS complex is an independent predictor of plaque burden in patients at intermediate risk of coronary artery disease. Indian Heart J. 2019, 71, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Elsevier. SCOPUS. 2019. Available online: https://www.scopus.com/freelookup/form/author.uri (accessed on 20 January 2022).
- Google. Google Patents. 2019. Available online: https://www.google.com/?tbm=pts (accessed on 20 January 2022).
- IMPI. SIGA. 2019. Available online: https://siga.impi.gob.mx/newSIGA/content/common/principal.jsf (accessed on 20 January 2022).
- WIPO. Divulgacion de Information. 2003. Available online: http://www.wipo.int/sme/es/documents/disclosing_inf.htm (accessed on 20 January 2022).
- OEP. ESPACENET. 2019. Available online: https://lp.espacenet.com/ (accessed on 20 January 2022).
- United States Government. USPTO. 2019. Available online: https://www.uspto.gov/ (accessed on 20 January 2022).
- WHO. WHO List of Priority Medical Devices for Management of Cardiovascular Diseases and Diabetes. 2021. Available online: https://apps.who.int/iris/bitstream/handle/10665/341967/9789240027978-eng.pdf (accessed on 20 January 2022).
- WHO. WIPO. 2019. Available online: https://www.wipo.int/portal/en/index.html (accessed on 20 January 2022).
- Narayan, W.-J.; Rappel, S. Machine and Process for The Automatic Localization of Sources of Biological Rhythm Disorders. 2010. Available online: https://patents.google.com/patent/WO2010042826A1/en (accessed on 20 January 2022).
- Arbel, M.O.R.; Tal, Y. Method and System for Cardiovascular System Diagnosis. 2010, Volume 2. Available online: https://patents.google.com/patent/US20070021673A1/en (accessed on 20 January 2022).
- Hersh, S.H. Method and Software for Cardiovascular Assessment and Risk Detection. 2011. Available online: https://patents.google.com/patent/US20110208434A1/en (accessed on 20 January 2022).
- Tzyy-Ping Jung, Jeng-Ren Duann, System and Method for Separating Cardiac Signals. 2011. Available online: https://patents.google.com/patent/US20050010120 (accessed on 20 January 2022).
- Afonso, V. System and Method for Mapping Complex Fractionated Electrocardiogram Information. 2012. Available online: https://patents.google.com/patent/US8229545 (accessed on 20 January 2022).
- Lewis, O.; William, D.; Musiol, T.; Ratingen, D.E.; Lewis, D.W.; Atlanta, G.A.; Tong Alvarez, N.M.U.; Reinartz, R.M.; Gonzalez, B.; Bhat, V. ECG Data Monitor. 2012. Available online: https://patents.google.com/patent/US20120311092A1 (accessed on 20 January 2022).
- Wendy, D.J.J.D.; Waanders, L.F.; Harsh, D.; Nagaraju, B.; Marcia, A.D.; Mani, A.K. System for Managing Cardiovascular Health Status. 2013. Available online: https://patents.google.com/patent/EP2544111A1 (accessed on 20 January 2022).
- Lux, R.L. Electrocardiographic Assessment of Arrhythmia Risk. 2013. Available online: https://patents.google.com/patent/US8437839B2 (accessed on 20 January 2022).
- Shennib, A. Programmable ECG Sensor Patch. 2014. Available online: https://patents.google.com/patent/US8688189B2 (accessed on 20 January 2022).
- Albert, D.E. Cardiac Performance Monitoring System for Use with Mobile Communication Devices. 2014. Available online: https://patents.google.com/patent/US8700137B2 (accessed on 20 January 2022).
- SLakshman, B.; Tamil, S.; Nourani, M.; Gupta, G. Method and Device for Early Detection of Heart Attack. 2015. Available online: https://patentscope.wipo.int/search/es/detail.jsf?docId=US73626273 (accessed on 20 January 2022).
- Domingez, R.C.H.; Bernardez, J.R.F.; Ojea, A.M.; Garcia, D.E.A. System, Computer-Implemented Method and Computer Program Product for Individualized Multiple-Disease Quantitative Risk Assessment. 2016. Available online: https://patents.google.com/patent/US20160342764A1 (accessed on 20 January 2022).
- Gurfinkel, Y.I.I.; Ostrozhinskij, V.A. Method and Device for Assessing the Risk of Cardiovascular Complications. 2015. Available online: https://patents.google.com/patent/US20150297098A1 (accessed on 20 January 2022).
- Bhaumik, K.J.S.B. QRS Complex Identification in Electrocardiogram Signals. 2016. Available online: https://patents.google.com/patent/US9414761B2 (accessed on 20 January 2022).
- Estes, N.E.H.; Durham, J.R. Method and Device to Predict Adverse Cardiovascular Events and Mortality from an Electrocardogram-Based Validated Risk Score. 2016. Available online: https://patents.google.com/patent/US20160256064A1 (accessed on 20 January 2022).
- Bighamian, R.; Hahn, J.-O.; Reisner, A.T.; Yapps, B. Systems and Methods for Monitoring and Controlling a Cardiovascular State of a Subject. 2015. Available online: https://patentscope.wipo.int/search/es/detail.jsf?docId=WO2016022989 (accessed on 20 January 2022).
- Gilwoo, G.L.C.L.; Taylor, C.A. System and Methods for Cardiovascular Blood Flow and Musculoskeletal Modeling for Predicting Device Failure or Clinical Events. 2016. Available online: https://patentscope.wipo.int/search/es/detail.jsf?docId=WO2016168017 (accessed on 20 January 2022).
- Rapin, J.; Li, J.; Massias, M. Automatic Method to Delineate or Categorize an Electrocardiogram. 2017. Available online: https://patents.google.com/patent/US20170112401A1 (accessed on 20 January 2022).
- Atashbar, A.E.M.Z.; Chlaihawi, A.A.; Narakathu, B.B. Printed ECG Electrode and Method. 2017. Available online: https://patents.google.com/patent/US20170332928A1 (accessed on 20 January 2022).
- Benjamin, S.; Shai, R.; Aaron, F. Analysis of Electrocardiogram Signals. 2015. Available online: https://patents.google.com/patent/US8934964B2 (accessed on 20 January 2022).
- Elazer, B.S.S.E.; Brian, C.; Noam, J. Cardiovascular Assist System that Quantifies Heart Function and Facilitates Heart Recovery. 2018. Available online: https://patentscope.wipo.int/search/es/detail.jsf?docId=WO2018053504 (accessed on 20 January 2022).
- Felix, J.; Dreisbach, E.M. Monitor Recorder-Implemented Method for Electrocardiography Value Encoding and Compression. 2017. Available online: https://patents.google.com/patent/US9730641B2 (accessed on 20 January 2022).
- Lim, L.S.; Wei, T.; Feng, S.G. System and Method for Assessing Clinical Event Risk Based on Heart Rate Complexity. Available online: https://patentscope.wipo.int/search/es/detail.jsf?docId=WO2019160504 (accessed on 20 January 2022).
- Mark, B.N.M. Continuous Detection and Monitoring of Heart Arrhythmia Using Both Wearable Sensors and Cloud-Resident Analyses. 2019. Available online: https://patentscope.wipo.int/search/es/detail.jsf?docId=WO2019108426 (accessed on 20 January 2022).
- Wei, D. Electrocardiograph with Extended Lead Function, and Extended Lead Electrocardiogram Deriving Method. 2013. Available online: https://patents.google.com/patent/US8359090B2 (accessed on 20 January 2022).
- Raven, J.S. ECG Analysis for Diagnosis of Heart Failure and Cardiovascular Disease Using Signals Obtained from an Implantable Monitor. 2019. Available online: https://patents.google.com/patent/US20190209037A1 (accessed on 20 January 2022).
- Wu, L.; Wan, L.M.; Yang, H.-W. Multi-Chanel Real-Time Cardiovascular Performance Evaluation System and Method. Available online: https://patentscope.wipo.int/search/es/detail.jsf?docId=WO2020086112 (accessed on 20 January 2022).
- Jiang, L.; Peterson, K.T. Systems, Devices, and Methods for Non-Invasive Cardiac Monitoring. 2020. Available online: https://patentscope.wipo.int/search/es/detail.jsf?docId=WO2020205987 (accessed on 20 January 2022).
- Thakur, P.H.; Ahmed, R.; Ruble, S.B. Systems and Methods for Predicting Atrial Arrhythmia. 2020. Available online: https://patentscope.wipo.int/search/es/detail.jsf?docId=WO2020190922 (accessed on 20 January 2022).
- Branislav, H.L.V.; Bosko, V. Compact Mobile Three-Lead Cardiac Monitoring Device. 2020. Available online: https://patentscope.wipo.int/search/es/detail.jsf?docId=WO2020232040 (accessed on 20 January 2022).
- Fang, D.Q. Cardiovascular Detection System and Method. 2017. Available online: https://patents.google.com/patent/US20170065194A1 (accessed on 20 January 2022).
- Gupta, S.; Korenberg, M. System and Method for Evaluating an Electrophysiological Signal. 2012. Available online: https://patents.google.com/patent/US20140194758A1 (accessed on 20 January 2022).

| Inclusion: | Exclusion |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores, N.; Reyna, M.A.; Avitia, R.L.; Cardenas-Haro, J.A.; Garcia-Gonzalez, C. Non-Invasive Systems and Methods Patents Review Based on Electrocardiogram for Diagnosis of Cardiovascular Diseases. Algorithms 2022, 15, 82. https://doi.org/10.3390/a15030082
Flores N, Reyna MA, Avitia RL, Cardenas-Haro JA, Garcia-Gonzalez C. Non-Invasive Systems and Methods Patents Review Based on Electrocardiogram for Diagnosis of Cardiovascular Diseases. Algorithms. 2022; 15(3):82. https://doi.org/10.3390/a15030082
Chicago/Turabian StyleFlores, Nellyzeth, Marco A. Reyna, Roberto L. Avitia, Jose Antonio Cardenas-Haro, and Conrado Garcia-Gonzalez. 2022. "Non-Invasive Systems and Methods Patents Review Based on Electrocardiogram for Diagnosis of Cardiovascular Diseases" Algorithms 15, no. 3: 82. https://doi.org/10.3390/a15030082
APA StyleFlores, N., Reyna, M. A., Avitia, R. L., Cardenas-Haro, J. A., & Garcia-Gonzalez, C. (2022). Non-Invasive Systems and Methods Patents Review Based on Electrocardiogram for Diagnosis of Cardiovascular Diseases. Algorithms, 15(3), 82. https://doi.org/10.3390/a15030082








