Numerical Simulation of Non-Linear Models of Reaction—Diffusion for a DGT Sensor
Abstract
1. Introduction
2. The Model
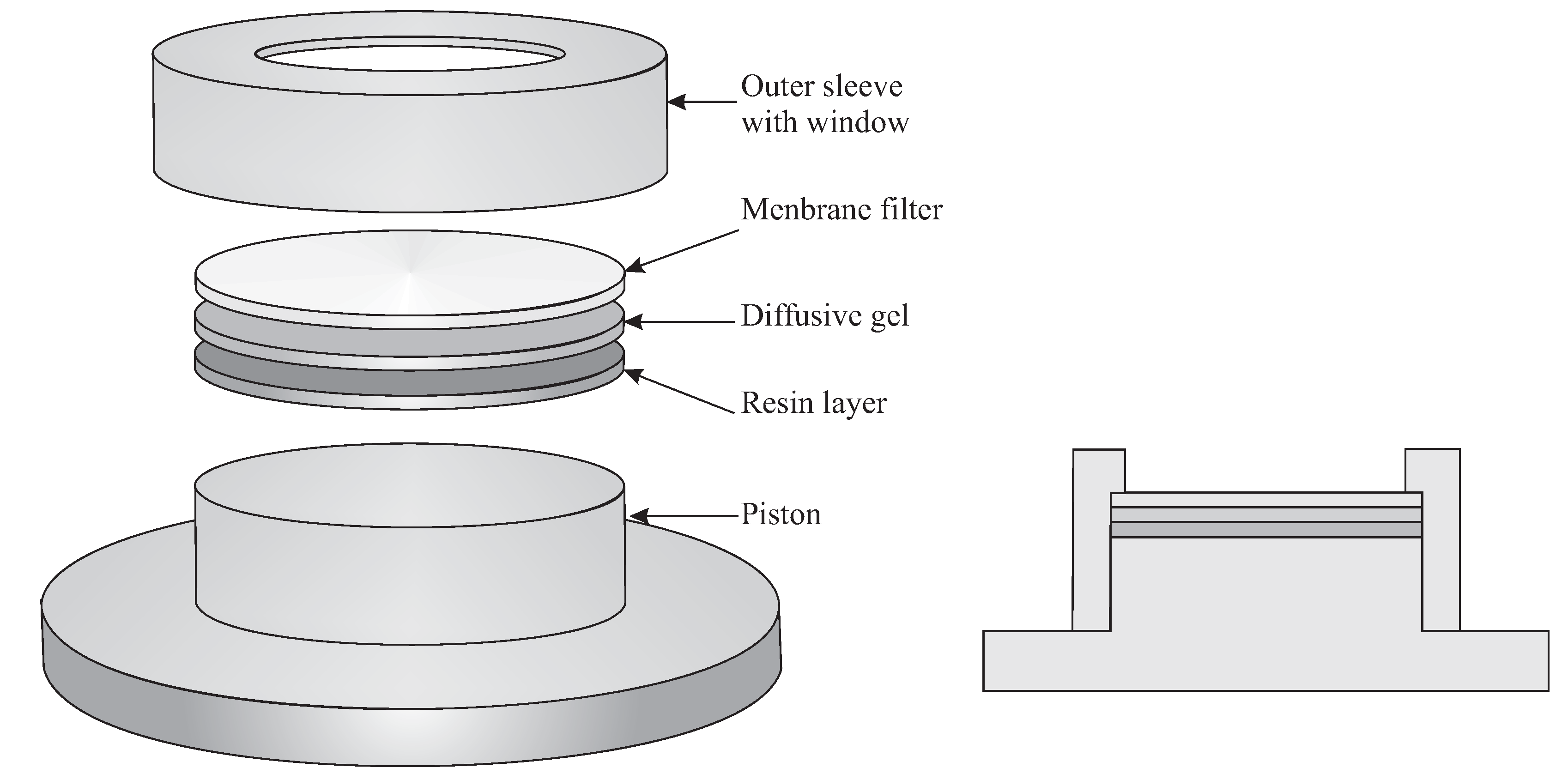
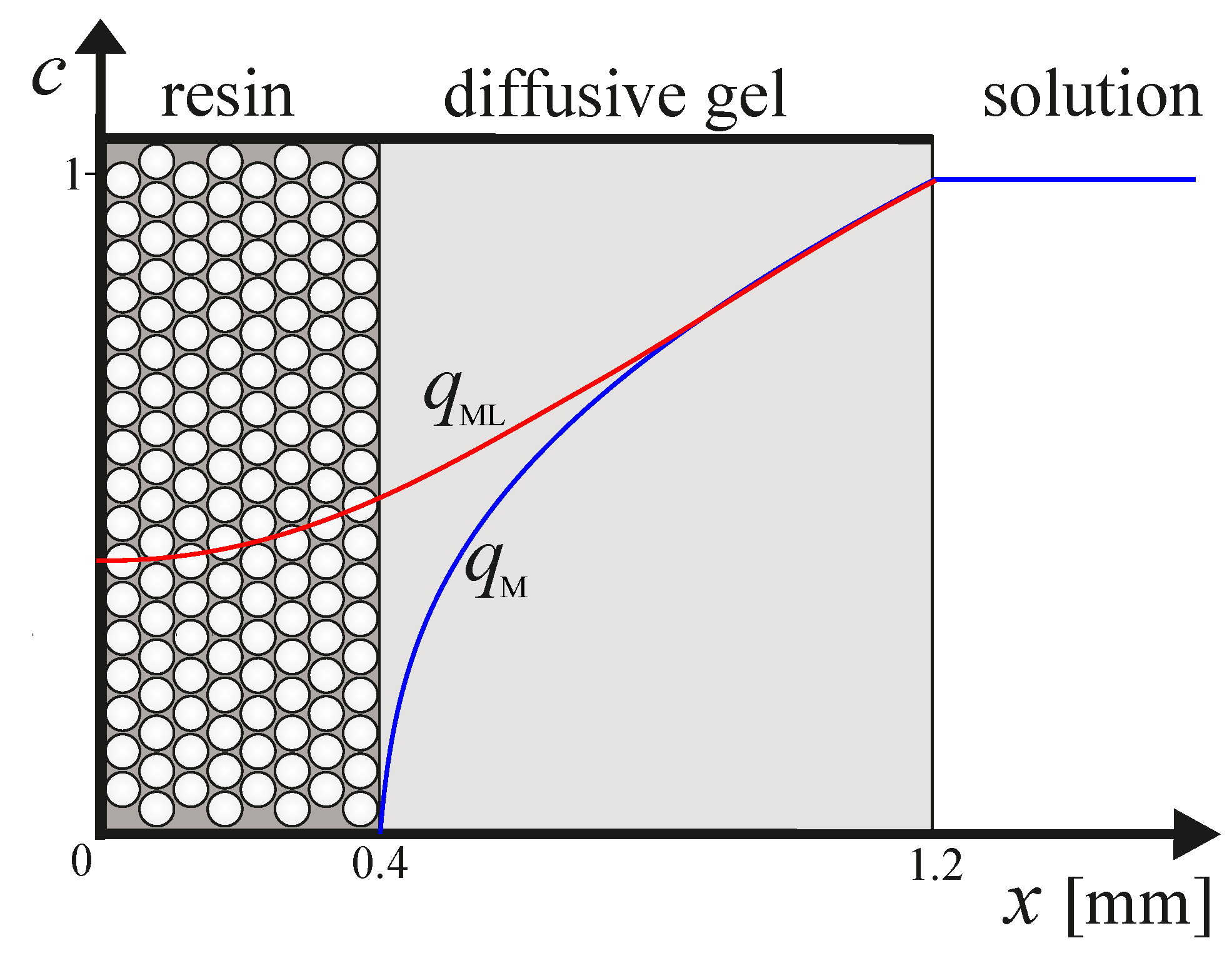
2.1. Diffusive Gradients in Thin Films (DGT)
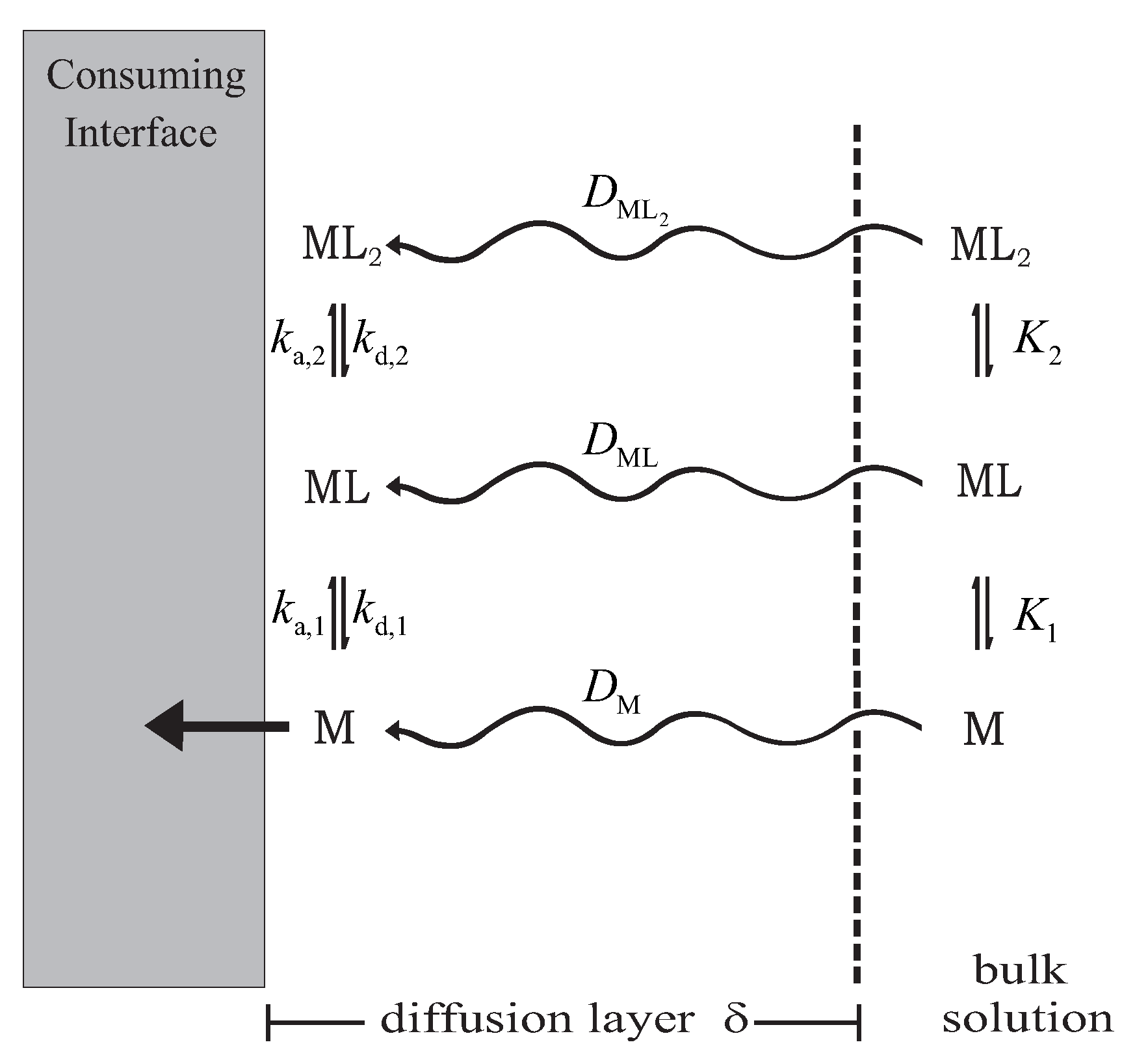
2.2. Mathematical Model
- Since the resin discs are made of complexing material embedded in the same type of gel that composes the diffusive layer, it is reasonable to consider that species that diffuse through the gel can penetrate the resin [8].
- Species that diffuse through the gel can bind the resin after. They do it by the described schema in Equation (9):where R denotes the free-sites in the interior of the resin, and , and represent the corresponding kinetic and stability constants.
- When the material used for the resin is Chelex, the grains of resin are located mainly in the layer adjacent to the diffusion gel [9]. In a first approach, we consider that binding sites in the resin are uniformly distributed.
- Migration forces or any electrostatic effects due to the charge of resin are not considered, i.e., the background salt is enough to screen these interactions.
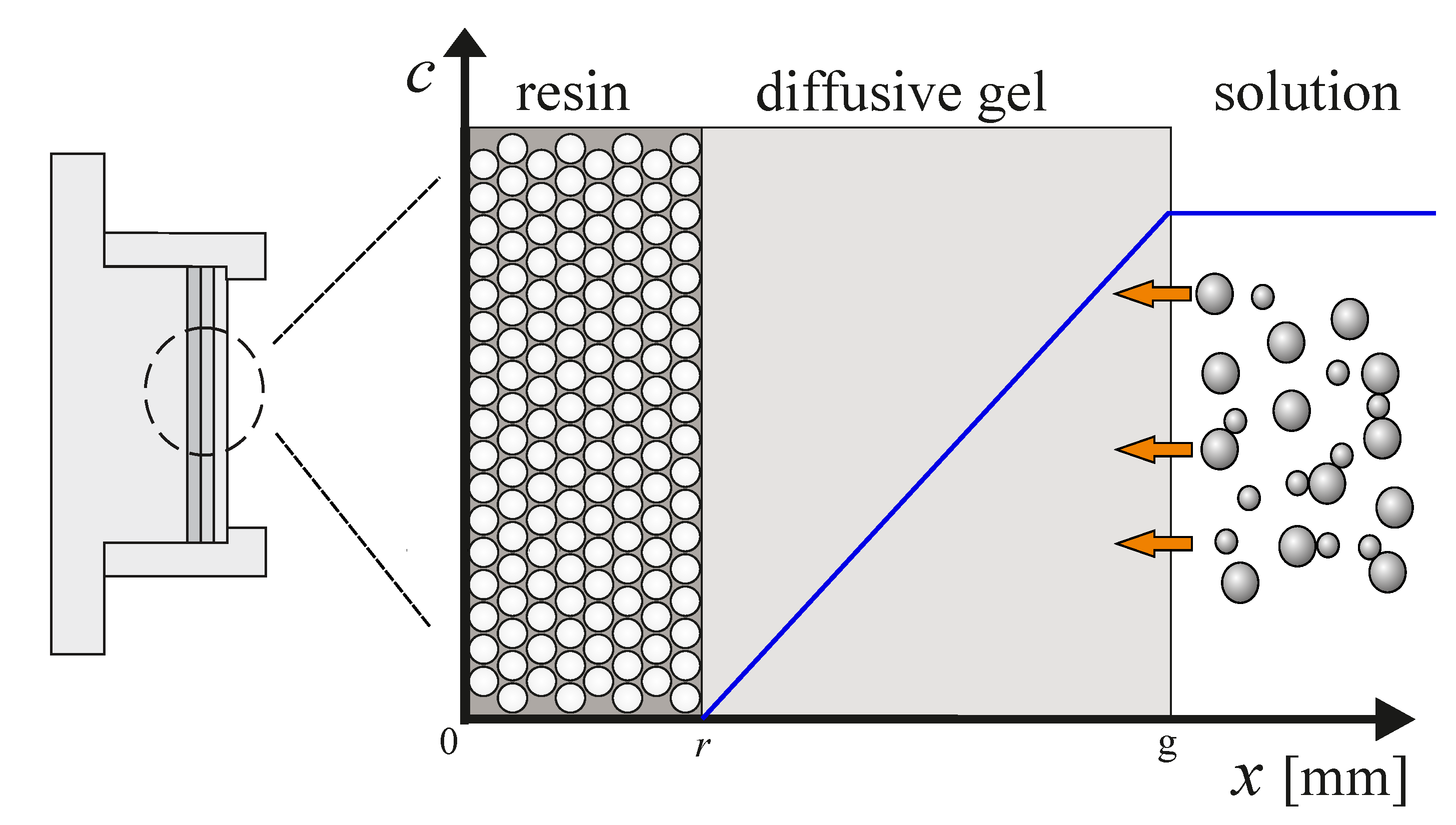
2.3. The Problem
2.4. Initial and Boundary Conditions
2.5. Dimensionless Formulation
- For all species in solution, that is, for all the species that have a bulk solution concentration, obtained from the solution of the equilibrium problem, we define standard concentrations,
- For the rest, that is to say, the resin and its complexes,
- For the spatial coordinate, instead of nondimensionalisation, a change of scale is used,where is the highest diffusion coefficient. It makes it necessary to define ; ; ; and .
- Given that the aim is to simulate experiments involving different time scales, the time axis is not changed.
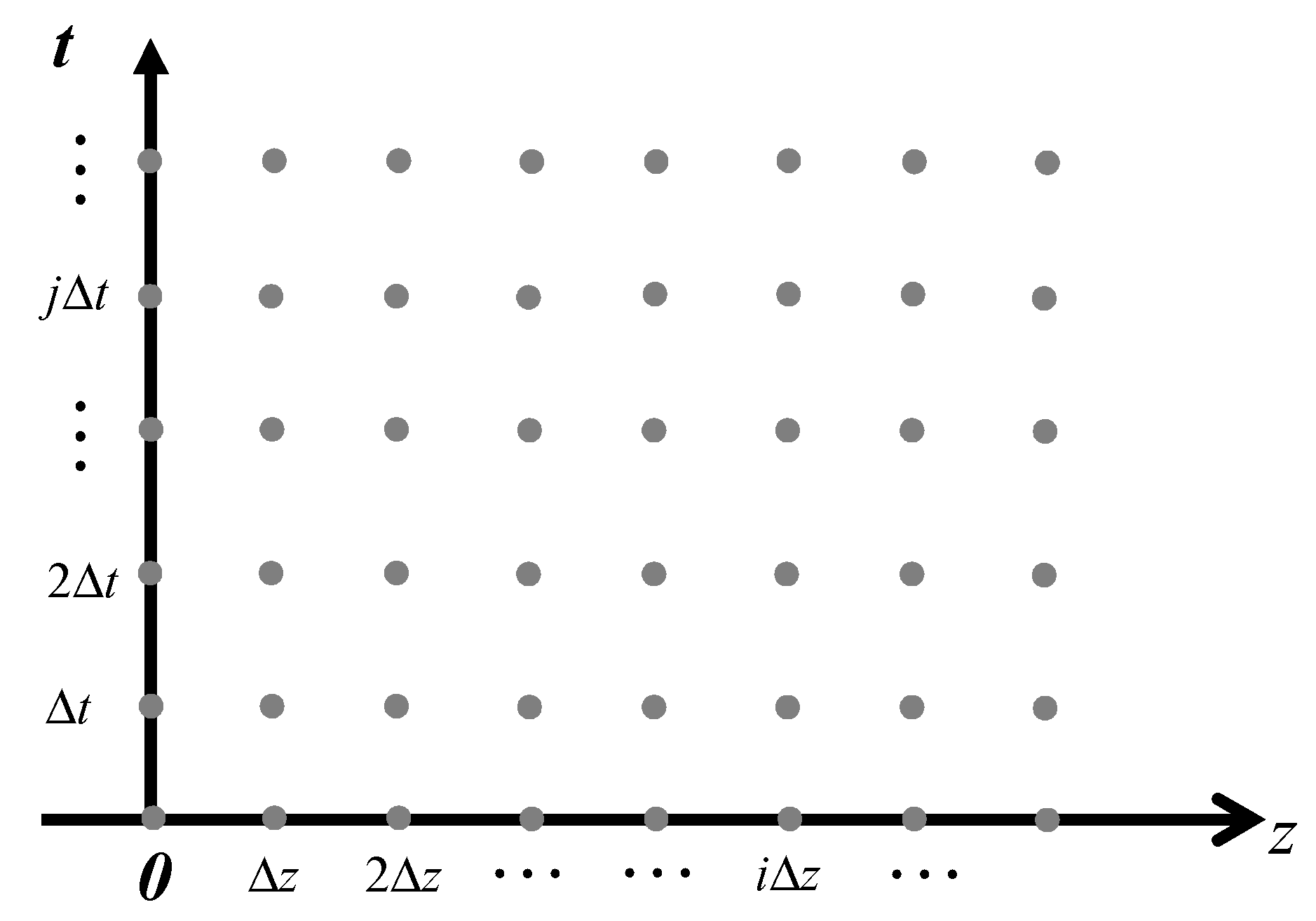
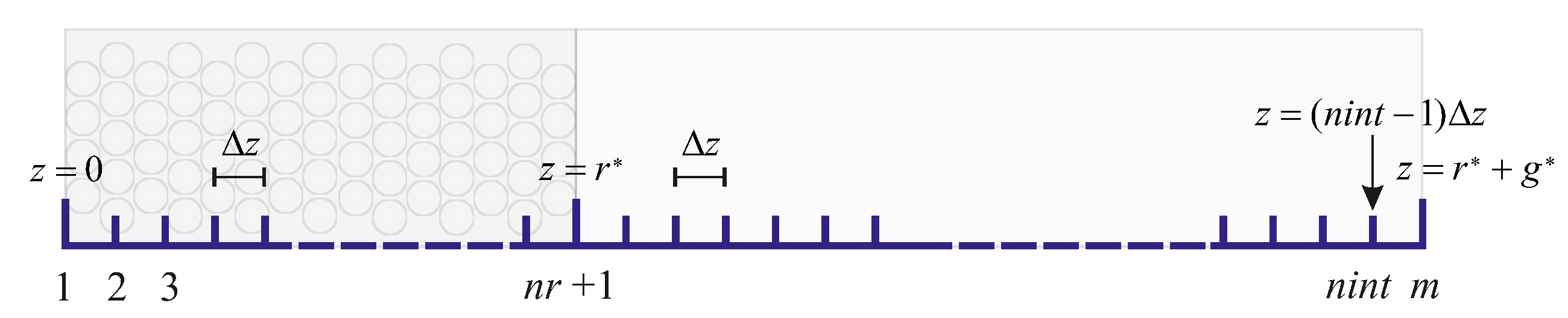
2.6. Discretization
3. Iterative Resolution of the Resulting System of Equations
- 1.
- Initialize all concentrations at with the solution at time t.
- 2.
- forTo solve discrete i-th equation (for the species i-th) considering all other j-th species () constant in this stage and whose value at being the corresponding to its most recent calculation.endfor
- 3.
- Repeat step 2 until the difference between concentrations in two consecutive iterations be less than a small fixed value.
4. Results and Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DGT | Diffusive Gradients in Thin Films |
| PDE | Partial differential equations |
Appendix A. General Resolution Algorithm
References
- Buffle, J.; Horvai, G. In Situ Monitoring of Aquatic Systems: Chemical Analysis and Speciation; John Wiley and Sons: Chichester, UK, 2000. [Google Scholar]
- Crank, J. The Mathematics of Diffusion; Clarendon Press: Oxford, UK, 1975. [Google Scholar]
- Mongin, S.; Uribe, R.; Puy, J.; Cecília, J.; Galceran, J.; Zhang, H.; Davison, W. Key role of the resin layer thickness in the lability of complexes measured by DGT. Environ. Sci. Technol. 2011, 45, 4869–4875. [Google Scholar] [CrossRef] [PubMed]
- Ames, W.F. Numerical Methods for Partial Differential Equations; Academic Press: London, UK, 1992. [Google Scholar]
- Hager, W.W. Applied Numerical Linear Algebra; Prentice-Hall: Upper Saddleriver, NJ, USA, 1988. [Google Scholar]
- Davison, W.; Zhang, H. In situ speciation measurements of trace components in natural waters using thin-film gels. Nature 1994, 367, 546–548. [Google Scholar] [CrossRef]
- Zhang, H.; Davison, W. Performance characteristics of Diffusive Gradients in Thin Films for the in situ measurement of trace metals in aqueous solution. Anal. Chem. 1995, 67, 3391–3400. [Google Scholar] [CrossRef]
- Garmo, O.A.; Lehto, N.J.; Zhang, H.; Davison, W.; Røyset, O.; Steinnes, E. Dynamic aspects of DGT as demonstrated by experiments with lanthanide complexes of a multidentate ligand. Environ. Sci. Technol. 2006, 40, 4754–4760. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Piedrahita, M.; Altier, A.; Cecilia, J.; Rey-Castro, C.; Galceran, J.; Puy, J. Influence of the settling of the resin beads on Diffusive Gradients in Thin Films measurements. Anal. Chim. Acta 2015, 885, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Uribe, R.; Mongin, S.; Puy, J.; Cecília, J.; Galceran, J.; Zhang, H.; Davison, W. Contribution of partially labile complexes to the DGT metal flux. Environ. Sci. Technol. 2011, 45, 5317–5322. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.; Griffiths, D. The Finite Difference Method in Partial Differential; John Wiley and Sons: Chichester, UK, 1980. [Google Scholar]
- Hughes, T.J. The Finite Element Method: Linear Static and Dynamic Finite Element Analysis; Prentice-Hall: Upper Saddleriver, NJ, USA, 1987. [Google Scholar]
- Salvador, J.; Garces, J.; Companys, E.; Cecilia, J.; Galceran, J.; Puy, J.; Town, R.M. Ligand mixture effects in metal complex lability. J. Phys. Chem. A 2007, 111, 4304–4311. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.L.; Zhang, H.; Davison, W.; Puy, J.; Galceran, J. Assessment of trace metal binding kinetics in the resin phase of Diffusive Gradients in Thin Films. Anal. Chim. Acta 2012, 717, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Shafaei, M.R.; Lehto, N.; Garmo, Ø.A.; Zhang, H. Kinetic studies of ni organic complexes using Diffusive Gradients in Thin Films (DGT) with double binding layers and a dynamic numerical model. Environ. Sci. Technol. 2013, 47, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Uribe, R.; Puy, J.; Cecília, J.; Galceran, J. Kinetic mixture effects in Diffusive Gradients in Thin Films (DGT). Phys. Chem. Chem. Phys. 2013, 15, 11349–11455. [Google Scholar] [CrossRef] [PubMed]
- Puy, J.; Uribe, R.; Mongin, S.; Galceran, J.; Cecília, J.; Levy, J.; Zhang, H.; Davison, W. Lability criteria in Diffusive Gradients in Thin Films. J. Phys. Chem. A 2012, 116, 6564–6573. [Google Scholar] [CrossRef] [PubMed]
- Puy, J.; Galceran, J.; Cruz-González, S.; David, C.; Uribe, R.; Lin, C.; Zhang, H.; Davison, W. Measurement of metals using DGT: Impact of ionic strength and kinetics of dissociation of complexes in the resin domain. Anal. Chem. 2014, 86, 7740–7748. [Google Scholar] [CrossRef] [PubMed]
- Altier, A.; Jiménez-Piedrahita, M.; Uribe, R.; Rey-Castro, C.; Galceran, J.; Puy, J. Time weighted average concentrations measured with Diffusive Gradients in Thin films (DGT). Anal. Chim. Acta 2019, 1060, 114–124. [Google Scholar] [CrossRef] [PubMed]
| 1 M + 1 L | 1 M + 2 L | 1 M + 14 L | |
|---|---|---|---|
| Program | 3’ 35” | 5’ 01” | 45’ 31” |
| COMSOL | 10’ 11” | 14’ 25” | 1 h 6’ 34” |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Averós, J.C.; Llorens, J.P.; Uribe-Kaffure, R. Numerical Simulation of Non-Linear Models of Reaction—Diffusion for a DGT Sensor. Algorithms 2020, 13, 98. https://doi.org/10.3390/a13040098
Averós JC, Llorens JP, Uribe-Kaffure R. Numerical Simulation of Non-Linear Models of Reaction—Diffusion for a DGT Sensor. Algorithms. 2020; 13(4):98. https://doi.org/10.3390/a13040098
Chicago/Turabian StyleAverós, Joan Cecilia, Jaume Puy Llorens, and Ramiro Uribe-Kaffure. 2020. "Numerical Simulation of Non-Linear Models of Reaction—Diffusion for a DGT Sensor" Algorithms 13, no. 4: 98. https://doi.org/10.3390/a13040098
APA StyleAverós, J. C., Llorens, J. P., & Uribe-Kaffure, R. (2020). Numerical Simulation of Non-Linear Models of Reaction—Diffusion for a DGT Sensor. Algorithms, 13(4), 98. https://doi.org/10.3390/a13040098





