Pulmonary Fissure Detection in 3D CT Images Using a Multiple Section Model
Abstract
1. Introduction
2. Related Works
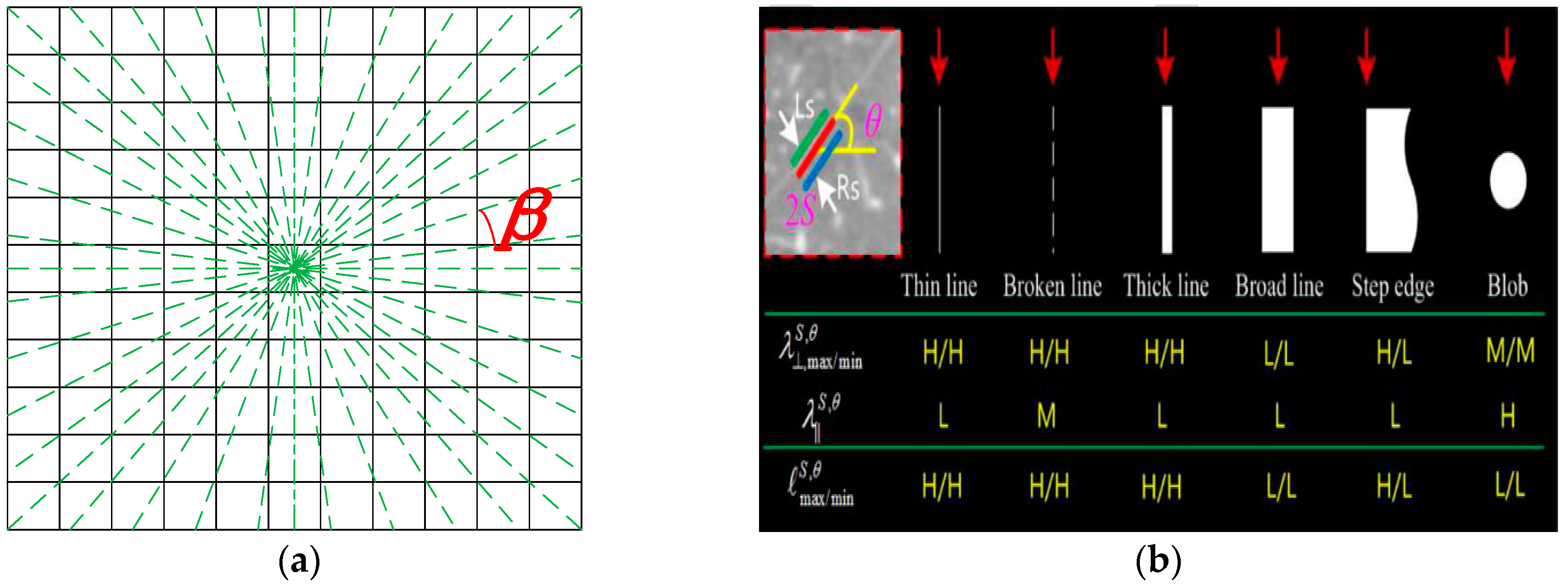
2.1. A Derivative of Stick Filter (DoS) for Fissure Enhancement
2.2. An Oriented Derivative of Stick (ODoS) Filter for Fissure Enhancement
3. Pulmonary Fissure Detection
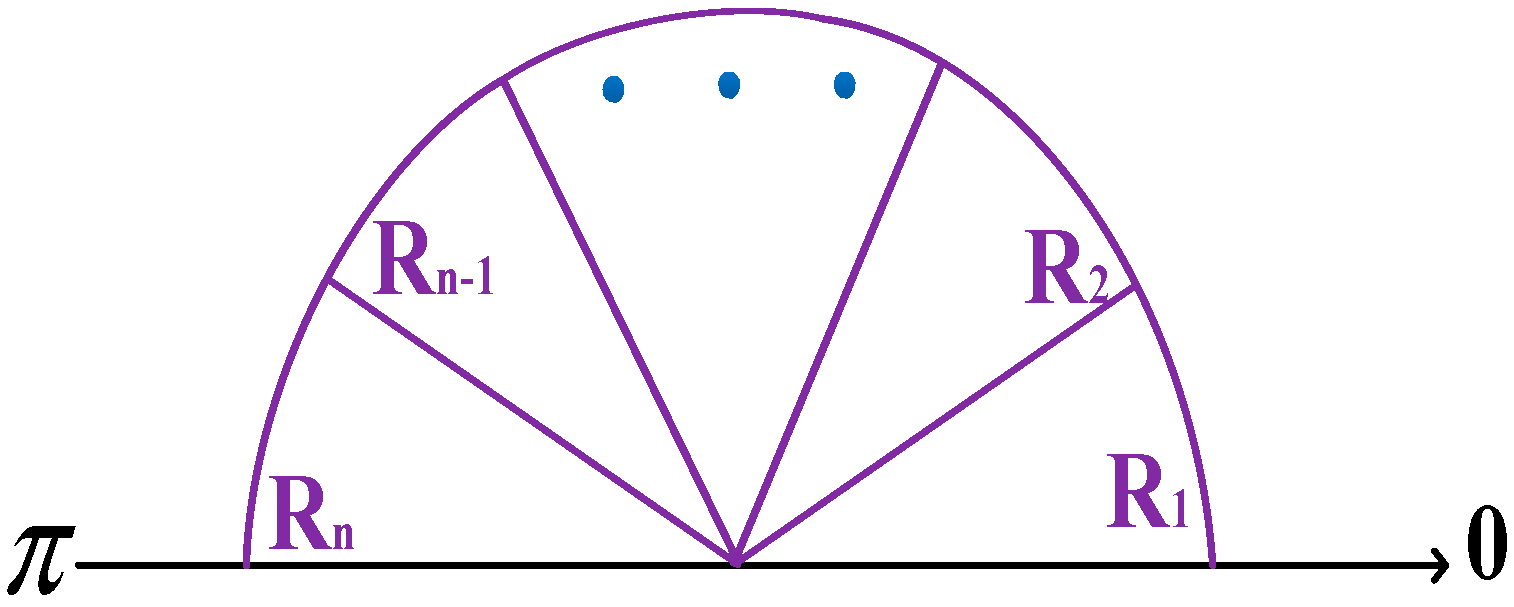
3.1. Improved Orientation Partition Scheme
3.2. Multiple Section Model
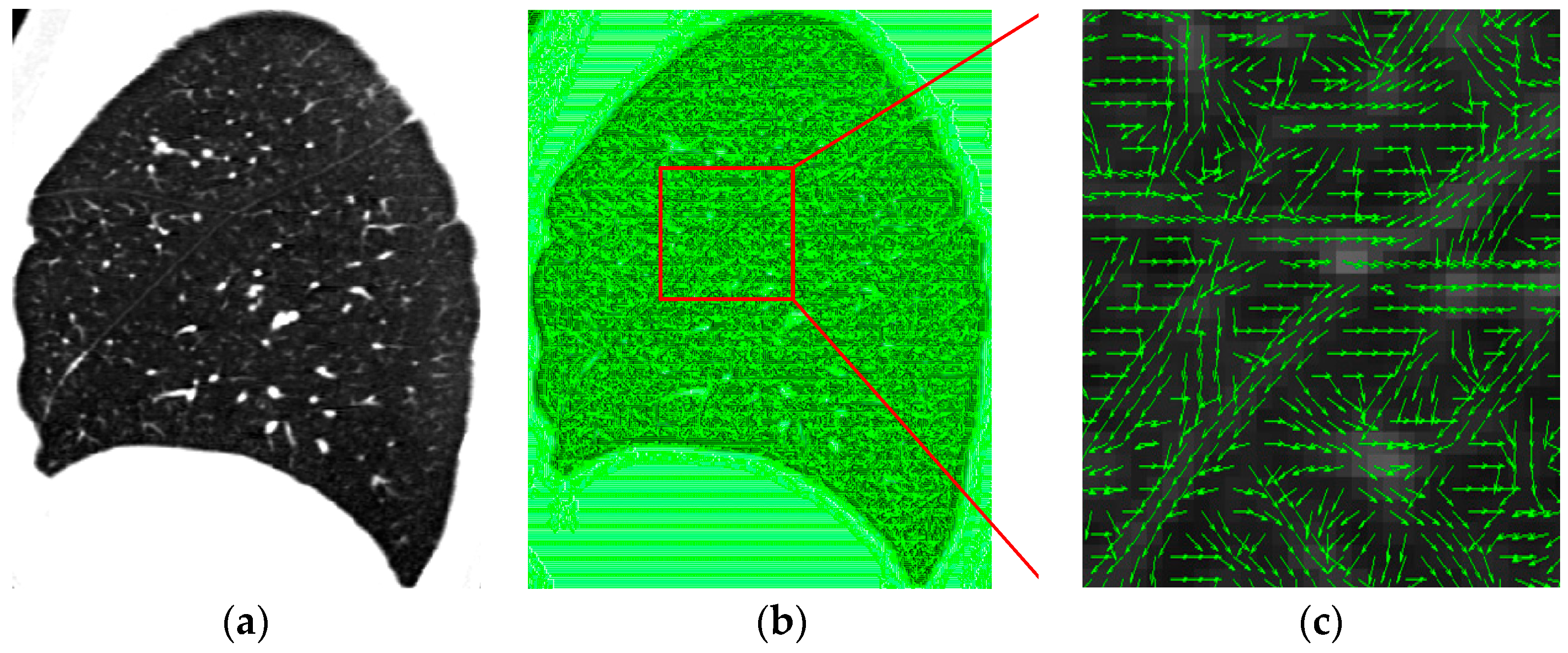
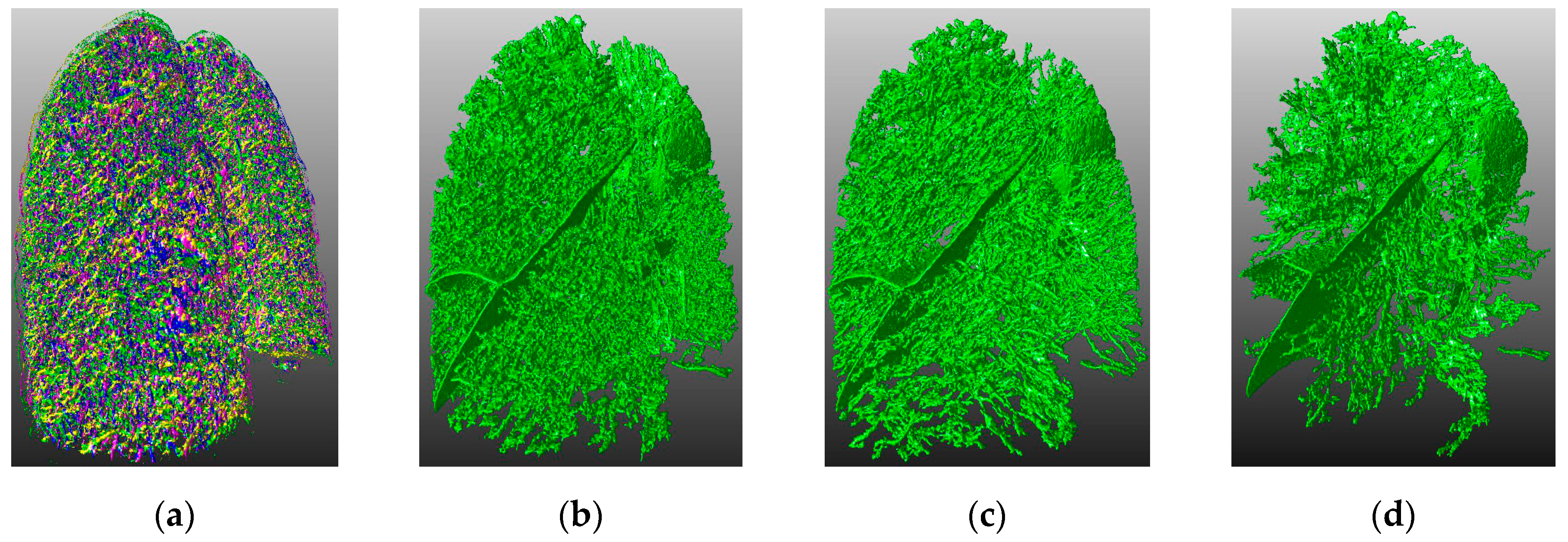
4. Experimental Results
4.1. Data and References
4.2. Evaluation Criteria
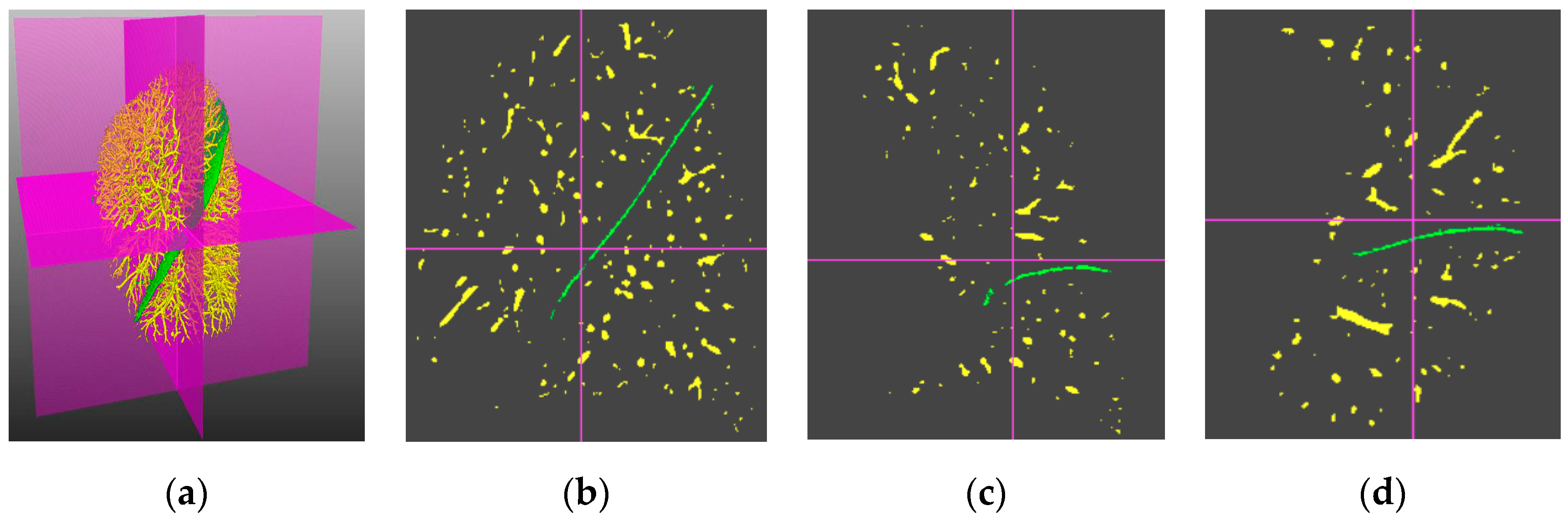
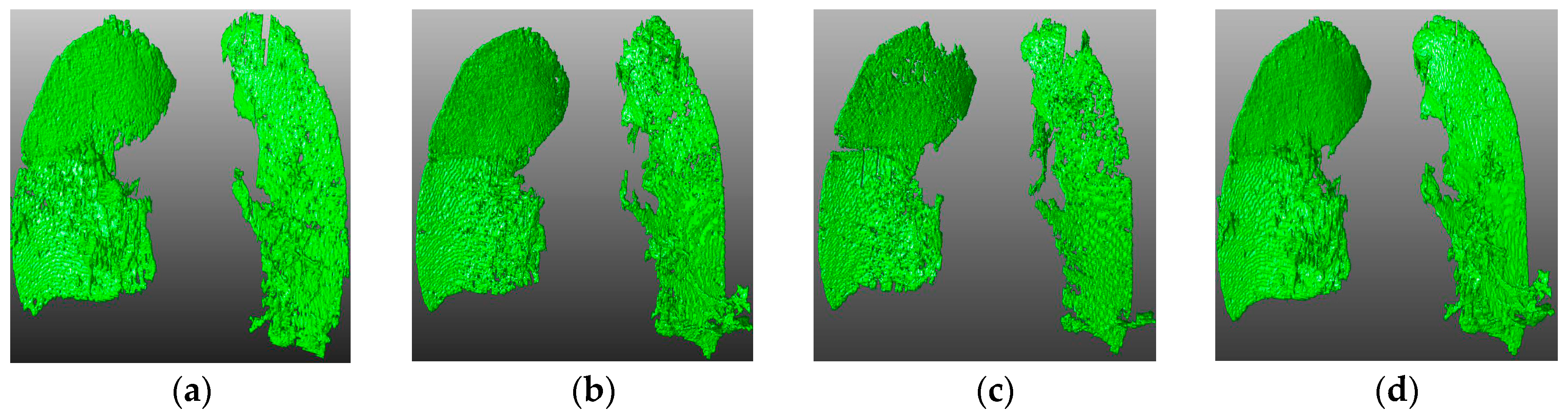
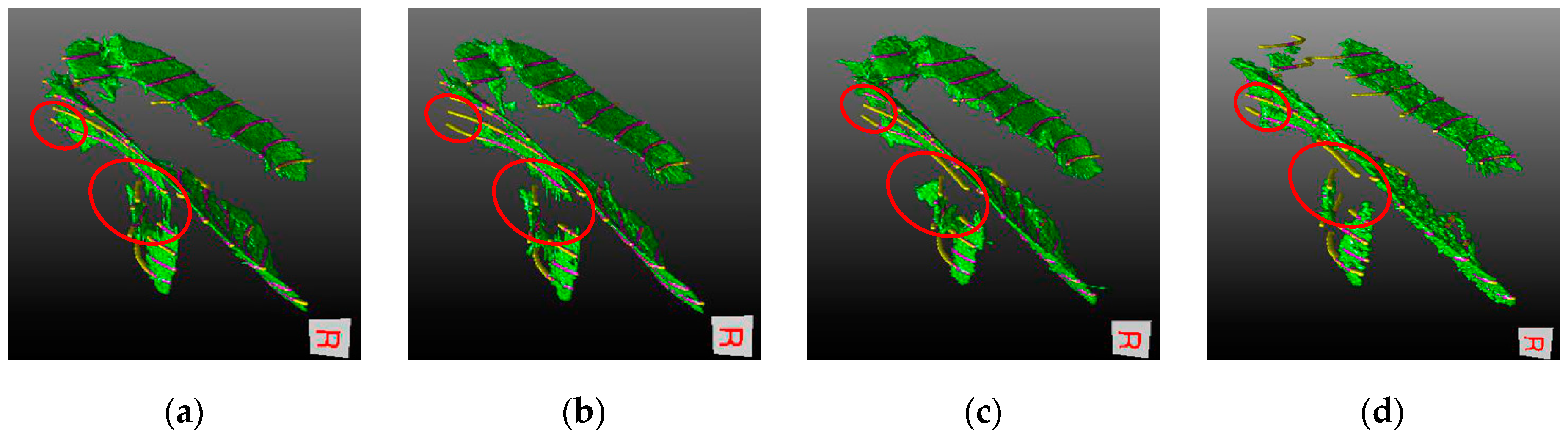
4.3. Visual Inspection
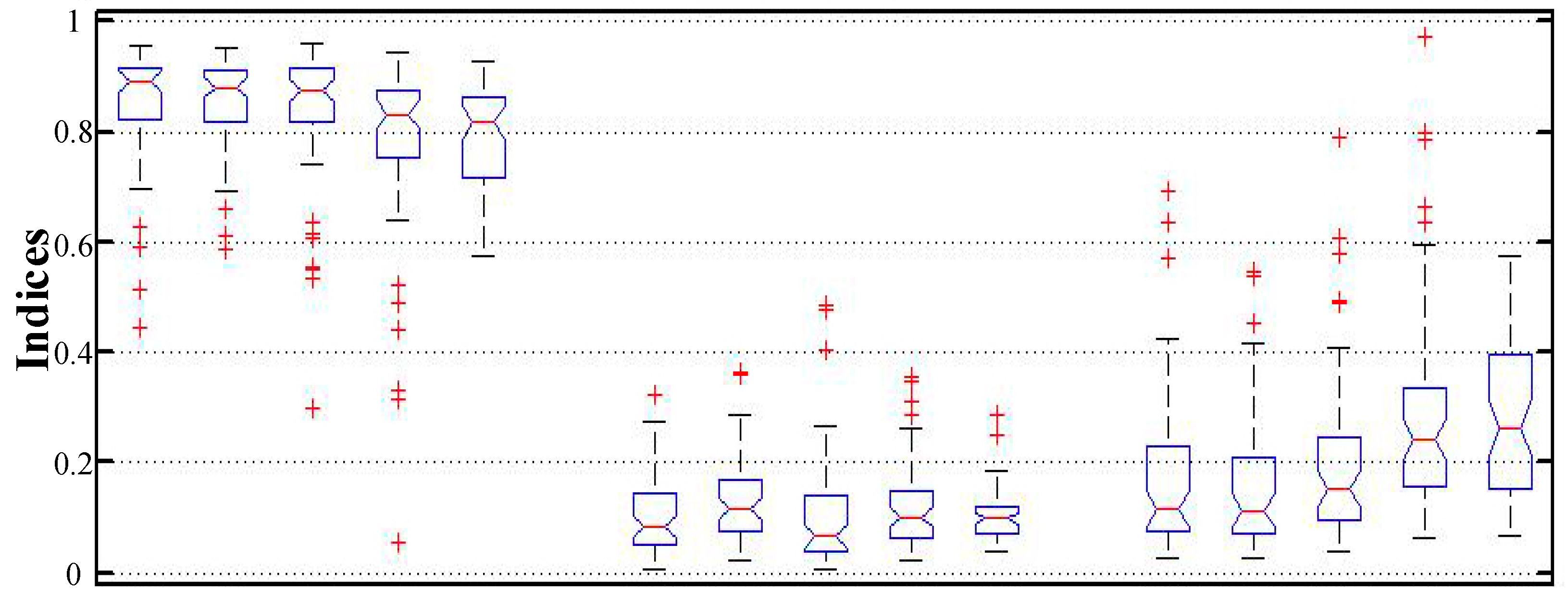
4.4. Quantitative Evaluation
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lassen-Schmidt, B.C.; Kuhnigk, J.M.; Konrad, O.; Van Ginneken, B.; Van Rikxoort, E.M. Fast interactive segmentation of the pulmonary lobes from thoracic computed tomography data. Phys. Med. Biol. 2017, 62, 6649. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhou, K.; Wang, M.; Lin, R.; Fan, J.; Che, G. Degree of pulmonary fissure completeness can predict postoperative cardiopulmonary complications and length of hospital stay in patients undergoing video-assisted thoracoscopic lobectomy for early-stage lung cancer. Interact. Cardiov. Thorac. Surg. 2017, 26, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Doel, T.; Gavaghan, D.J.; Grau, V. Review of automatic pulmonary lobe segmentation methods from CT. Comput. Med. Imag. Graph. 2015, 40, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Wiemker, R.; Bülow, T.; Blaffert, T. Unsupervised extraction of the pulmonary interlobar fissures from high resolution thoracic CT data. In International Congress Series; Elsevier: Amsterdam, The Netherlands, 2005; Volume 1281, pp. 1121–1126. [Google Scholar]
- Lassen, B.; van Rikxoort, E.M.; Schmidt, M.; Kerkstra, S.; van Ginneken, B.; Kuhnigk, J.M. Automatic segmentation of the pulmonary lobes from chest CT scans based on fissures, vessels, and bronchi. IEEE Trans. Med. Imaging 2013, 32, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Shamonin, D.P.; Staring, M.; Bakker, M.E.; Xiao, C.; Stolk, J.; Reiber, J.H.; Stoel, B.C. Automatic lung lobe segmentation of COPD patients using iterative B-spline fitting. In Medical Imaging 2012: Image Processing; International Society for Optics and Photonics: Bellingham, WA, USA, 2012; Volume 8314, p. 83140W. [Google Scholar]
- Doel, T.; Matin, T.N.; Gleeson, F.V.; Gavaghan, D.J.; Grau, V. Pulmonary lobe segmentation from CT images using fissureness, airways, vessels and multilevel B-splines. In Proceedings of the 2012 9th IEEE International Symposium on Biomedical Imaging, Barcelona, Spain, 2–5 May 2012; pp. 1491–1494. [Google Scholar]
- Chen, C.J.; Wang, Y.W.; Shen, W.C.; Chen, C.Y.; Fang, W.P. The lobe fissure tracking by the modified ant colony optimization framework in CT images. Algorithms 2014, 7, 635–649. [Google Scholar] [CrossRef]
- Qi, S.; van Triest, H.J.; Yue, Y.; Xu, M.; Kang, Y. Extraction algorithm of pulmonary fissures from thin-section CT images based on linear feature detector method. IEEE Trans. Nucl. Sci. 1999, 46, 2128–2133. [Google Scholar]
- Zhang, L.; Hoffman, J.M.; Reinhardt, J.M. Atlas-driven lung lobe segmentation in volumetric X-ray CT images. IEEE Trans. Med. Imaging 2006, 25, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Betke, M.; Ko, J.P. Pulmonary fissure segmentation on CT. Med. Image Anal. 2006, 10, 530–547. [Google Scholar] [CrossRef] [PubMed]
- Klinder, T.; Wendland, H.; Wiemker, R. Lobar fissure detection using line enhancing filters. In Medical Imaging 2013: Image Processing; International Society for Optics and Photonics: Bellingham, WA, USA, 2013; Volume 8669, p. 86693C. [Google Scholar]
- Xiao, C.; Stoel, B.C.; Bakker, M.E.; Peng, Y.; Stolk, J.; Staring, M. Pulmonary fissure detection in CT images using a derivative of stick filter. IEEE Trans. Med. Imaging 2016, 35, 1488–1500. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Xiao, C. An oriented derivative of stick filter and post-processing segmentation algorithms for pulmonary fissure detection in CT images. Biomed. Signal Process. Control 2018, 43, 278–288. [Google Scholar] [CrossRef]
- Pu, J.; Leader, J.K.; Zheng, B.; Knollmann, F.; Fuhrman, C.; Sciurba, F.C.; Gur, D. A computational geometry approach to automated pulmonary fissure segmentation in CT examinations. IEEE Trans. Med. Imaging 2009, 28, 710–719. [Google Scholar] [PubMed]
- Gu, S.; Wilson, D.; Wang, Z.; Bigbee, W.L.; Siegfried, J.; Gur, D.; Pu, J. Identification of pulmonary fissures using a piecewise plane fitting algorithm. Comput. Med. Imaging Graph. 2012, 36, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, W. Orientation image analysis of electrospun submicro-fibers based on Hough transform and Regionprops function. Text. Res. J. 2017, 87, 2263–2274. [Google Scholar] [CrossRef]
- Mredhula, L.; Dorairangaswamy, M.A. An effective filtering technique for image denoising using probabilistic principal component analysis (PPCA). J. Med. Imaging Health Inform. 2016, 6, 194–203. [Google Scholar] [CrossRef]
- Ukil, S.; Reinhardt, J.M. Anatomy-guided lung lobe segmentation in X-ray CT images. IEEE Trans. Med. Imaging 2009, 28, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; van Triest, H.J.; Yue, Y.; Xu, M.; Kang, Y. Automatic pulmonary fissure detection and lobe segmentation in CT chest images. Biomed. Eng. Online 2014, 13, 59. [Google Scholar] [CrossRef] [PubMed]
- Van Rikxoort, E.M.; Van Ginneken, B. Automatic segmentation of the lungs and lobes from thoracic CT scans. In Proceedings of the 4th International Workshop Pulmonary Image Analysis, Toronto, ON, Canada, 18 September 2011; pp. 261–268. [Google Scholar]
- Bragman, F.J.; McClelland, J.R.; Jacob, J.; Hurst, J.R.; Hawkes, D.J. Pulmonary lobe segmentation with probabilistic segmentation of the fissures and a groupwise fissure prior. IEEE Trans. Med. Imaging 2017, 36, 1650–1663. [Google Scholar] [CrossRef] [PubMed]

| 400 × 512 × 512 | Proposed method | ODoS | DoS | Fissureness |
| Runtime | 1410s | 1390s | 1470s | 600s |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, R.; Zhou, J. Pulmonary Fissure Detection in 3D CT Images Using a Multiple Section Model. Algorithms 2019, 12, 75. https://doi.org/10.3390/a12040075
Xiao R, Zhou J. Pulmonary Fissure Detection in 3D CT Images Using a Multiple Section Model. Algorithms. 2019; 12(4):75. https://doi.org/10.3390/a12040075
Chicago/Turabian StyleXiao, Runing, and Jinzhi Zhou. 2019. "Pulmonary Fissure Detection in 3D CT Images Using a Multiple Section Model" Algorithms 12, no. 4: 75. https://doi.org/10.3390/a12040075
APA StyleXiao, R., & Zhou, J. (2019). Pulmonary Fissure Detection in 3D CT Images Using a Multiple Section Model. Algorithms, 12(4), 75. https://doi.org/10.3390/a12040075





