Efficient Deep Learning-Based Automated Pathology Identification in Retinal Optical Coherence Tomography Images
Abstract
1. Introduction
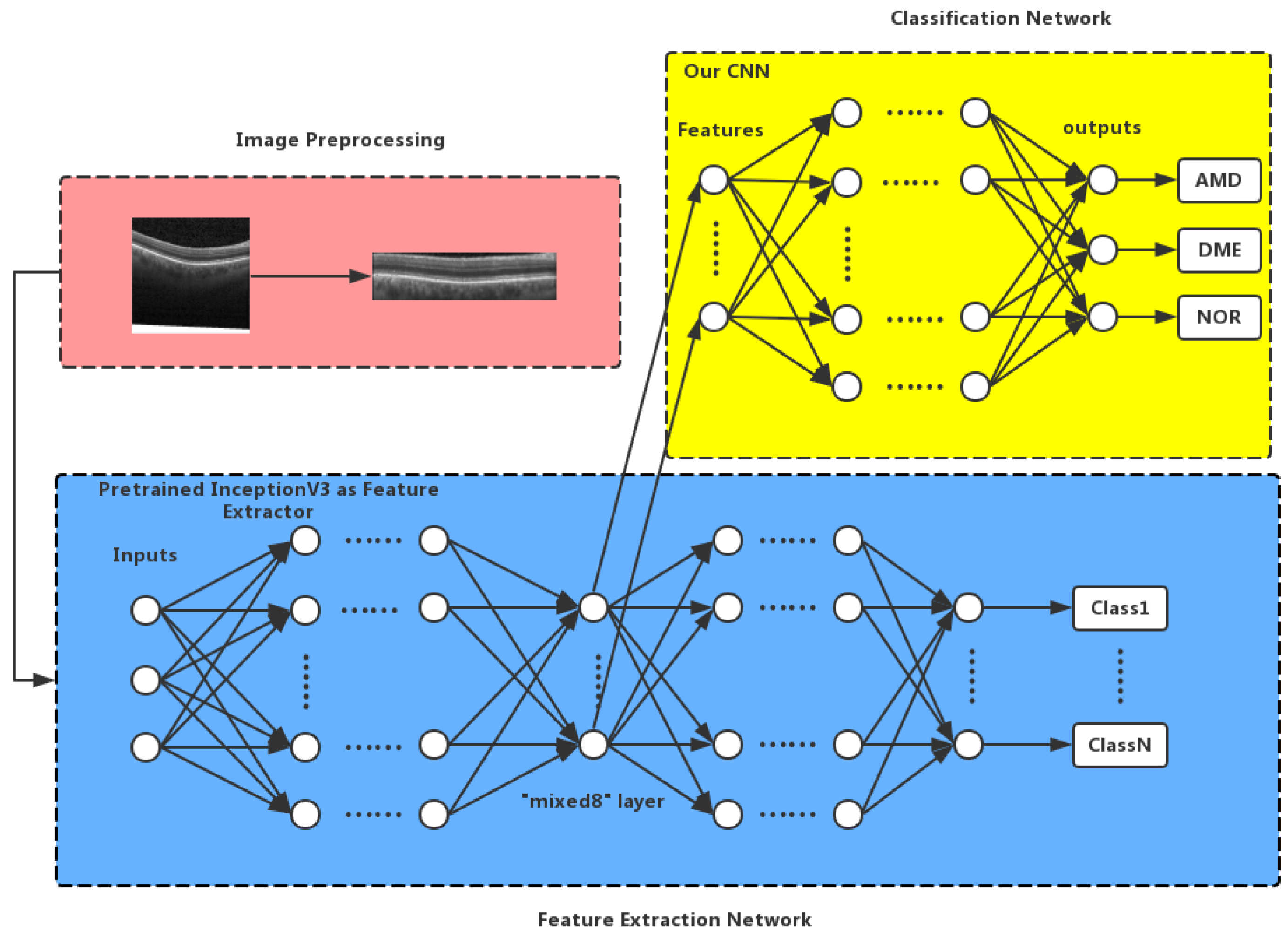
2. Proposed Method
2.1. Image Preprocessing
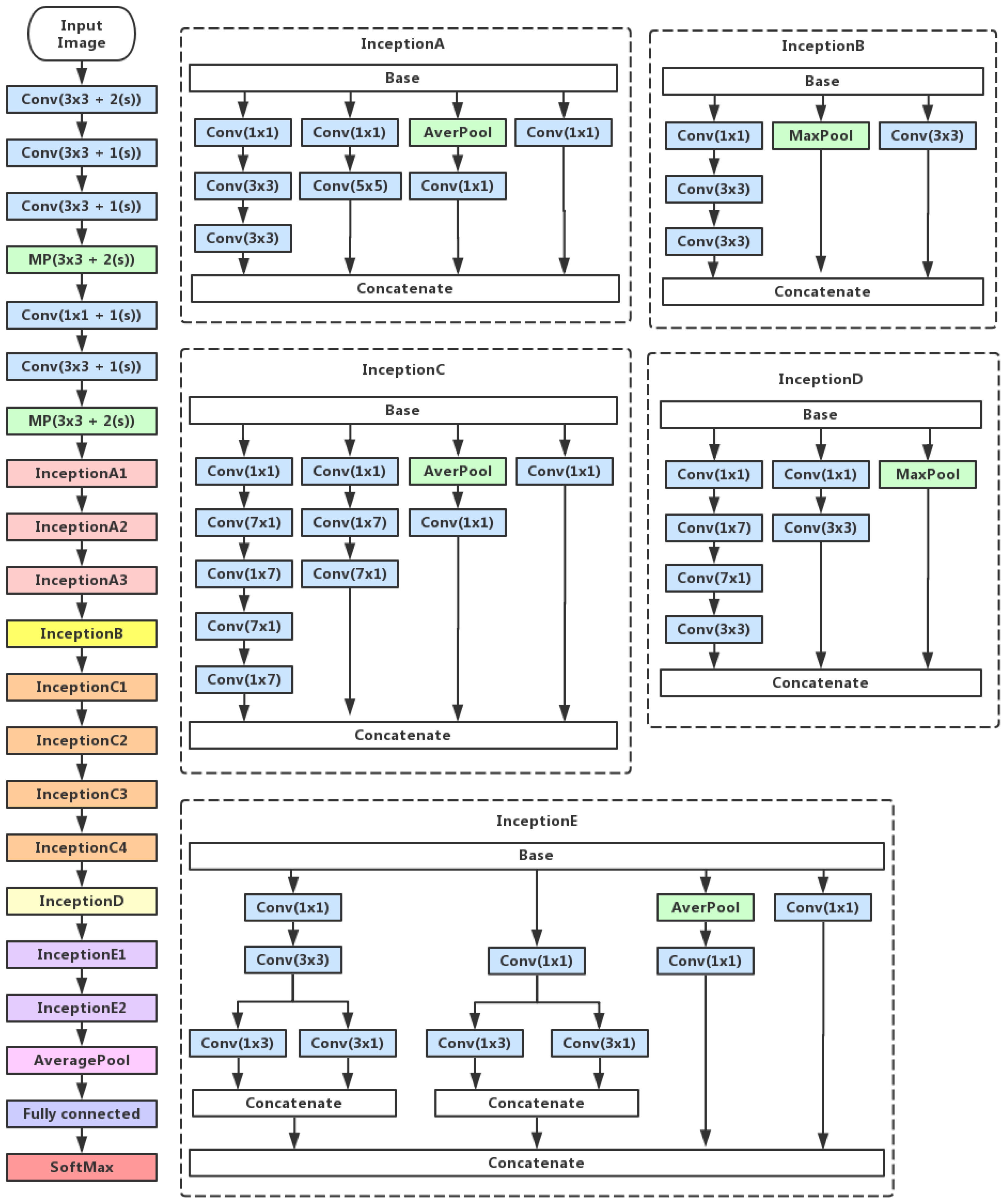
2.2. Inception V3 Feature Extraction
2.3. Convolutional Neural Network
3. Experiments and Results
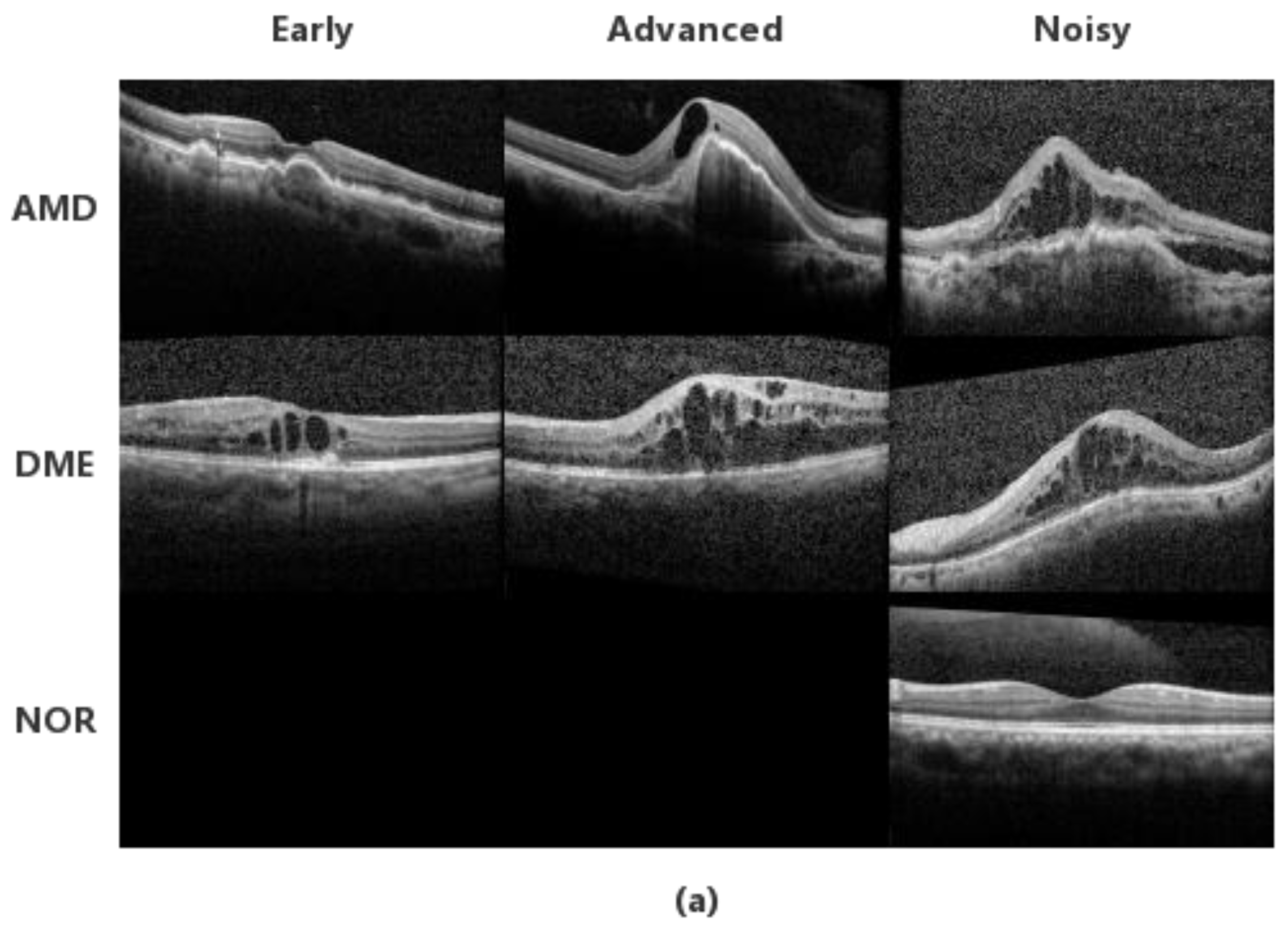
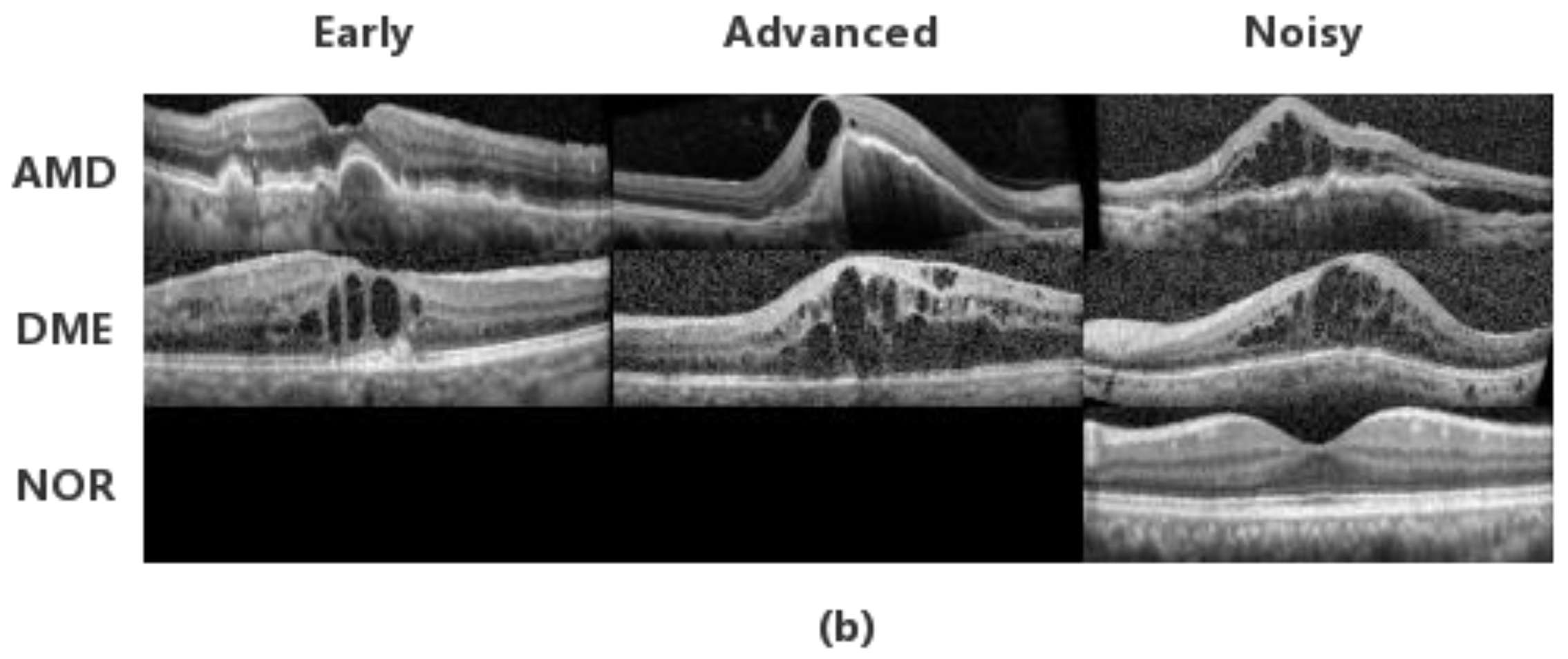
3.1. Datasets
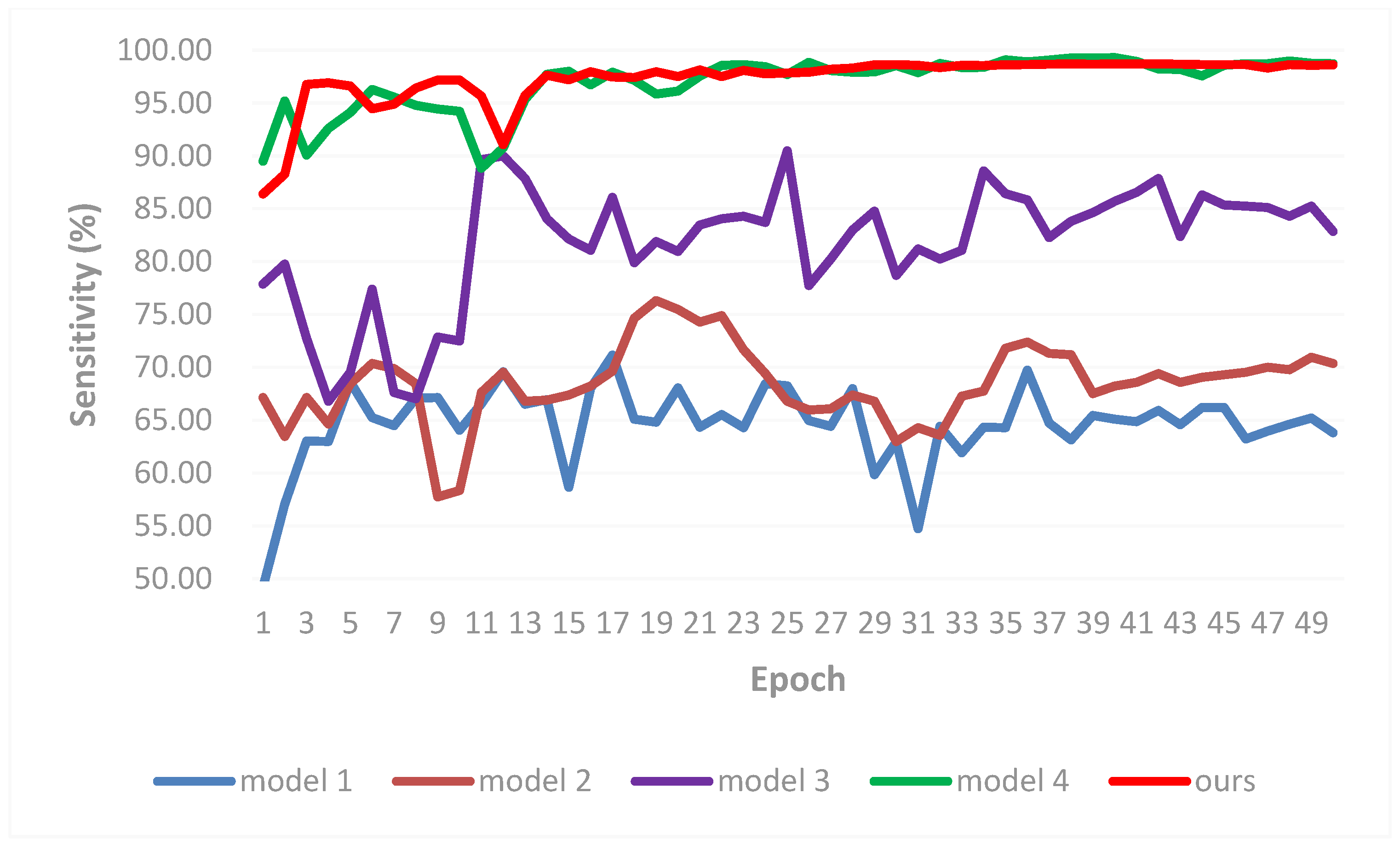
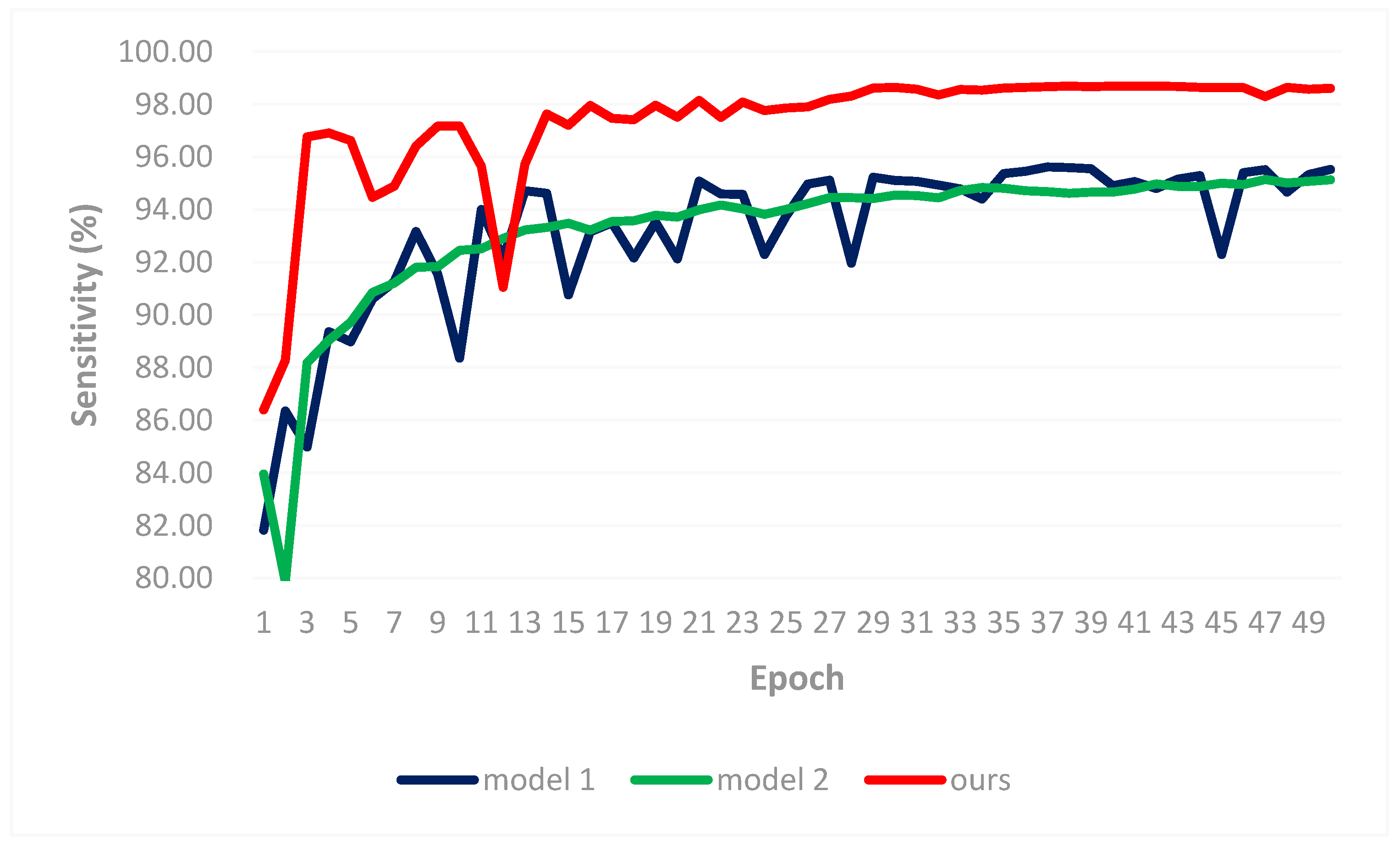
3.2. Result Comparisons
- Experiment 2: Comparison to transfer learning-based method [31]
- Experiment 3: Comparisons on classification performance of retinal OCT B-scans
- Experiment 4: Comparison with the efficiency of the fine-tuning
- Experiment 5: Effectiveness of different architectures
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Grogory, K.; Puliafito, C.A.; et al. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Podoleanu, A.G.; Rosen, R.B. Combinations of techniques in imaging the retina with high resolution. Prog. Retinal Eye Res. 2008, 27, 464–499. [Google Scholar] [CrossRef] [PubMed]
- Cogliati, A.; Canavesi, C.; Hayes, A.; Tankam, P.; Duma, V.-F.; Santhanam, A.; Thompson, K.P.; Rolland, J.P. MEMS-based handheld scanning probe with pre-shaped input signals for distortion-free images in Gabor-Domain Optical Coherence Microscopy. Opt. Express 2016, 24, 13365–13374. [Google Scholar] [CrossRef] [PubMed]
- Choma, M.A.; Sarunic, M.V.; Yang, C.; Izatt, J.A. Sensitivity advantage of swept-source and Fourier-domain optical coherence tomography. Opt. Express 2003, 11, 2183–2189. [Google Scholar] [CrossRef] [PubMed]
- Virgili, G.; Menchini, F.; Casazza, G.; Hogg, R.; Das, R.R.; Wang, X.; Michelessi, M. Optical coherence tomography (OCT) for detection of macular oedema in patients with diabetic retinopathy. Cochrane Database Syst. 2015, 1, CD008081. [Google Scholar] [CrossRef] [PubMed]
- Keane, P.A.; Patel, P.J.; Liakopoulos, S.; Heussen, F.M.; Sadda, S.R.; Tufail, A. Evaluation of age-related macular degeneration with optical coherence tomography. Surv. Ophthalmol. 2012, 57, 389–414. [Google Scholar] [CrossRef] [PubMed]
- Antony, B.J.; Abràmoff, M.D.; Harper, M.M.; Jeong, W.; Sohn, E.H.; Kwon, Y.H.; Kardon, R.; Garvin, M.K. A combined machine-learning and graph-based framework for the segmentation of retinal surfaces in SD-OCT volumes. Biomed. Opt. Express 2013, 4, 2712–2728. [Google Scholar] [CrossRef] [PubMed]
- Carass, A.; Lang, A.; Hauser, M.; Calabresi, P.A.; Ying, H.S.; Prince, J.L. Multiple-object geometric deformable model for segmentation of macular OCT. Biomed. Opt. Express 2014, 5, 1062–1074. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.J.; Izatt, J.A.; O’Connell, R.V.; Winter, K.P.; Toth, C.A.; Farsiu, S. Validated automatic segmentation of AMD pathology including drusen and geographic atrophy in SD-OCT images. Investig. Ophthalmol. Vis. Sci. 2012, 53, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.J.; Li, X.T.; Nicholas, P.; Toth, C.A.; Izatt, J.A.; Farsiu, S. Automatic segmentation of seven retinal layers in SDOCT images congruent with expert manual segmentation. Opt. Express 2010, 18, 19413–19428. [Google Scholar] [CrossRef] [PubMed]
- DeBuc, D.C.; Somfai, G.M.; Ranganathan, S.; Tátrai, E.; Ferencz, M.; Puliafito, C.A. Reliability and reproducibility of macular segmentation using a custom-built optical coherence tomography retinal image analysis software. J. Biomed. Opt. 2009, 14, 064023. [Google Scholar] [CrossRef] [PubMed]
- Fernández, D.C.; Salinas, H.M.; Puliafito, C.A. Automated detection of retinal layer structures on optical coherence tomography images. Opt. Express 2005, 13, 10200–10216. [Google Scholar] [CrossRef]
- Ishikawa, H.; Stein, D.M.; Wollstein, G.; Beaton, S.; Fujimoto, J.G.; Schuman, J.S. Macular segmentation with optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2012–2017. [Google Scholar] [CrossRef] [PubMed]
- Lang, A.; Carass, A.; Hauser, M.; Sotirchos, E.S.; Calabresi, P.A.; Ying, H.S.; Prince, J.L. Retinal layer segmentation of macular OCT images using boundary classification. Biomed. Opt. Express 2013, 4, 1133–1152. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.A.; Hornegger, J.; Mardin, C.Y.; Tornow, R.P. Retinal nerve fiber layer segmentation on FD-OCT scans of normal subjects and glaucoma patients. Biomed. Opt. Express 2010, 1, 1358–1383. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Wong, A.; Bizheva, K.; Clausi, D.A. Intra-retinal layer segmentation in optical coherence tomography images. Opt. Express 2009, 17, 23719–23728. [Google Scholar] [CrossRef] [PubMed]
- Mujat, M.; Chan, R.; Cense, B.; Park, B.; Joo, C.; Akkin, T.; Chen, T.; de Boer, J. Retinal nerve fiber layer thickness map determined from optical coherence tomography images. Opt. Express 2005, 13, 9480–9491. [Google Scholar] [CrossRef] [PubMed]
- Paunescu, L.A.; Schuman, J.S.; Price, L.L.; Stark, P.C.; Beaton, S.; Ishikawa, H.; Wollstein, G.; Fujimoto, J.G. Reproducibility of nerve fiber thickness, macular thickness, and optic nerve head measurements using StratusOCT. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1716–1724. [Google Scholar] [CrossRef]
- Shahidi, M.; Wang, Z.; Zelkha, R. Quantitative thickness measurement of retinal layers imaged by optical coherence tomography. Am. J. Ophthalmol. 2005, 139, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, T.; Zhao, Y.; He, Y. 3D automatic segmentation method for retinal optical coherence tomography volume data using boundary surface enhancement. J. Innov. Opt. Health Sci. 2016, 9, 1650008. [Google Scholar] [CrossRef]
- Vermeer, K.A.; van der Schoot, J.; Lemij, H.G.; de Boer, J.F. Automated segmentation by pixel classification of retinal layers in ophthalmic OCT images. Biomed. Opt. Express 2011, 2, 1743–1756. [Google Scholar] [CrossRef] [PubMed]
- Reisman, C.A.; Chan, K.; Ramachandran, R.; Raza, A.; Hood, D.C. Automated segmentation of outer retinal layers in macular OCT images of patients with retinitis pigmentosa. Biomed. Opt. Express 2011, 2, 2493–2503. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Chen, M.; Ishikawa, H.; Wollstein, G.; Schuman, J.S.; Rehg, J.M. Automated macular pathology diagnosis in retinal OCT images using multi-scale spatial pyramid and local binary patterns in texture and shape encoding. Med. Image Anal. 2011, 15, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Sugruk, J.; Kiattisin, S.; Leelasantitham, A. Automated classification between age-related macular degeneration and diabetic macular edema in OCT image using image segmentation. In Proceedings of the 7th Biomedical Engineering International Conference, Fukuoka, Japan, 26–28 November 2014; pp. 1–4. [Google Scholar]
- Srinivasan, P.P.; Kim, L.A.; Mettu, P.S.; Cousins, S.W.; Comer, G.M.; Izatt, J.A.; Farsiu, S. Fully automated detection of diabetic macular edema and dry age-related macular degeneration from optical coherence tomography images. Biomed. Opt. Express 2014, 5, 3568–3577. [Google Scholar] [CrossRef] [PubMed]
- Hassan, B.; Raja, G.; Hassan, T.; Usman Akram, M. Structure tensor based automated detection of macular edema and central serous retinopathy using optical coherence tomography images. J. Opt. Soc. Am. A 2016, 33, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.K.; Li, S.; Sun, Z.Y. Fully automated macular pathology detection in retina optical coherence tomography images using sparse coding and dictionary learning. J. Biomed. Opt. 2017, 22, 16012. [Google Scholar] [CrossRef] [PubMed]
- Venhuizen, F.G.; van Ginneken, B.; Bloemen, B.; van Grinsven, M.J.J.P.; Philipsen, R.; Hoyng, C.; Theelen, T.; Sánchez, C.I. Automated age-related macular degeneration classification in OCT using unsupervised feature learning. Med. Imaging Comput.-Aided Diagn. 2015, 9414. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Yao, Z.; Zhao, R.; Zhou, F. Machine learning based detection of age-related macular degeneration (AMD) and diabetic macular edema (DME) from optical coherence tomography (OCT) images. Biomed. Opt. Express 2016, 7, 4928–4940. [Google Scholar] [CrossRef] [PubMed]
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. Imagenet classification with deep convolutional neural networks. Adv. Neural Inf. Process. Syst. 2012, 1097–1105. [Google Scholar] [CrossRef]
- Karri, S.; Chakraborty, D.; Chatterjee, J. Transfer learning based classification of optical coherence tomography images with diabetic macular edema and dry age-related macular degeneration. Biomed. Opt. Express 2017, 8, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Szegedy, C.; Liu, W.; Jia, Y.; Sermanet, P.; Reed, S.; Anguelov, D.; Erhan, D.; Vanhoucke, V.; Rabinovich, A. Going deeper with convolutions. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Boston, MA, USA, 8–10 June 2015; pp. 1–9. [Google Scholar]
- Rasti, R.; Mehridehnavi, A. Macular OCT Classification using a Multi-Sacle Convolutional Neural Network Ensemble. IEEE Trans. Med. Imaging 2018. [Google Scholar] [CrossRef] [PubMed]
- Kermany, D.S.; Goldbaum, M.; Cai, W.; Valentim, C.C.S.; Liang, H.; Baxter, S.L.; McKeown, A.; Yang, G.; Wu, X.; Yan, F.; et al. Identifying Medical Diagnoses and Treatable Diseases by Image-Based Deep Learning. Cell 2018, 172, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Albarrak, A.; Coenen, F.; Zheng, Y.; Yu, W. Volumetric image mining based on decomposition and graph analysis: An application to retinal optical coherence tomography. Comput. Intell. Inform. 2012, 263–268. [Google Scholar] [CrossRef]
- Fang, L.; Wang, C.; Li, S.; Yan, J.; Chen, X.; Rabbani, H. Automatic classification of retinal three-dimensional optical coherence tomography images using principal component analysis network with composite kernels. J. Biomed. Opt. 2017, 22, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Dong, W.; Socher, R.; Li, L.J.; Li, K.; Li, F.-F. Imagenet: A large-scale hierarchical image database. In Proceedings of the Computer Vision and Pattern Recognition, Miami Beach, FL, USA, 22–25 June 2009; pp. 248–255. [Google Scholar]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep residual learning for image recognition. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition (CVPR 2016), Las Vegas, NV, USA, 27–30 June 2016. [Google Scholar]
- Zhou, Z.; Shin, J.; Zhang, L.; Gurudu, S.; Gotway, M.; Liang, J. Fine-tuning convolutional neural networks for biomedical image analysis: Actively and incrementally. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition (CVPR 2017), Honolulu, HI, USA, 21–26 July 2017; pp. 7340–7349. [Google Scholar]
- Tajbakhsh, N.; Shin, J.Y.; Gurudu, S.R.; Hurst, R.T.; Kendall, C.B.; Gotway, M.B.; Liang, J. Convolutional neural networks for medical image analysis: Full training or fine tuning? IEEE Trans. Med. Imaging 2016, 35, 1299–1312. [Google Scholar] [CrossRef] [PubMed]
- Szegedy, C.; Vanhoucke, V.; Ioffe, S.; Shlens, J.; Wojna, Z. Rethinking the inception architecture for computer vision. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition (CVPR 2016), Las Vegas, NV, USA, 27–30 June 2016. [Google Scholar]
- Dabov, K.; Foi, A.; Katkovnik, V.; Egiazarian, K. Image denoising by sparse 3-D transform-domain collaborative filtering. IEEE Trans. Image Process. 2007, 16, 2080–2095. [Google Scholar] [CrossRef] [PubMed]
- Matthew, D.Z. ADADELTA: An adaptive learning rate method. Tech. Rep. 2012, arXiv:1212.5701. [Google Scholar]
| Name | Patch Size/Stride | Padding | Output Size |
|---|---|---|---|
| Convolution1 | 3 × 3/1 | same | 128 × 8 × 8 |
| Max Pooling1 | 2 × 2/2 | valid | 128 × 4 × 4 |
| BatchNormalization1 | 128 × 4 × 4 | ||
| Convolution2 | 3 × 3/1 | same | 128 × 4 × 4 |
| Max Pooling2 | 2 × 2/2 | valid | 128 × 2 × 2 |
| BatchNormalization2 | 128 × 2 × 2 | ||
| Convolution3 | 3 × 3/1 | same | 128 × 2 × 2 |
| BatchNormalization3 | 128 × 2 × 2 | ||
| Flatten | 512 | ||
| Dense | 3 |
| HOG-SVM [25] | ScSPM [27] | Ours | |
|---|---|---|---|
| AMD | 15/15 = 100.00% | 15/15 = 100.00% | 15/15 = 100.00% |
| DME | 15/15 = 100.00% | 15/15 = 100.00% | 15/15 = 100.00% |
| NOR | 13/15 = 86.67% | 14/15 = 93.33% | 15/15 = 100.00% |
| Overall | 43/45 = 95.56% | 44/45 = 97.78% | 45/45 = 100.00% |
| HOG-SVM [25] | Deep CNN [31] | Ours | |
|---|---|---|---|
| AMD | 89 | 89 | 89 |
| DME | 83 | 86 | 92 |
| NOR | 90 | 99 | 100 |
| Methods | Classes | Accuracy | Sensitivity | Specificity |
|---|---|---|---|---|
| ScSPM | AMD | 97.35 ± 0.58 | 96.19 ± 0.85 | 97.94 ± 0.45 |
| DME | 97.17 ± 0.44 | 93.81 ± 0.51 | 98.87 ± 0.46 | |
| NOR | 97.87 ± 0.15 | 98.73 ± 0.49 | 97.44 ± 0.08 | |
| IBDL | AMD | 91.23 ± 0.38 | 85.40 ± 0.81 | 94.25 ± 0.58 |
| DME | 94.77 ± 0.29 | 96.83 ± 0.22 | 93.65 ± 0.57 | |
| NOR | 91.63 ± 0.40 | 85.32 ± 0.40 | 94.92 ± 0.66 | |
| Ours | AMD | 98.51 ± 0.19 | 98.14 ± 0.49 | 98.69 ± 0.44 |
| DME | 97.80 ± 0.35 | 94.57 ± 0.76 | 99.43 ± 0.31 | |
| NOR | 98.35 ± 0.20 | 99.33 ± 0.41 | 97.85 ± 0.38 |
| Methods | Classes | Accuracy | Sensitivity | Specificity |
|---|---|---|---|---|
| ScSPM | AMD | 97.75 ± 0.21 | 96.43 ± 0.58 | 98.43 ± 0.08 |
| DME | 97.60 ± 0.29 | 95.48 ± 0.89 | 98.67 ± 0.09 | |
| NOR | 97.91 ± 0.31 | 98.10 ± 0.84 | 97.81 ± 0.40 | |
| IBDL | AMD | 93.36 ± 0.32 | 88.84 ± 2.78 | 95.66 ± 1.02 |
| DME | 96.96 ± 0.14 | 98.13 ± 0.46 | 96.33 ± 0.24 | |
| NOR | 93.39 ± 0.25 | 89.11 ± 2.21 | 95.57 ± 0.99 | |
| Ours | AMD | 99.01 ± 0.30 | 99.02 ± 0.39 | 99.01 ± 0.37 |
| DME | 98.51 ± 0.27 | 96.34 ± 1.08 | 99.60 ± 0.20 | |
| NOR | 99.07 ± 0.21 | 99.55 ± 0.46 | 98.83 ± 0.32 |
| Partition | Methods | Overall-Acc | Overall-Se | Overall-Sp |
|---|---|---|---|---|
| 1/4 dataset | ScSPM | 97.46 | 96.24 | 98.08 |
| IBDL | 92.54 | 89.18 | 94.27 | |
| Ours | 98.22 | 97.35 | 98.66 | |
| 1/2 dataset | ScSPM | 97.75 | 96.67 | 98.30 |
| IBDL | 94.57 | 92.03 | 95.85 | |
| Ours | 98.86 | 98.30 | 99.15 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Q.; He, W.; Huang, J.; Sun, Y. Efficient Deep Learning-Based Automated Pathology Identification in Retinal Optical Coherence Tomography Images. Algorithms 2018, 11, 88. https://doi.org/10.3390/a11060088
Ji Q, He W, Huang J, Sun Y. Efficient Deep Learning-Based Automated Pathology Identification in Retinal Optical Coherence Tomography Images. Algorithms. 2018; 11(6):88. https://doi.org/10.3390/a11060088
Chicago/Turabian StyleJi, Qingge, Wenjie He, Jie Huang, and Yankui Sun. 2018. "Efficient Deep Learning-Based Automated Pathology Identification in Retinal Optical Coherence Tomography Images" Algorithms 11, no. 6: 88. https://doi.org/10.3390/a11060088
APA StyleJi, Q., He, W., Huang, J., & Sun, Y. (2018). Efficient Deep Learning-Based Automated Pathology Identification in Retinal Optical Coherence Tomography Images. Algorithms, 11(6), 88. https://doi.org/10.3390/a11060088




