Pickering Particles Prepared from Food Waste
Abstract
:1. Introduction
2. Results and Discussion
2.1. Properties of Prepared Coffee Pickering Particles
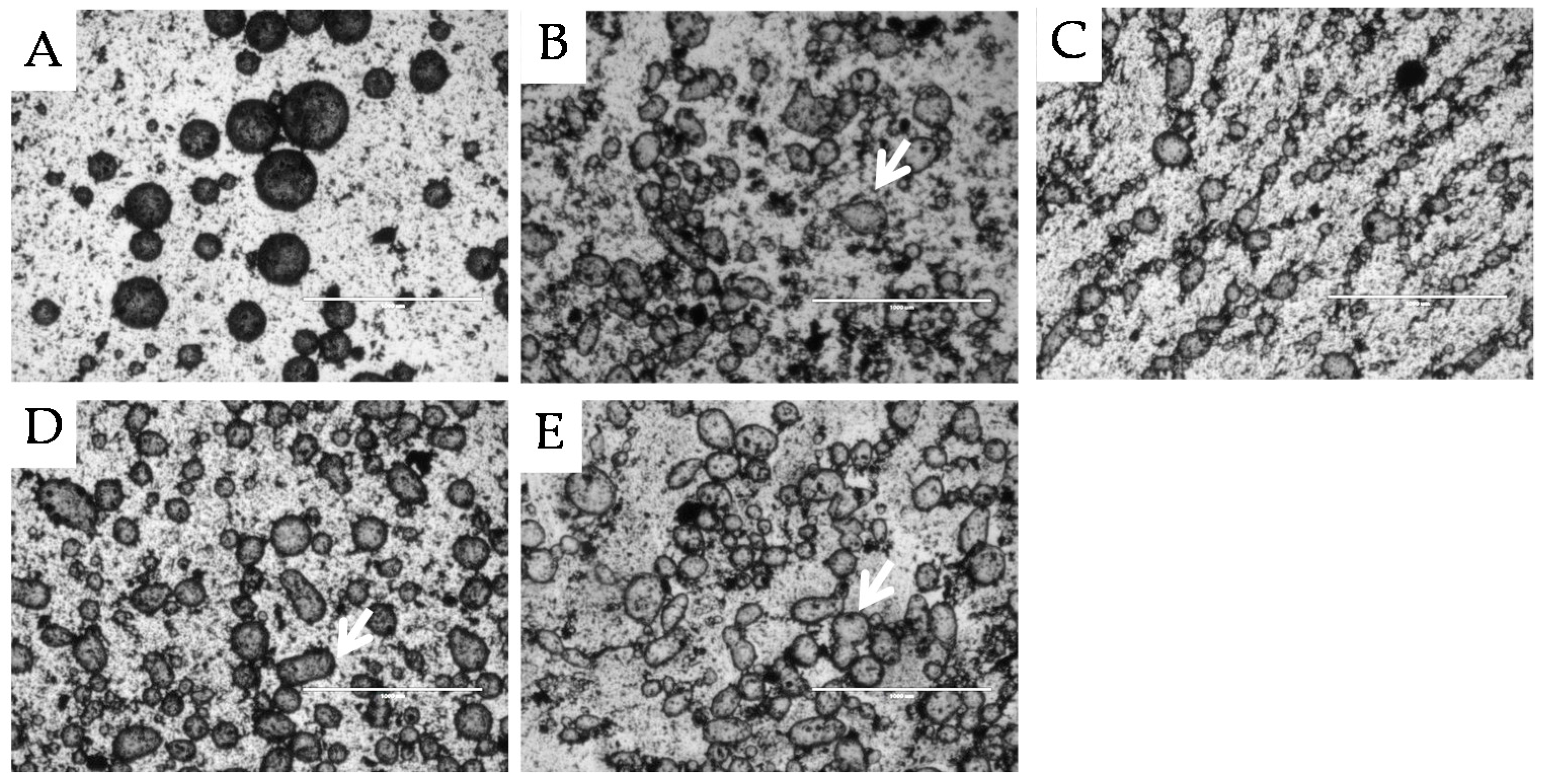
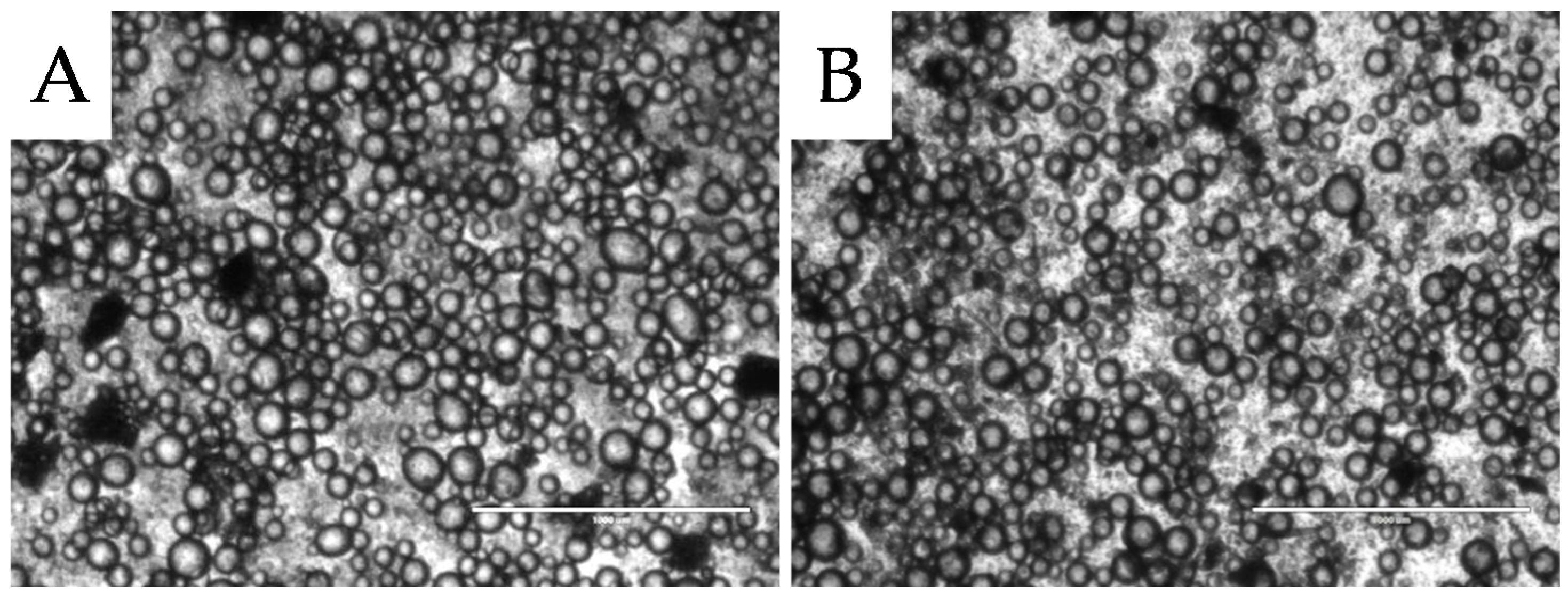
2.1.1. Surface Morphology
2.1.2. Lignin Content
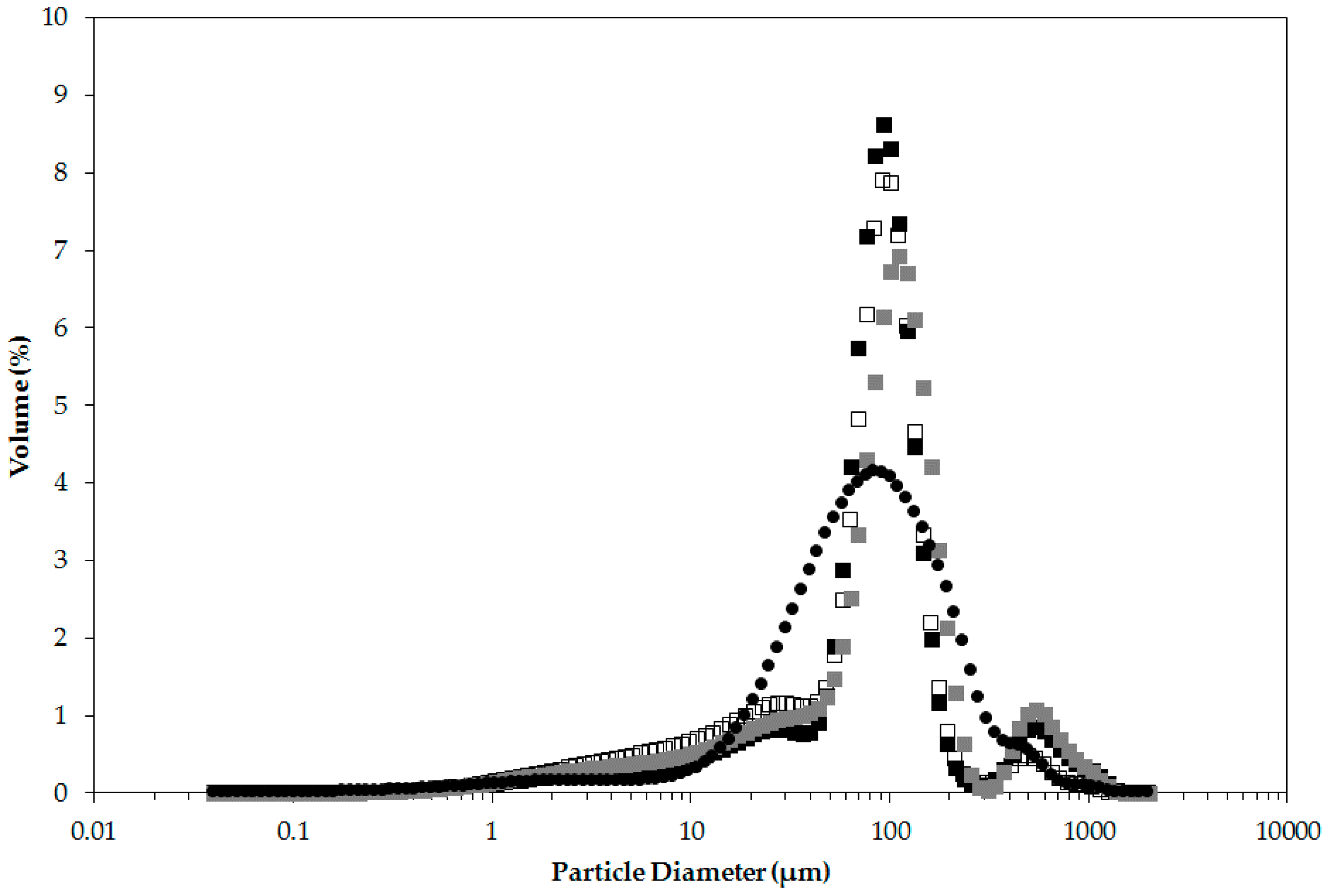
2.1.3. Particle Size
2.1.4. Particle Hydrophobicity
2.2. Application of Coffee Pickering Particles in Food Emulsions
2.2.1. Microstructure Stability
2.2.2. Temperature Stability
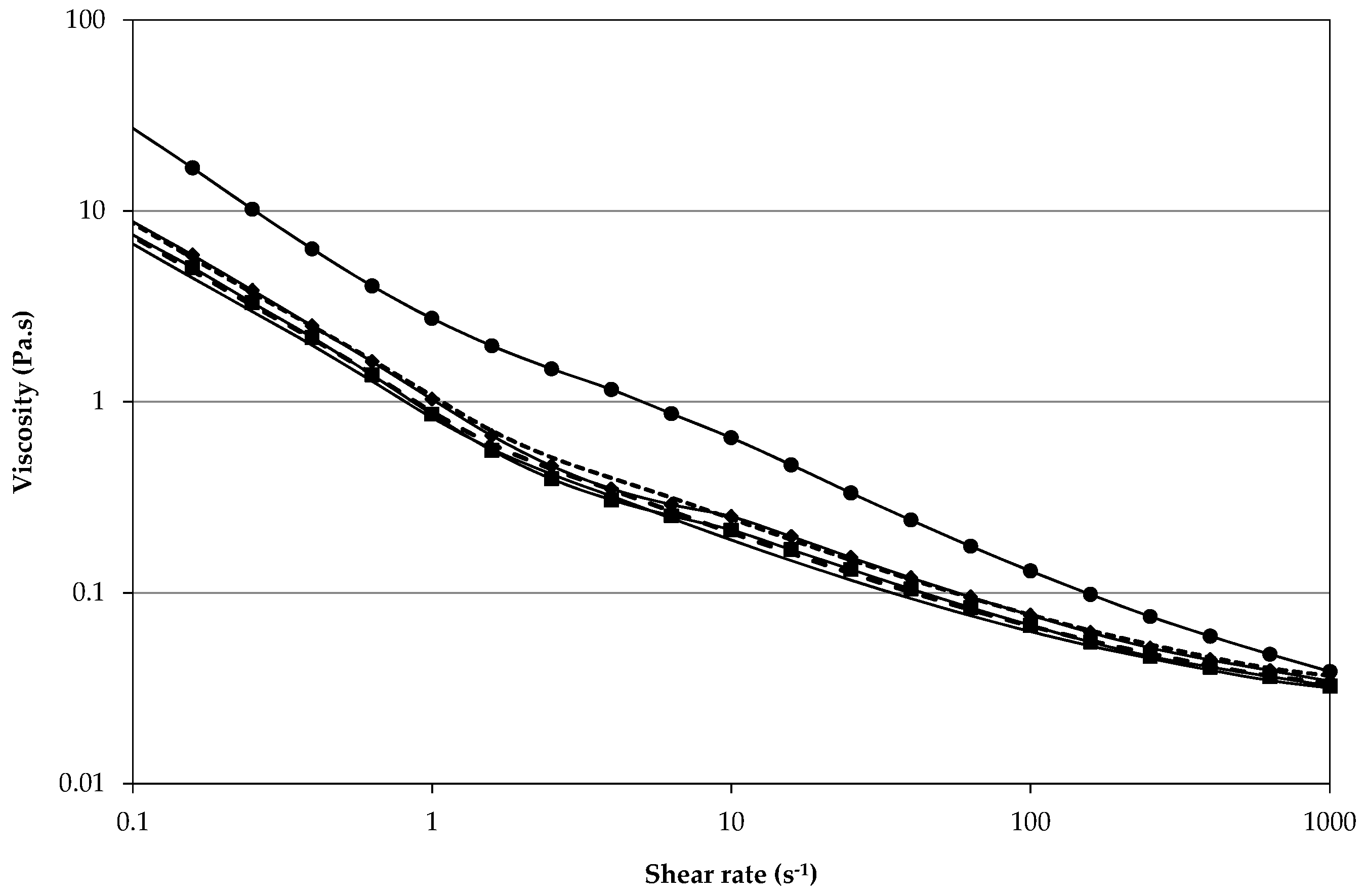
2.2.3. Shear Stability
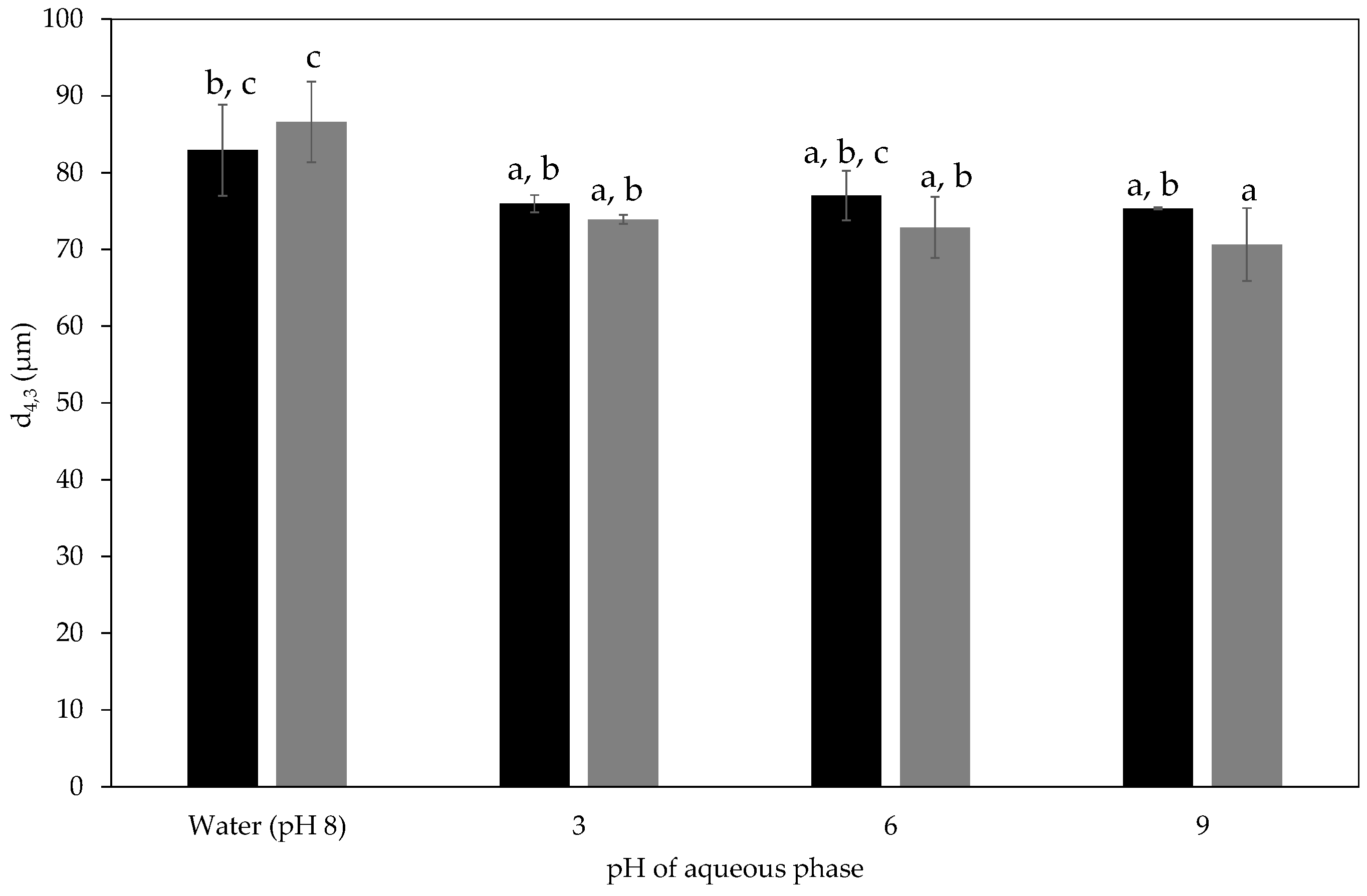
2.2.4. pH Stability of Milled Waste Coffee Pickering Emulsions
3. Materials and Methods
3.1. Materials
3.2. Prepartation of Ground Coffee Waste Particle Preparation
3.3. Emulsion Processing
3.4. Characterisation of Ground Coffee Waste Pickering Particles and Pickering Emulsions
3.4.1. Lignin Quantification
3.4.2. Microstructure Imaging
3.4.3. Particle Sizing
3.4.4. Particle Hydrophobicity
3.4.5. Process Stability of o/w Emulsions
3.5. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Binks, B.P. Particles as surfactants-similarities and differences. Curr. Opin. Coll. Interface Sci. 2002, 7, 21–41. [Google Scholar] [CrossRef]
- Dickinson, E. Use of nanoparticles and microparticles in the formation and stabilization of food emulsions. Trends Food Sci. Technol. 2012, 24, 4–12. [Google Scholar] [CrossRef]
- Aveyard, R.; Binks, B.P.; Clint, J.H. Emulsions stabilised solely by colloidal particles. Adv. Colloid Interface Sci. 2003, 100, 503–546. [Google Scholar] [CrossRef]
- Finkle, P.; Draper, H.D.; Hildebrand, J.H. The theory of emulsification. J. Am. Chem. Soc. 1923, 45, 2780–2788. [Google Scholar] [CrossRef]
- Yusoff, A.; Murray, B.S. Modified starch granules as particle-stabilizers of oil-in-water emulsions. Food Hydrocoll. 2011, 25, 42–55. [Google Scholar] [CrossRef]
- Luo, Z.J.; Murray, B.S.; Yusoff, A.; Morgan, M.R.A.; Povey, M.J.W.; Day, A.J. Particle-stabilizing effects of flavonoids at the oil-water interface. J. Agric. Food Chem. 2011, 59, 2636–2645. [Google Scholar] [CrossRef] [PubMed]
- Tzoumaki, M.V.; Moschakis, T.; Kiosseoglou, V.; Biliaderis, C.G. Oil-in-water emulsions stabilized by chitin nanocrystal particles. Food Hydrocoll. 2011, 25, 1521–1529. [Google Scholar] [CrossRef]
- Paunov, V.N.; Paunov, V.N.; Cayre, O.; Nobel, P.F.; Stoyanov, S.D.; Velikov, K.P.; Golding, M. Emulsions stabilised by food colloid particles: Role of particle adsorption and wettability at the liquid interface. J. Colloid Interface Sci. 2007, 312, 381–389. [Google Scholar] [CrossRef] [PubMed]
- De Folter, J.W.J.; van Ruijven, M.W.M.; Velikov, K.P. Oil-in-water Pickering emulsions stabilized by colloidal particles from the water-insoluble protein zein. Soft Matter 2012, 8, 2807–2815. [Google Scholar] [CrossRef]
- Destribats, M.; Rouvet, M.; Gehin-delval, C.; Schmitt, C.; Binks, B.P. Emulsions stabilised by whey protein microgel particles: Towards food-grade Pickering emulsions. Soft Matter 2014, 10, 6941–6954. [Google Scholar] [CrossRef] [PubMed]
- Rayner, M.; Marku, D.; Eriksson, M.; Sjöö, M.; Dejmek, P.; Wahlgren, M. Biomass-based particles for the formulation of Pickering type emulsions in food and topical applications. Colloid Surf. A Physicochem. Eng. Appl. 2014, 458, 48–62. [Google Scholar] [CrossRef]
- Campbell, A.L.; Holt, B.L.; Stoyanov, S.D.; Paunov, V.N. Scalable fabrication of anisotropic micro-rods from food-grade materials using an in shear flow dispersion-solvent attrition technique. J. Mater. Chem. 2008, 18, 4074–4078. [Google Scholar] [CrossRef]
- Gould, J.; Vieira, J.; Wolf, B. Cocoa particles for food emulsion stabilisation. Food Funct. 2013, 4, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
- Vieira, J.B.; Husson, J.; Wolf, B.; Gould, J. Emulsion Stabilisation. Patent EP2589297, 2013. [Google Scholar]
- Doherty, W.O.; Mousavioun, P.; Fellows, C.M. Value-adding to cellulosic ethanol: Lignin polymers. Ind. Crops Prod. 2011, 33, 259–276. [Google Scholar] [CrossRef] [Green Version]
- Tortora, M.; Cavalieri, F.; Mosesso, P.; Ciaffardini, F.; Melone, F.; Crestini, C. Ultrasound driven assembly of lignin into microcapsules for storage and delivery of hydrophobic molecules. Biomacromolecules 2014, 15, 1634–1643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afanas’ev, N.; Selyanina, S.; Selivanova, N. Stabilization of the oleic acid-water emulsion with various kraft lignins. Russ. J. Appl. Chem. 2008, 81, 1851–1855. [Google Scholar] [CrossRef]
- Gundersen, S.A.; Sæther, Ø.; Sjöblom, J. Salt effects on lignosulfonate and Kraft lignin stabilized O/W-emulsions studied by means of electrical conductivity and video-enhanced microscopy. Colloid Surf. A Physicochem. Eng. Asp. 2001, 186, 141–153. [Google Scholar] [CrossRef]
- Gan, M.; Pan, J.; Zhang, Y.; Dai, X.; Yin, Y.; Qu, Q.; Yan, Y. Molecularly imprinted polymers derived from lignin-based Pickering emulsions and their selectively adsorption of lambda-cyhalothrin. Chem. Eng. J. 2014, 257, 317–327. [Google Scholar] [CrossRef]
- Stewart, H.E. Development of Food-Grade Microparticles from Lignin. Ph.D. Thesis, Massey University, Palmerston North, New Zealand, 2015. [Google Scholar]
- Wei, Z.; Yang, Y.; Yang, R.; Wang, C. Alkaline lignin extracted from furfural residues for pH-responsive Pickering emulsions and their recyclable polymerization. Green Chem. 2012, 14, 3230–3236. [Google Scholar] [CrossRef]
- Redgwell, R.J.; Trovato, V.; Curti, D. Cocoa bean carbohydrates: Roasting-induced changes and polymer interactions. Food Chem. 2003, 80, 511–516. [Google Scholar] [CrossRef]
- Pujol, D.; Gominho, J.; Olivella, M.A.; Fiol, N.; Villaescusa, I.; Pereira, H. The chemical composition of exhausted coffee waste. Ind. Crops Prod. 2013, 50, 423–429. [Google Scholar] [CrossRef]
- Business Waste: How to Keep Coffee Grounds Out of the Ground. Avaliable online: http://www.gd-environmental.co.uk/blog/business-waste-how-keep-coffee-grounds-out-ground/ (accessed on 1 June 2016).
- Chapagain, A.; James, K. The Water and Carbon Footprint of Household Food and Drink Waste in the UK; WRAP and WWF: Banbury, UK, 2011. [Google Scholar]
- European Coffee Report 2013/2014: European Chapter and Key National Data; European Coffee Federation: Brussels, Belgium, 2014.
- Obruca, S.; Benesova, P.; Kucera, D.; Petrik, S.; Marova, I. Biotechnological conversion of spent coffee grounds into polyhydroxyalkanoates and carotenoids. New Biotechnol. 2015, 32, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.Q.; Hu, F.; Huang, F.; Ragauskas, A.J. Lignin Structural Alterations in Thermochemical Pretreatments with Limited Delignification. Bioenergy Res. 2015, 8, 992–1003. [Google Scholar] [CrossRef]
- Dean, J.F. Lignin analysis. In Methods in Plant Biochemistry and Molecular Biology, 1st ed.; CRC Press: Cleveland, OH, USA, 1997; pp. 199–215. [Google Scholar]
- Donohoe, B.S.; Decker, S.R.; Tuvker, M.P.; Himmel, M.E.; Vinzant, T.B. Visualizing Lignin Coalescence and Migration Through Maize Cell Walls Following Thermochemical Pretreatment. Biotechnol. Bioeng. 2008, 101, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Araya, F.; Troncoso, E.; Mendonca, R.T.; Freer, J. Condensed lignin structures and re-localization achieved at high severities in autohydrolysis of Eucalyptus globulus wood and their relationship with cellulose accessibility. Biotechnol. Bioeng. 2015, 112, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhang, X.; Zhou, X.; Xu, F. Revealing the Changes in Topochemical Characteristics of Poplar Cell Wall During Hydrothermal Pretreatment. BioEnergy Res. 2014, 7, 1358–1368. [Google Scholar] [CrossRef]
- Ma, X.J.; Cao, S.L.; Luo, X.L.; Chen, L.H.; Huang, L.L. Surface characterizations of bamboo substrates treated by hot water extraction. Bioresour. Technol. 2013, 136, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Sannigrahi, P.; Kim, D.H.; Jung, S.; Ragauskas, A. Pseudo-lignin and pretreatment chemistry. Energy Environ. Sci. 2011, 4, 1306–1310. [Google Scholar] [CrossRef]
- Hu, F.; Jung, S.; Ragauskas, A. Impact of Pseudolignin versus Dilute Acid-Pretreated Lignin on Enzymatic Hydrolysis of Cellulose. ACS Sustain. Chem. Eng. 2013, 1, 62–65. [Google Scholar] [CrossRef]
- Reddy, P.; Lekha, P.; Reynolds, W.; Kirsch, C. Structural characterisation of pretreated solids from flow-through liquid hot water treatment of sugarcane bagasse in a fixed-bed reactor. Bioresour. Technol. 2015, 183, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Ibbett, R.; Gaddipati, S.; Greetham, D.; Hill, S.; Tucker, G. The kinetics of inhibitor production resulting from hydrothermal deconstruction of wheat straw studied using a pressurised microwave reactor. Biotechnol. Biofuels 2014, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.K.; Kim, Y.; Ximenes, E.; Ladisch, M.R. Effect of liquid hot water pretreatment severity on properties of hardwood lignin and enzymatic hydrolysis of cellulose. Biotechnol. Bioeng. 2015, 112, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Heiss-Blanquet, S.; Zheng, D.; Ferreira, N.L.; Lapierre, C.; Baumberger, S. Effect of pretreatment and enzymatic hydrolysis of wheat straw on cell wall composition, hydrophobicity and cellulase adsorption. Bioresour. Technol. 2011, 102, 5938–5946. [Google Scholar] [CrossRef] [PubMed]
- Binks, B.; Lumsdon, S. Pickering emulsions stabilized by monodisperse latex particles: Effects of particle size. Langmuir 2001, 17, 4540–4547. [Google Scholar] [CrossRef]
- Pawar, A.B.; Caggioni, M.; Ergun, R.; Hartel, R.W.; Spicer, P.T. Arrested coalescence in Pickering emulsions. Soft Matter 2011, 7, 7710–7716. [Google Scholar] [CrossRef]
- Binks, B.P.; Fletcher, P.D.I.; Holt, B.L.; Beaussoubre, P.; Wong, K. Phase inversion of particle-stabilised perfume oil-water emulsions: Experiment and theory. Phys. Chem. Chem. Phys. 2010, 12, 11954–11966. [Google Scholar] [CrossRef] [PubMed]
- Binks, B.P.; Lumsdon, S.O. Effects of oil type and aqueous phase composition on oil-water mixtures containing particles of intermediate hydrophobicity. Phys. Chem. Chem. Phys. 2000, 2, 2959–2967. [Google Scholar] [CrossRef]

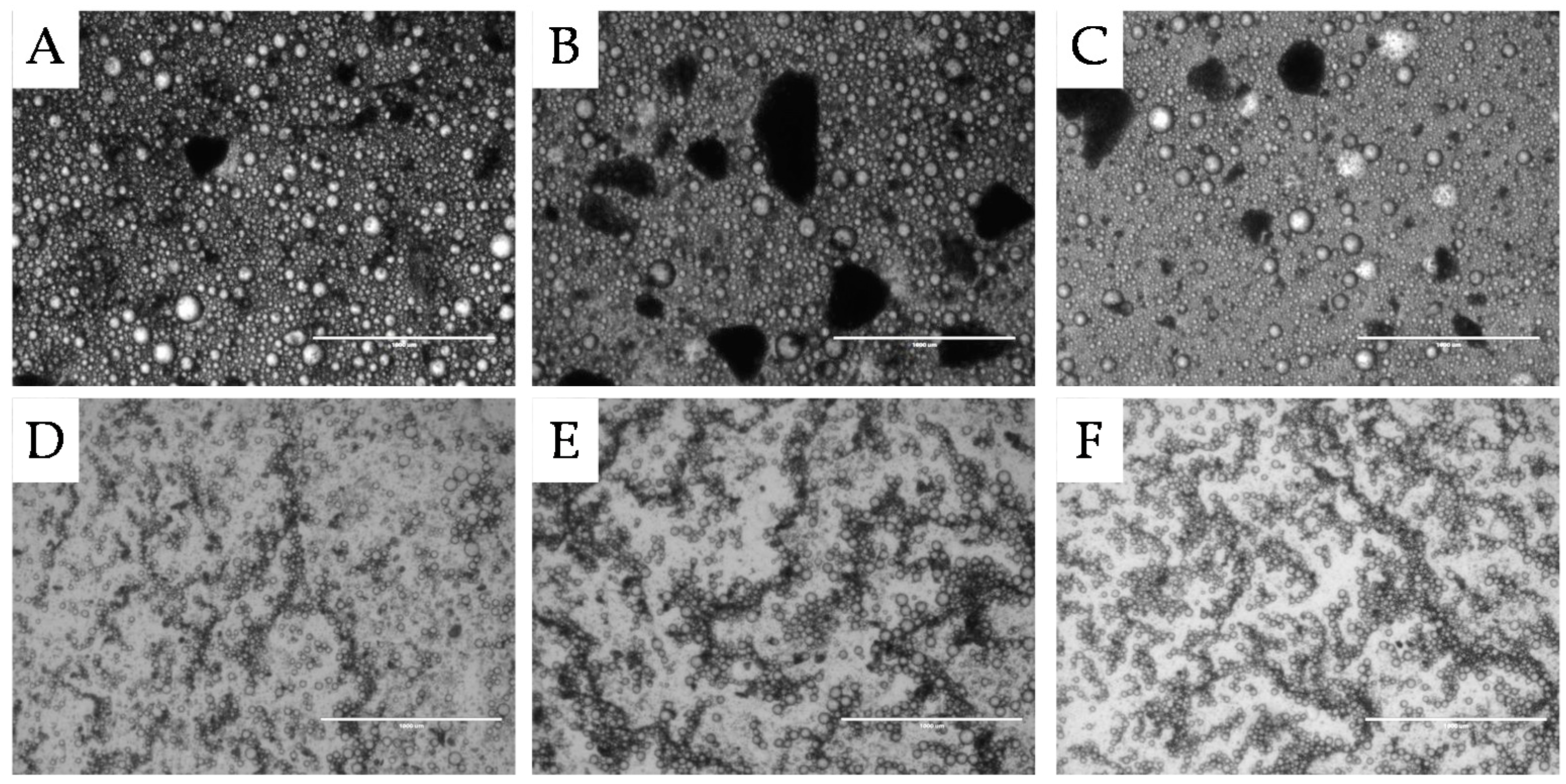
 , 2nd
, 2nd  , and 3rd
, and 3rd  ).
).
 , 2nd
, 2nd  , and 3rd
, and 3rd  ).
).
| Sample | d4,3 (μm) | d10,3 (μm) |
|---|---|---|
| Unmilled | 341.11 ± 21.67 | 35.91 ± 5.65 |
| Milled | 117.12 ± 16.15 | 21.90 ± 2.55 |
| Milled and Hydrothermally Treated | 144.25 ± 6.14 | 19.24 ± 0.20 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gould, J.; Garcia-Garcia, G.; Wolf, B. Pickering Particles Prepared from Food Waste. Materials 2016, 9, 791. https://doi.org/10.3390/ma9090791
Gould J, Garcia-Garcia G, Wolf B. Pickering Particles Prepared from Food Waste. Materials. 2016; 9(9):791. https://doi.org/10.3390/ma9090791
Chicago/Turabian StyleGould, Joanne, Guillermo Garcia-Garcia, and Bettina Wolf. 2016. "Pickering Particles Prepared from Food Waste" Materials 9, no. 9: 791. https://doi.org/10.3390/ma9090791
APA StyleGould, J., Garcia-Garcia, G., & Wolf, B. (2016). Pickering Particles Prepared from Food Waste. Materials, 9(9), 791. https://doi.org/10.3390/ma9090791







