Passivation Characteristics of Alloy Corrosion-Resistant Steel Cr10Mo1 in Simulating Concrete Pore Solutions: Combination Effects of pH and Chloride
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials
2.1.1. Test Solutions
2.1.2. Steel Samples
2.2. Electrochemical Measurements
2.3. Surface Analysis
3. Results and Discussion
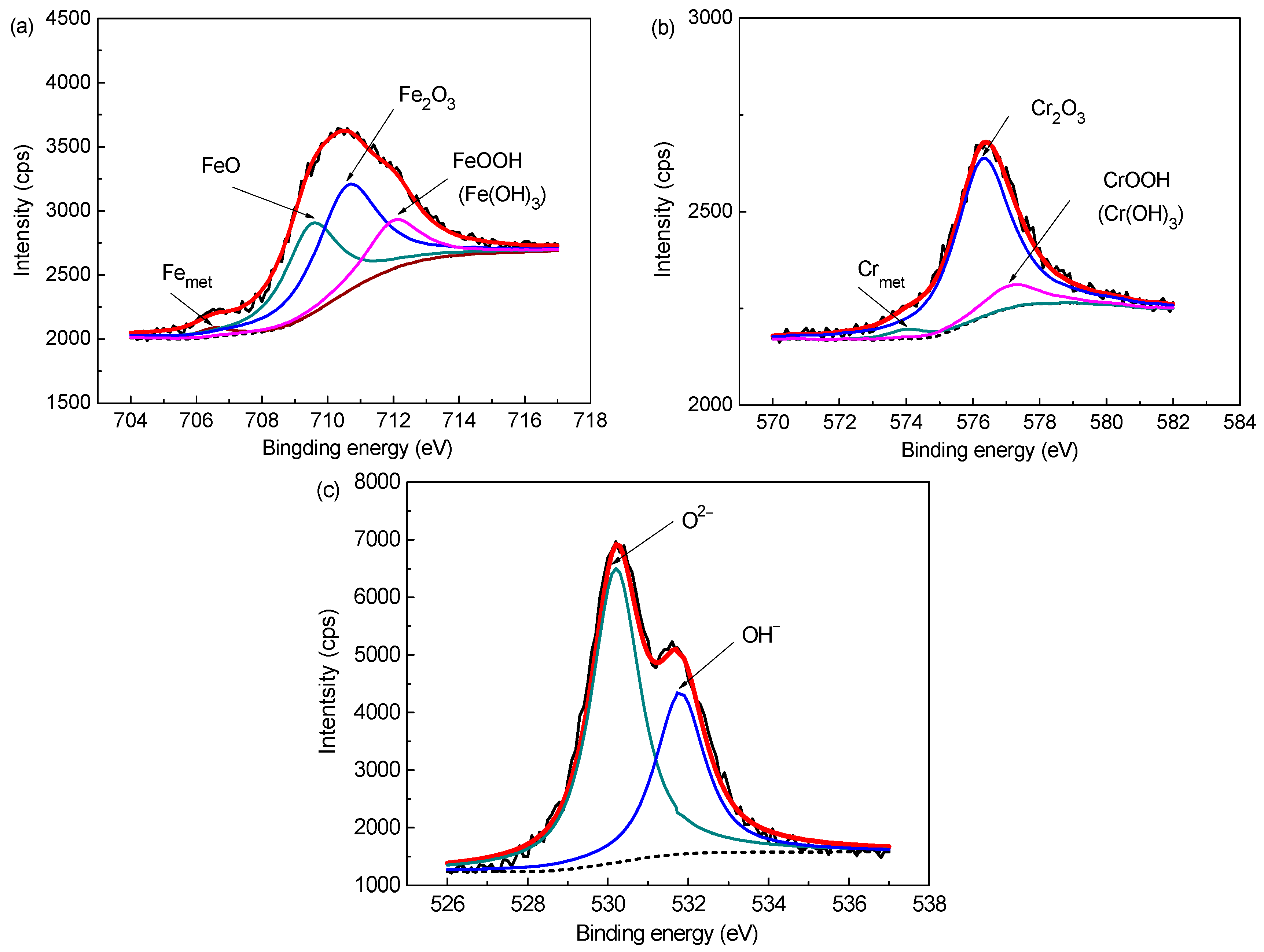
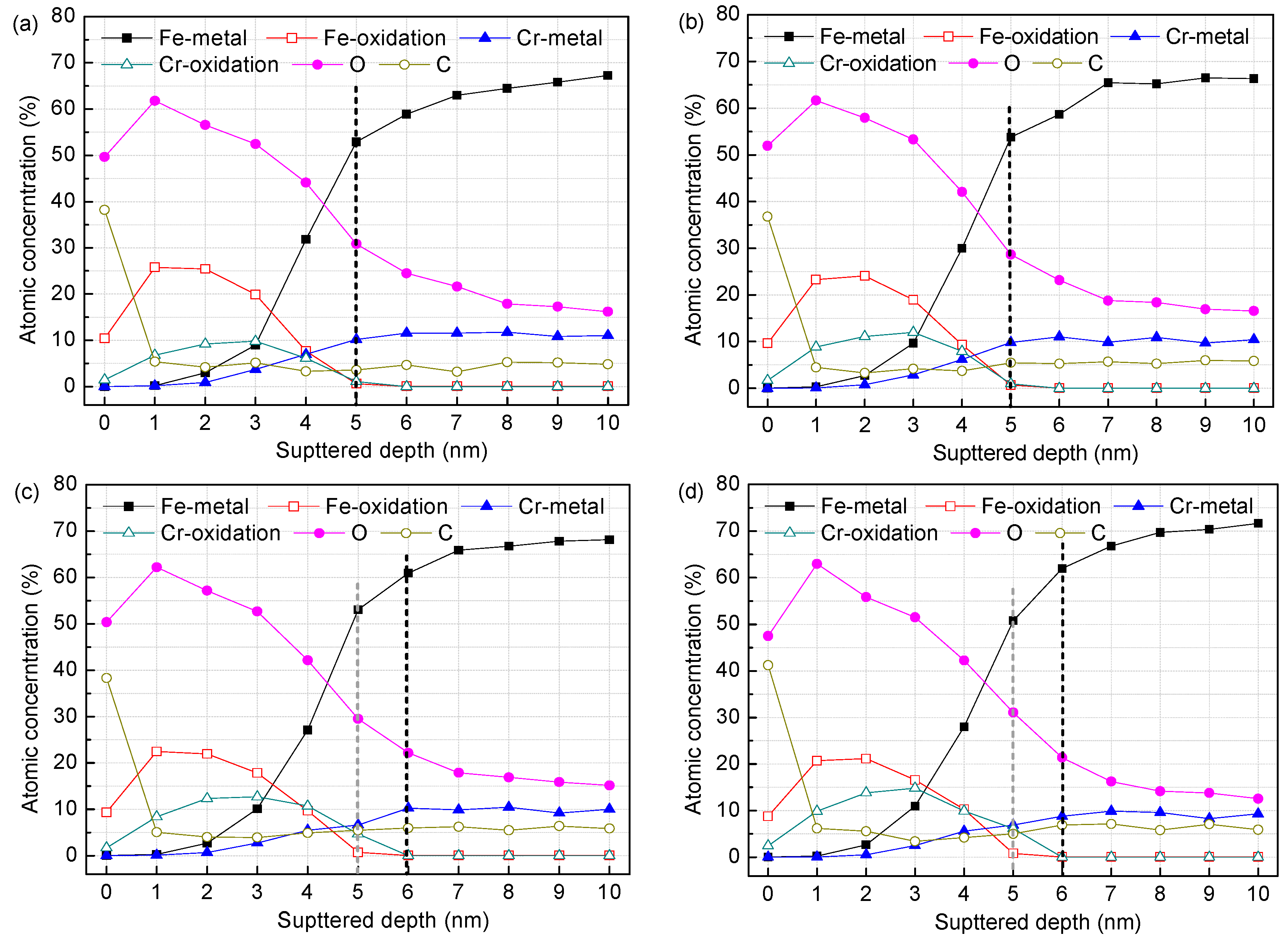
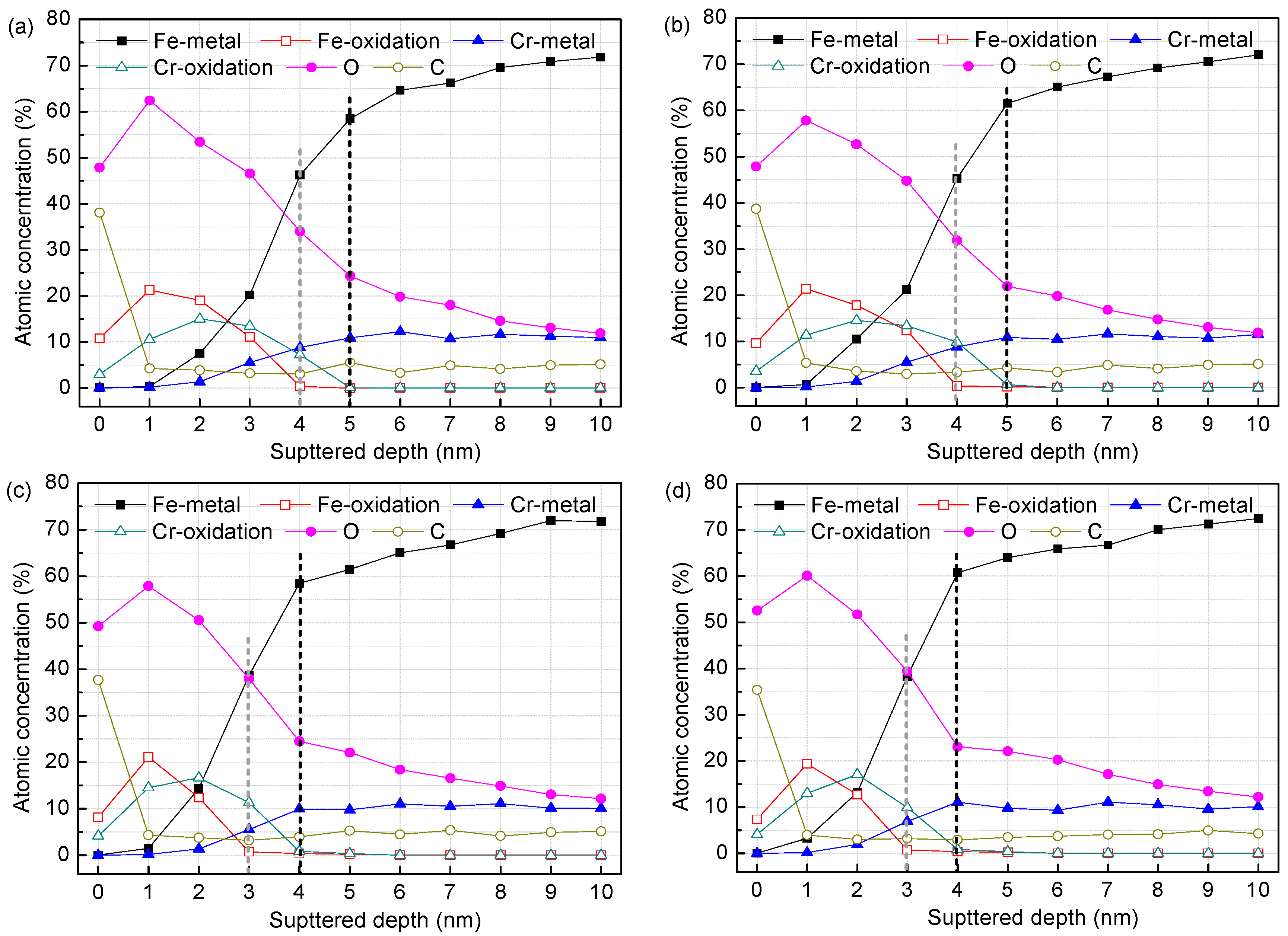
3.1. Surface Film Composition and Structure (XPS Analysis)
3.2. Electrochemical Results
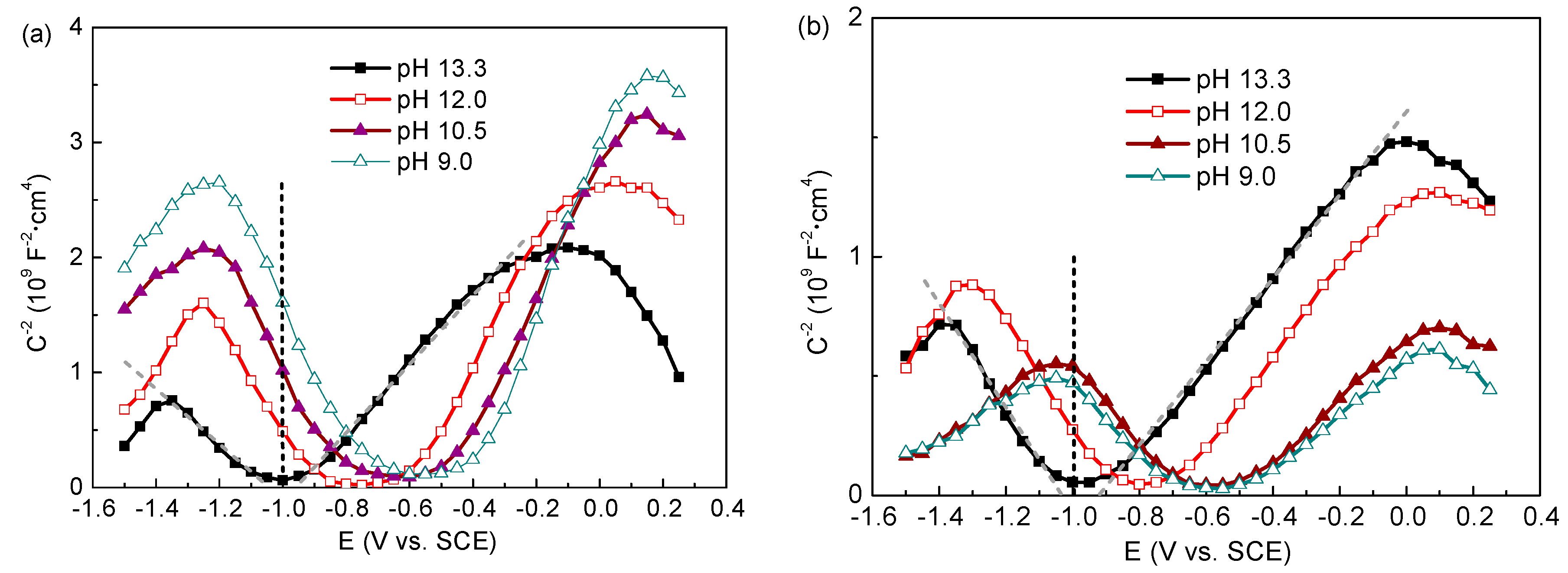
3.2.1. Capacitance Measurements (Mott–Schottky Plots)
- for n-type semiconductor
- for p-type semiconductor
3.2.2. Linear Polarization Resistance
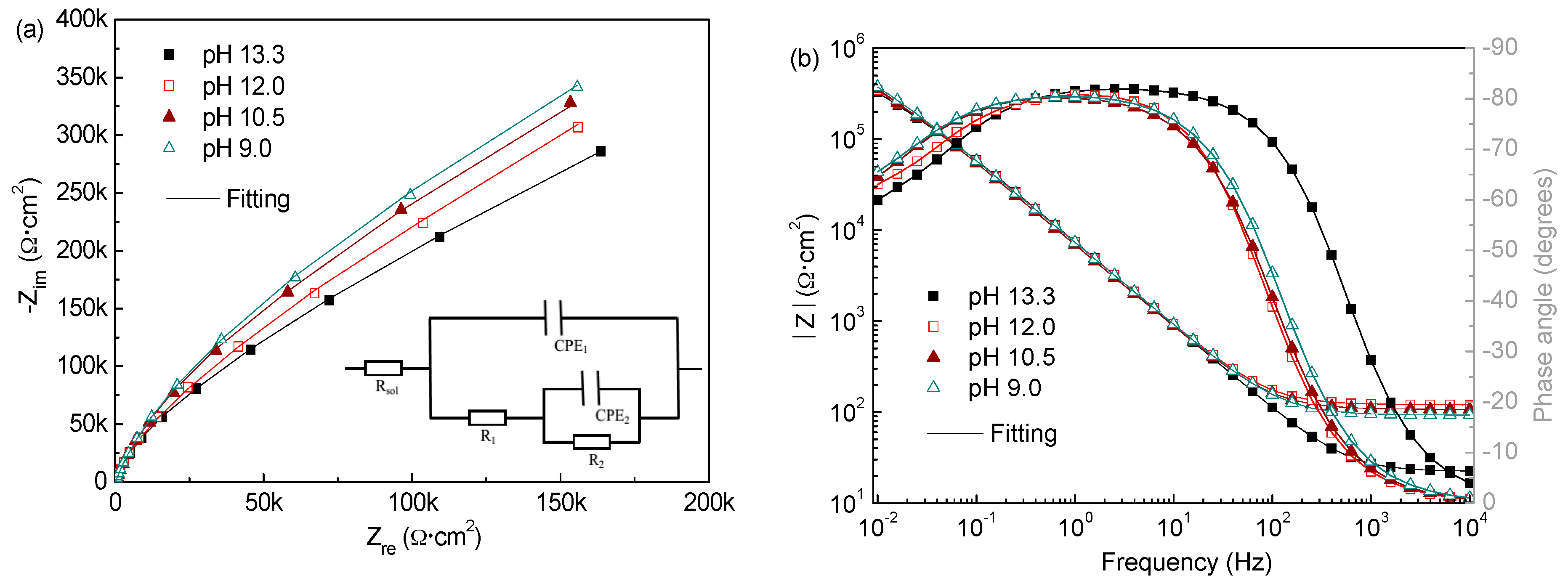
3.2.3. Electrochemical Impedance Spectroscopy
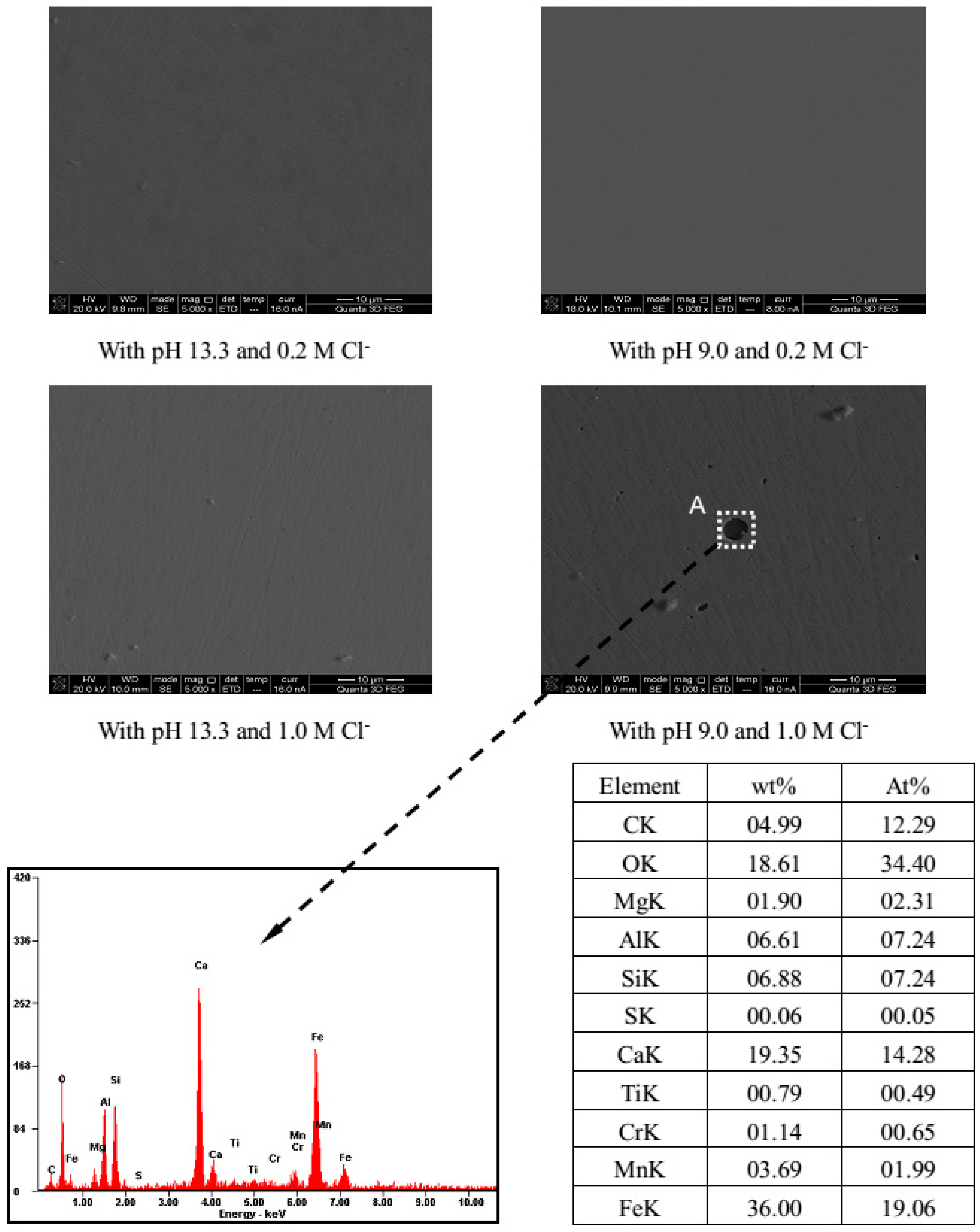
3.3. Surface Morphology
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pfeifer, D.W. High performance concrete and reinforcing steel with a 100-year service life. PCI J. 2000, 45, 46–54. [Google Scholar] [CrossRef]
- Broomfield, J.P. Steel Corrosion in Concrete—Understanding, Investigation and Repair, 2nd ed.; Taylor & Francis: London, UK, 2007. [Google Scholar]
- Revie, R.W.; Uhlig, H.H. Corrosion and Corrosion Control—An Introduction to Corrosion Science and Engineering, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Smith, F.N.; Tulimin, M. Using stainless steel as long-lasting rebar material. Mater. Perform. 1999, 38, 72–76. [Google Scholar]
- Castro-Borges, P.; Rincón, O.T.D.; Moreno, E.I.; Torres-Acosta, A.A.; Martínez Madrid, M.; Knudsen, A. Performance of a 60-year-old concrete pier with stainless steel reinforcement. Mater. Perform. 2002, 41, 50–55. [Google Scholar]
- Presuel-Moreno, F.; Scully, J.R.; Sharp, S.R. Literature review of commercially available alloys that have potential as low-cost, corrosion-resistant concrete reinforcement. Corrosion 2010, 66, 1–13. [Google Scholar] [CrossRef]
- Zhang, J.C.; Huang, W.K.; Li, Y.; Ma, H. A Highly Corrosion-Resistant Reinforcing Steel with High Strength and Its Preparation Technologies. Chinese Patent CN103789677A, 14 May 2014. (In Chinese)[Google Scholar]
- Trejo, D.; Pillai, R.G. Accelerated chloride threshold testing—Part II: Corrosion-resistant reinforcement. ACI Mater. J. 2004, 101, 57–64. [Google Scholar]
- Mohamed, N.; Boulfiza, M.; Evitts, R. Corrosion of carbon steel and corrosion-resistant rebars in concrete structures under chloride ion attack. J. Mater. Eng. Perform. 2013, 22, 787–795. [Google Scholar] [CrossRef]
- Ghods, P.; Isgor, O.B.; Bensebaa, F.; Kingston, D. Angle-resolved XPS study of carbon steel passivity and chloride-induced depassivation in simulated concrete pore solution. Corros. Sci. 2012, 58, 159–167. [Google Scholar] [CrossRef]
- Ghods, P.; Isgor, O.B.; Brown, J.R.; Bensebaa, F.; Kingston, D. XPS depth profiling study on the passive oxide film of carbon steel in saturated calcium hydroxide solution and the effect of chloride on the film properties. Appl. Surf. Sci. 2011, 257, 4669–4677. [Google Scholar] [CrossRef]
- Moreno, M.; Morris, W.; Alvarez, M.G.; Duffó, G.S. Corrosion of reinforcing steel in simulated concrete pore solutions: Effect of carbonation and chloride content. Corros. Sci. 2004, 46, 2681–2699. [Google Scholar] [CrossRef]
- Kouřil, M.; Novák, P.; Bojko, M. Threshold chloride concentration for stainless steels activation in concrete pore solutions. Cem. Concr. Res. 2010, 40, 431–436. [Google Scholar] [CrossRef]
- Ai, Z.; Jiang, J.; Sun, w.; Song, D.; Ma, H.; Zhang, J.; Wang, D. Passive behaviour of alloy corrosion-resistant steel Cr10Mo1 in simulating concrete pore solutions with different pH. Appl. Surf. Sci. 2016, 389, 1126–1136. [Google Scholar] [CrossRef]
- Papadakis, V.; Fardis, M.; Vayenas, V. Effect of composition, environmental factors and cement-lime mortar coating on concrete carbonation. Mater. Struct. 1992, 25, 293–304. [Google Scholar] [CrossRef]
- Ghods, P.; Isgor, O.B.; McRae, G.; Miller, T. The effect of concrete pore solution composition on the quality of passive oxide films on black steel reinforcement. Cem. Concr. Compos. 2009, 31, 2–11. [Google Scholar] [CrossRef]
- Poursaee, A.; Hansson, C.M. Reinforcing steel passivation in mortar and pore solution. Cem. Concr. Res. 2007, 37, 1127–1133. [Google Scholar] [CrossRef]
- Freire, L.; Ferreira, M.G.S.; Carmezim, M.J.; Montemor, M.F. The passive behaviour of AISI 316 in alkaline media and the effect of pH: A combined electrochemical and analytical study. Electrochim. Acta 2010, 55, 6174–6181. [Google Scholar] [CrossRef]
- Escrivà-Cerdán, C.; Blasco-Tamarit, E.; García-Garcíaa, D.M.; García-Antóna, J.; Guenbour, A. Effect of potential formation on the electrochemical behaviour of a highly alloyed austenitic stainless steel in contaminated phosphoric acid at different temperatures. Electrochim. Acta 2012, 80, 248–256. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Physical Electronics: Chanhassen, MN, USA, 1995. [Google Scholar]
- Guo, L.Q.; Lin, M.C.; Qiao, L.J.; Volinsky, A.A. Duplex stainless steel passive film electrical properties studied by in situ current sensing atomic force microscopy. Corros. Sci. 2014, 78, 55–62. [Google Scholar] [CrossRef]
- Montemor, M.F.; Simões, A.M.P.; Ferreira, M.G.S.; Da Cunha Belo, M. The role of Mo in the chemical composition and semiconductive behaviour of oxide films formed on stainless steels. Corros. Sci. 1999, 41, 17–34. [Google Scholar] [CrossRef]
- Abreu, C.M.; Cristóbal, M.J.; Losada, R.; Nóvoa, X.R.; Pena, G.; Pérez, M.C. Long-term behaviour of AISI 304L passive layer in chloride containing medium. Electrochim. Acta 2006, 51, 1881–1890. [Google Scholar] [CrossRef]
- Kirchheim, R.; Heine, B.; Fischmeister, H.; Hofmann, S.; Knote, H.; Stolz, U. The passivity of iron—Chromium alloys. Corros. Sci. 1989, 29, 899–917. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas d'equilibres Electrochimiques; Gauthier-villars et Cie: Paris, France, 1963. [Google Scholar]
- Bautista, A.; Blanco, G.; Velasco, F.; Gutiérrez, A.; Soriano, L.; Palomares, F.J.; Takenout, H. Changes in the passive layer of corrugated austenitic stainless steel of low nickel content due to exposure to simulated pore solutions. Corros. Sci. 2009, 51, 785–792. [Google Scholar] [CrossRef]
- Seyeux, A.; Maurice, V.; Marcus, P. Breakdown Kinetics at Nanostructure Defects of Passive Films. Electrochem. Solid-State Lett. 2009, 12, C25–C27. [Google Scholar] [CrossRef]
- Sherif, E.M. A Comparative study on the electrochemical corrosion behavior of iron and x-65 steel in 4.0 wt % sodium chloride solution after different exposure intervals. Molecules 2014, 19, 9962–9974. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Li, Y.; Wang, F.H. The improvement of the corrosion resistance of 309 stainless steel in the transpassive region by nans-crystallization. Electrochim. Acta 2009, 54, 1339–1349. [Google Scholar] [CrossRef]
- Ramasubramanian, N.; Preocanin, N.; Davidson, R.D. Analysis of passive films on stainless steel by cyclic voltammetry and Auger spectroscopy. J. Electrochem. Soc. 1985, 132, 793–798. [Google Scholar] [CrossRef]
- Olsson, C.O.A.; Landolt, D. Passive films on stainless steels-chemistry, structure and growth. Electrochim. Acta 2003, 48, 1093–1104. [Google Scholar] [CrossRef]
- Mathieu, H.J.; Landolt, D. An investigation of thin oxide films thermally grown in situ on Fe24Cr and Fe24Cr11Mo by auger electron spectroscopy and X-ray photoelectron spectroscopy. Corros. Sci. 1986, 26, 547–559. [Google Scholar] [CrossRef]
- Hakiki, N.B.; Boudin, S.; Rondot, B.; Da Cunha Belo, M. The electronic structure of passive films formed on stainless steels. Corros. Sci. 1995, 37, 1809–1822. [Google Scholar] [CrossRef]
- Gryse, R.D.; Gomes, W.P.; Cardon, F.; Vennik, J. On the interpretation of Mott-Schottky plots determined at semiconductor/electrolyte systems. J. Electrochem. Soc. 1975, 122, 711–712. [Google Scholar] [CrossRef]
- Di Paola, A. Semiconducting properties of passive films on stainless steels. Electrochim. Acta 1989, 34, 203–210. [Google Scholar] [CrossRef]
- Chao, C.Y.; Lin, L.F.; MacDonald, D.D. A point defect model for anodic passive film. J Electrochem. Soc. 1981, 128, 1187–1194. [Google Scholar] [CrossRef]
- Hakiki, N.E.; Montemor, M.F.; Ferreira, M.G.S.; da Cunha Belo, M. Semiconducting properties of thermally grown oxide films on AISI 304 stainless steel. Corros. Sci. 2000, 42, 687–702. [Google Scholar] [CrossRef]
- Gurdián, H.; García-Alcocel, E.; Baeza-Brotons, F.; Garcés, P.; Zornoza, E. Corrosion behavior of steel reinforcement in concrete with recycled aggregates, fly ash and spent cracking catalyst. Materials 2014, 7, 3176–3197. [Google Scholar] [CrossRef] [Green Version]
- Standard Test Method for Half-Cell Potentials of Uncoated Reinforcing Steel in Concrete; ASTM C876–91; ASTM International: Philadelphia, PA, USA, 1999.
- Wu, J.W.; Bai, D.; Baker, A.P.; Li, Z.H.; Liu, X.B. Electrochemical techniques correlation study of on-line corrosion monitoring probes. Mater. Corros. 2015, 66, 143–151. [Google Scholar] [CrossRef]
- Barsoukov, E.; Macdonald, J.R. Impedance Spectroscopy Theory, Experiment, and Applications, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
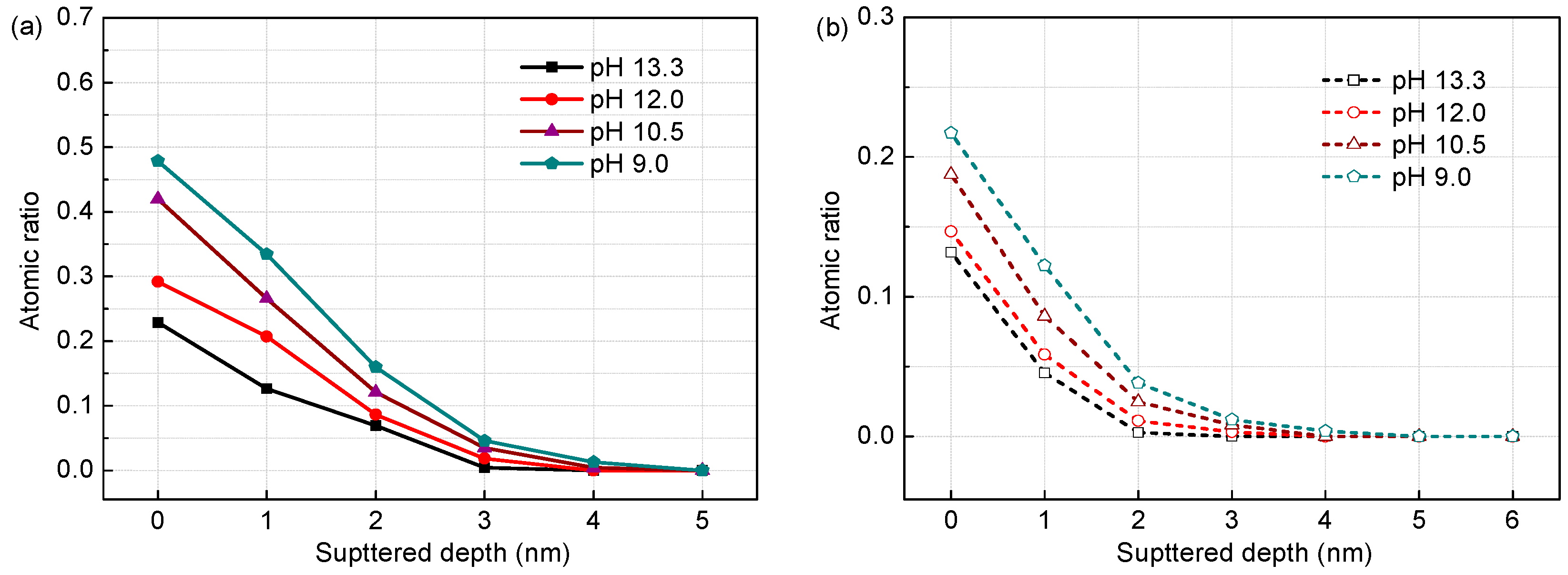
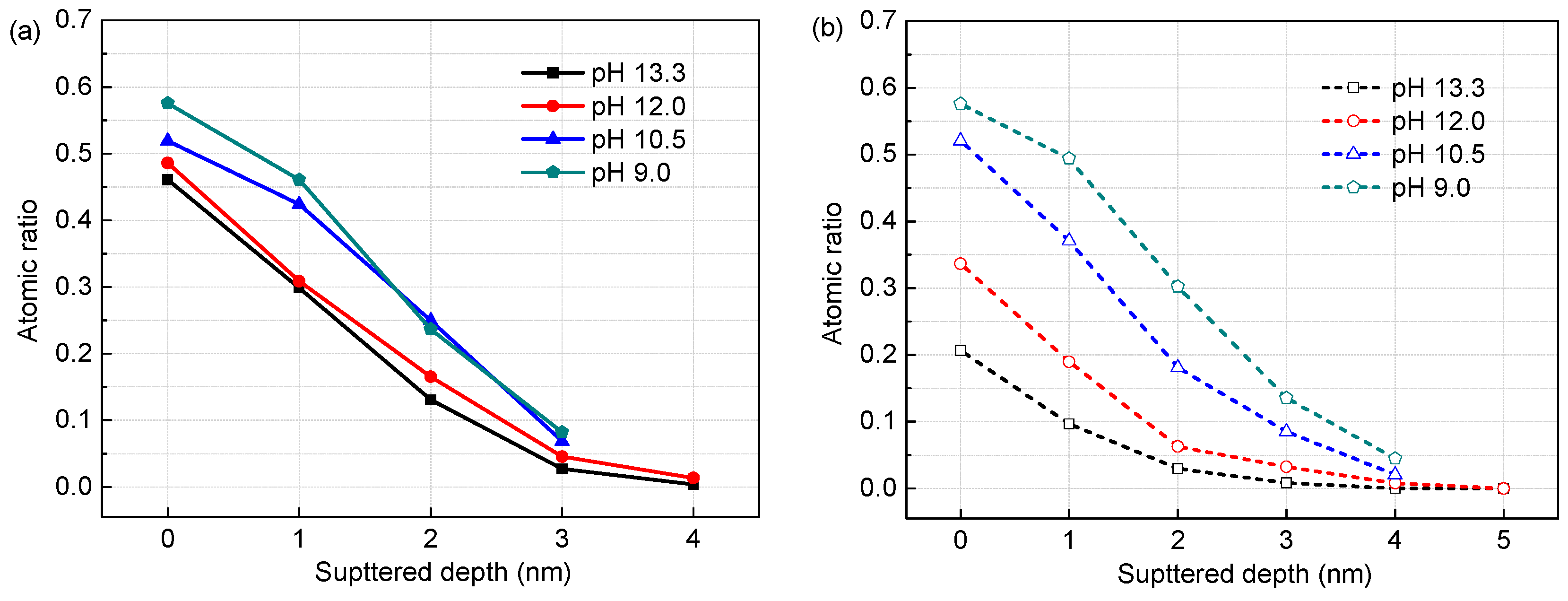
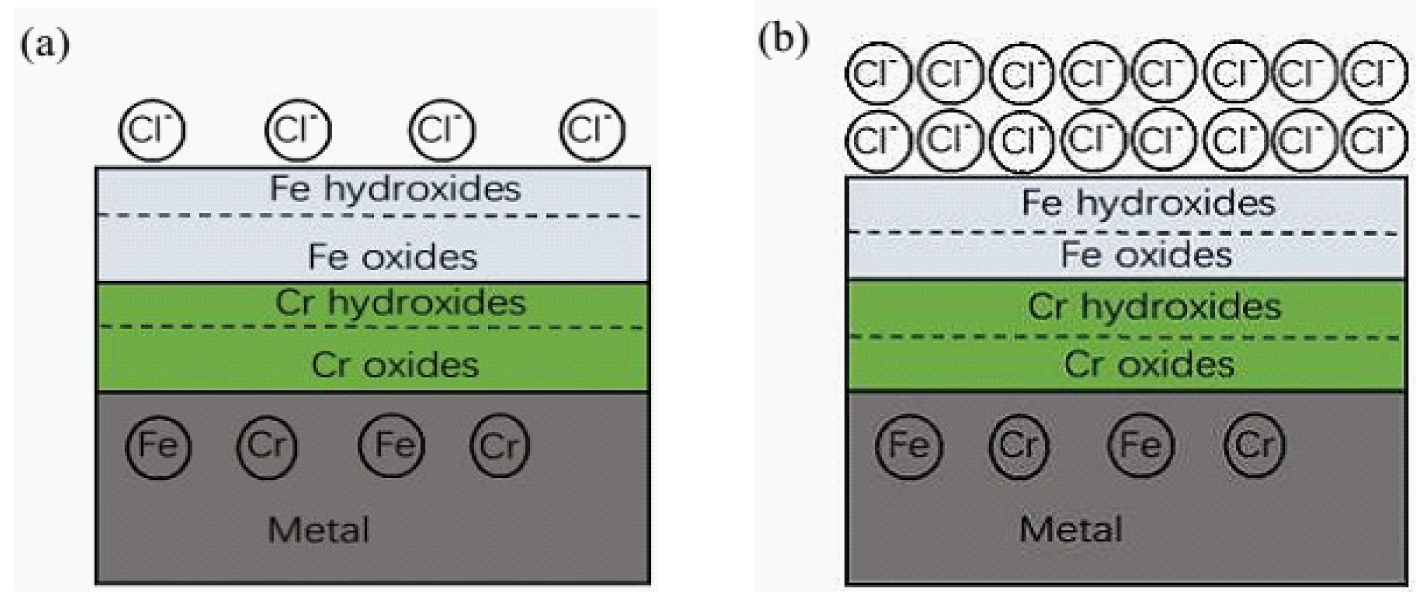
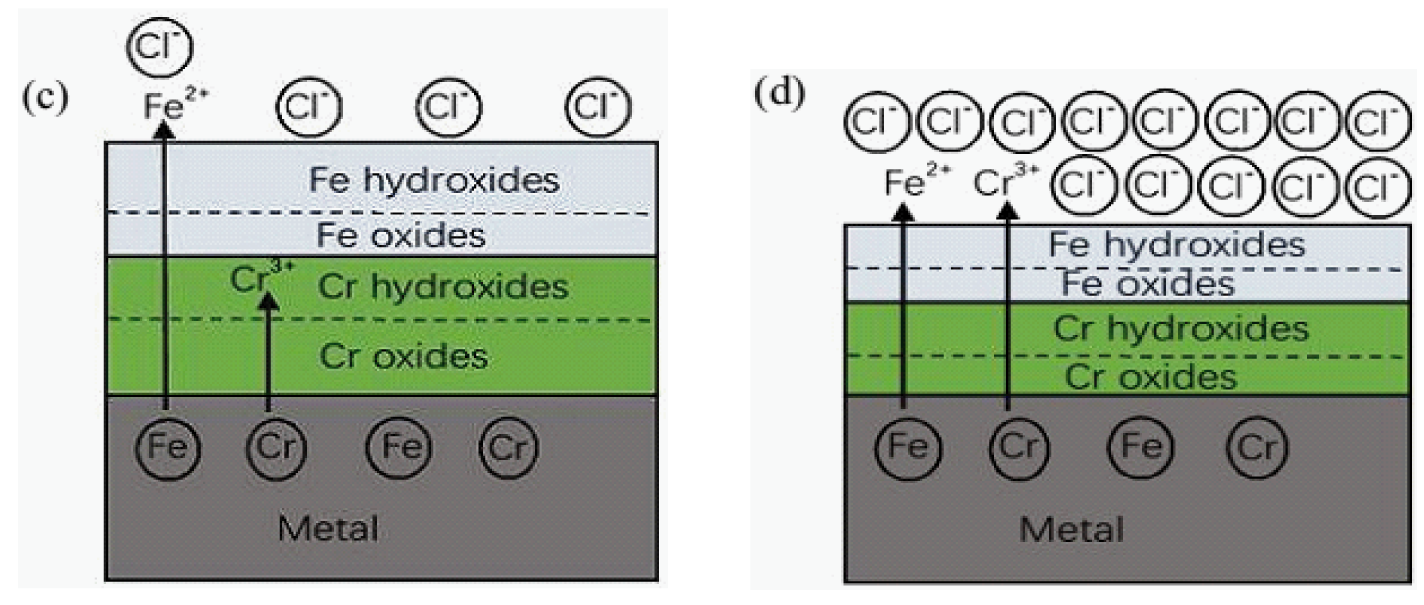



| pH | 0.2 M | 1.0 M | ||
|---|---|---|---|---|
| Nd (1020 cm−3) | Na (1020 cm−3) | Nd (1020 cm−3) | Na (1020 cm−3) | |
| 13.3 | 29.65 | 36.53 | 49.11 | 41.26 |
| 12.0 | 18.21 | 19.72 | 45.90 | 43.47 |
| 10.5 | 16.41 | 14.61 | 59.51 | 68.28 |
| 9.0 | 15.98 | 12.54 | 63.26 | 78.02 |
| Cl− Contents (M) | pH | Rsol (Ω·cm2) | R1 (Ω·cm2) | CPE1 | R2 (Ω·cm2) | CPE2 | ||
|---|---|---|---|---|---|---|---|---|
| Y0 (Ω−1·cm−2·sn) | n | Y0 (Ω−1·cm−2·sn) | n | |||||
| 0.2 | 13.3 | 18.2 | 3.54 × 105 | 2.47 × 10−5 | 0.92 | 11.37 × 105 | 2.07 × 10−5 | 0.82 |
| 12.0 | 121.2 | 4.93 × 105 | 2.54 × 10−5 | 0.91 | 13.23 × 105 | 1.94 × 10−5 | 0.82 | |
| 10.5 | 106.3 | 6.84 × 105 | 2.75 × 10−5 | 0.90 | 15.62 × 105 | 1.85 × 10−5 | 0.80 | |
| 9.0 | 96.8 | 7.31 × 105 | 2.87 × 10−5 | 0.89 | 17.36 × 105 | 1.73 × 10−5 | 0.80 | |
| 1.0 | 13.3 | 11.8 | 2.96 × 105 | 2.58 × 10−5 | 0.91 | 8.85 × 105 | 2.23 × 10−5 | 0.81 |
| 12.0 | 35.7 | 2.31 × 105 | 2.91 × 10−5 | 0.90 | 6.13 × 105 | 2.07 × 10−5 | 0.81 | |
| 10.5 | 30.4 | 1.06 × 104 | 2.12 × 10−4 | 0.81 | 1.89 × 104 | 1.63 × 10−4 | 0.59 | |
| 9.0 | 23.2 | 3.21 × 103 | 2.93 × 10−4 | 0.79 | 9.76 × 103 | 2.41 × 10−4 | 0.53 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ai, Z.; Sun, W.; Jiang, J.; Song, D.; Ma, H.; Zhang, J.; Wang, D. Passivation Characteristics of Alloy Corrosion-Resistant Steel Cr10Mo1 in Simulating Concrete Pore Solutions: Combination Effects of pH and Chloride. Materials 2016, 9, 749. https://doi.org/10.3390/ma9090749
Ai Z, Sun W, Jiang J, Song D, Ma H, Zhang J, Wang D. Passivation Characteristics of Alloy Corrosion-Resistant Steel Cr10Mo1 in Simulating Concrete Pore Solutions: Combination Effects of pH and Chloride. Materials. 2016; 9(9):749. https://doi.org/10.3390/ma9090749
Chicago/Turabian StyleAi, Zhiyong, Wei Sun, Jinyang Jiang, Dan Song, Han Ma, Jianchun Zhang, and Danqian Wang. 2016. "Passivation Characteristics of Alloy Corrosion-Resistant Steel Cr10Mo1 in Simulating Concrete Pore Solutions: Combination Effects of pH and Chloride" Materials 9, no. 9: 749. https://doi.org/10.3390/ma9090749







