One-Step Synthesis of Single-Wall Carbon Nanotube-ZnS Core-Shell Nanocables
Abstract
:1. Introduction
2. Experimental
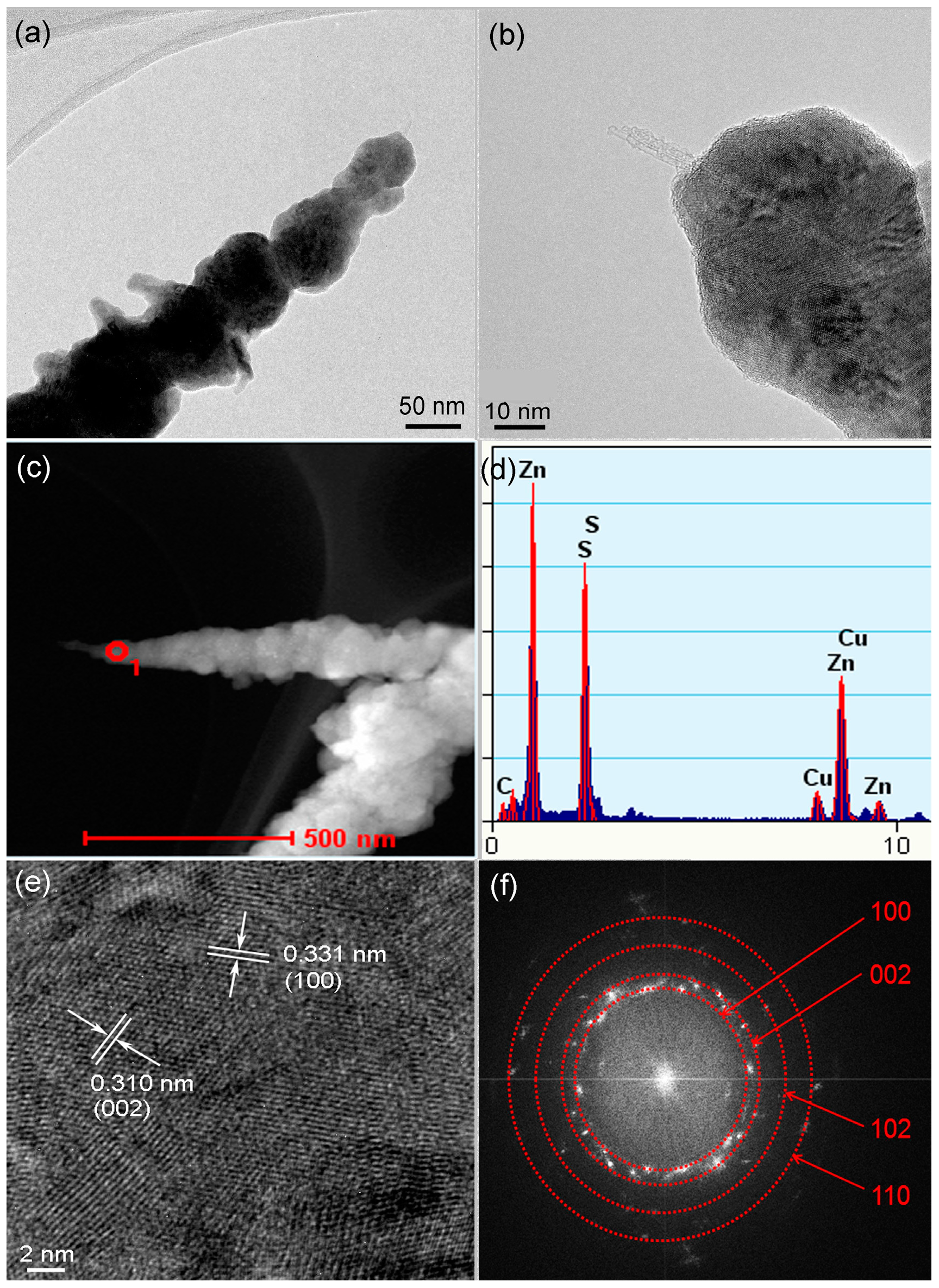
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Deep, J.; Vinod, K.S.; Lincoln, J.L.; Tobin, J.M.; Mark, C.H. Carbon nanomaterials for electronics, optoelectronics, photovoltaics, and sensing. Chem. Soc. Rev. 2013, 42, 2824–2860. [Google Scholar]
- Kumar, A.; Zhou, C.W. The race to replace tin-doped indium oxide: Which material will win? ACS Nano 2010, 4, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Haggenmueller, R.; Guthy, C.; Lukes, J.R.; Fischer, J.E.; Winey, K.I. Single wall carbon nanotube/polyethylene nanocomposites: Thermal and electrical conductivity. Macromolecules 2007, 40, 2417–2421. [Google Scholar] [CrossRef]
- Wu, H.P.; Meng, Q.H.; Yang, Q.; Zhang, M.; Lu, K.; Wei, Z.X. Large-area polyimide/SWCNT nanocable cathode for flexible lithium-ion batteries. Adv. Mater. 2015, 27, 6504–6510. [Google Scholar] [CrossRef] [PubMed]
- Eder, D. Carbon nanotube-inorganic hybrids. Chem. Rev. 2010, 110, 1348–1385. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Ly, J.; Han, S.; Zhang, D.H.; Requicha, A.; Thompson, M.E.; Zhou, C.W. Synthesis and electronic properties of individual single-walled carbon nanotube/polypyrrole composite nanocables. Adv. Mater. 2005, 17, 2727–2732. [Google Scholar] [CrossRef]
- Rodrigues, O.E.D.; Saraiva, G.D.; Nascimento, R.O.; Barros, E.B.; Mendes, J.; Kim, Y.A.; Muramatsu, H.; Endo, M.; Terrones, M.; Dresselhaus, M.S.; et al. Synthesis and characterization of selenium-carbon nanocables. Nano. Lett. 2008, 8, 3651–3655. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Shi, J.P.; Zhang, H.W.; Sun, M. Highly controllable synthesis of near-infrared persistent luminescence SiO2/CaMgSi2O6 composite nanospheres for imaging in vivo. Opt. Express 2014, 22, 10509–10518. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Zhang, Y.W.; Wu, X.; Huang, L.; Li, D.S.; Fan, W.; Han, G. Direct aqueous-phase synthesis of sub-10 nm “luminous pearls” with enhanced in vivo renewable near-infrared persistent luminescence. J. Am. Chem. Soc. 2015, 137, 5304–5307. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Zhang, Y.W.; Wu, X.; Wu, X.Q.; Maudgal, R.; Zhang, H.W.; Han, G. In vivo repeatedly charging near-infrared-emitting mesoporous SiO2/ZnGa2O4:Cr3+ persistent luminescence nanocomposites. Adv. Sci. 2015, 2, 1500001. [Google Scholar] [CrossRef] [PubMed]
- Monroy, E.; Omnes, F.; Calle, F. Wide-bandgap semiconductor ultraviolet photodetectors. Semicond. Sci. Technol. 2003, 18, 33–51. [Google Scholar] [CrossRef]
- Yu, X.X.; Yu, J.G.; Cheng, B.; Huang, B.B. One-pot template-free synthesis of monodisperse zinc sulfide hollow spheres and their photocatalytic properties. Chem. Eur. J. 2009, 15, 6731–6739. [Google Scholar] [CrossRef] [PubMed]
- Dabbousi, B.O.; RodriguezViejo, J.; Mikulec, F.V.; Heine, J.R.; Mattoussi, H.; Ober, R.; Jensen, K.F.; Bawendi, M.G. (CdSe)ZnS core–shell quantum dots: Synthesis and characterization of a size series of highly luminescent nanocrystallites. J. Phys. Chem. B 1997, 101, 9463–9475. [Google Scholar] [CrossRef]
- Fang, X.S.; Bando, Y.; Liao, M.Y.; Gautam, U.K.; Zhi, C.Y.; Dierre, B.; Liu, B.D.; Zhai, T.Y.; Sekiguchi, T.; Koide, Y.; et al. Single-crystalline ZnS nanobelts as ultraviolet-light sensors. Adv. Mater. 2009, 21, 2034–2039. [Google Scholar] [CrossRef]
- Wang, X.W.; Li, M.X.; Chang, Z.; Yang, Y.Q.; Wu, Y.P.; Liu, X. Co3O4@MWCNT nanocable as cathode with superior electrochemical performance for supercapacitors. Appl. Mater. Interfaces 2015, 5, 2280–2285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Hou, P.X.; Liu, C. Synthesis of coaxial nanocables of single-walled carbon nanotubes sheathed with amorphous silicon oxide. New Carbon Mater. 2013, 28, 8–13. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Saito, R.; Jorio, A. Raman spectroscopy of carbon nanotubes. Phys. Rep. 2005, 409, 47–99. [Google Scholar] [CrossRef]
- Richter, E.; Subbaswamy, K.R. Theory of size-dependent resonance Raman scattering from carbon nanotubes. Phys. Rev. Lett. 1997, 79, 2738–2741. [Google Scholar] [CrossRef]
- Cheng, H.M.; Li, F.; Sun, X.; Brown, S.D.M.; Pimenta, M.A.; Marucci, A.; Dresselhaus, G.; Dresselhaus, M.S. Bulk morphology and diameter distribution of single-walled carbon nanotubes synthesized by catalytic decomposition of hydrocarbons. Chem. Phys. Lett. 1998, 289, 602–610. [Google Scholar] [CrossRef]
- Ren, W.C.; Li, F.; Chen, J.A.; Bai, S.; Cheng, H.M. Morphology, diameter distribution and Raman scattering measurements of double-walled carbon nanotubes synthesized by catalytic decomposition of methane. Chem. Phys. Lett. 2002, 359, 196–202. [Google Scholar] [CrossRef]
- Ren, W.C.; Cheng, H.M. Aligned double-walled carbon nanotube long ropes with a narrow diameter distribution. J. Phys. Chem. B 2005, 109, 7169–7173. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, L.; Righi, A.; Guillard, T.; Rols, S.; Anglaret, E.; Laplaze, D.; Sauvajol, J.L. Resonant Raman study of the structure and electronic properties of single-wall carbon nanotubes. Chem. Phys. Lett. 2000, 316, 186–190. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Jorio, A.; Souza, A.G.; Saito, R. Raman spectroscopy on isolated single wall carbon nanotubes. Carbon 2002, 40, 2043–2061. [Google Scholar] [CrossRef]
- Kataura, H.; Kumazawa, Y.; Maniwa, Y.; Umezu, I.; Suzuki, S.; Ohtsuka, Y.; Achiba, Y. Optical properties of single-wall carbon nanotubes. Synth. Met. 1999, 103, 2555–2558. [Google Scholar] [CrossRef]
- Samsonidze, G.G.; Saito, R.; Kobayashi, N.; Gruneis, A.; Jiang, J.; Jorio, A.; Chou, S.G.; Dresselhaus, G.; Dresselhaus, M.S. Family behavior of the optical transition energies in single-wall carbon nanotubes of smaller diameters. Appl. Phys. Lett. 2004, 85, 5703–5705. [Google Scholar] [CrossRef]
- Brown, S.D.M.; Jorio, A.; Corio, P.; Dresselhaus, M.S.; Dresselhaus, G.; Saito, R.; Kneipp, K. Origin of the Breit-Wigner-Fano lineshape of the tangential G-band feature of metallic carbon nanotubes. Phys. Rev. B 2001, 63, 155414. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Resonant Raman spectroscopy of disordered, amorphous, and diamondlike carbon. Phys. Rev. B 2001, 64, 075414. [Google Scholar] [CrossRef]
- Ren, W.C.; Li, F.; Cheng, H.M. Evidence for, and an understanding of, the initial nucleation of carbon nanotubes produced by a floating catalyst method. J. Phys. Chem. B 2006, 110, 16941–16946. [Google Scholar] [CrossRef] [PubMed]
- Gorbunov, A.; Jost, O.; Pompe, W.; Graff, A. Solid-liquid-solid growth mechanism of single-wall carbon nanotubes. Carbon 2002, 40, 113–118. [Google Scholar] [CrossRef]



© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; He, X.; Wang, L.; Gao, J.; Li, J. One-Step Synthesis of Single-Wall Carbon Nanotube-ZnS Core-Shell Nanocables. Materials 2016, 9, 718. https://doi.org/10.3390/ma9090718
Zhang Y, He X, Wang L, Gao J, Li J. One-Step Synthesis of Single-Wall Carbon Nanotube-ZnS Core-Shell Nanocables. Materials. 2016; 9(9):718. https://doi.org/10.3390/ma9090718
Chicago/Turabian StyleZhang, Yanli, Xiangming He, Li Wang, Jian Gao, and Jianjun Li. 2016. "One-Step Synthesis of Single-Wall Carbon Nanotube-ZnS Core-Shell Nanocables" Materials 9, no. 9: 718. https://doi.org/10.3390/ma9090718






