Synthesis of Acylated Xylan-Based Magnetic Fe3O4 Hydrogels and Their Application for H2O2 Detection
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
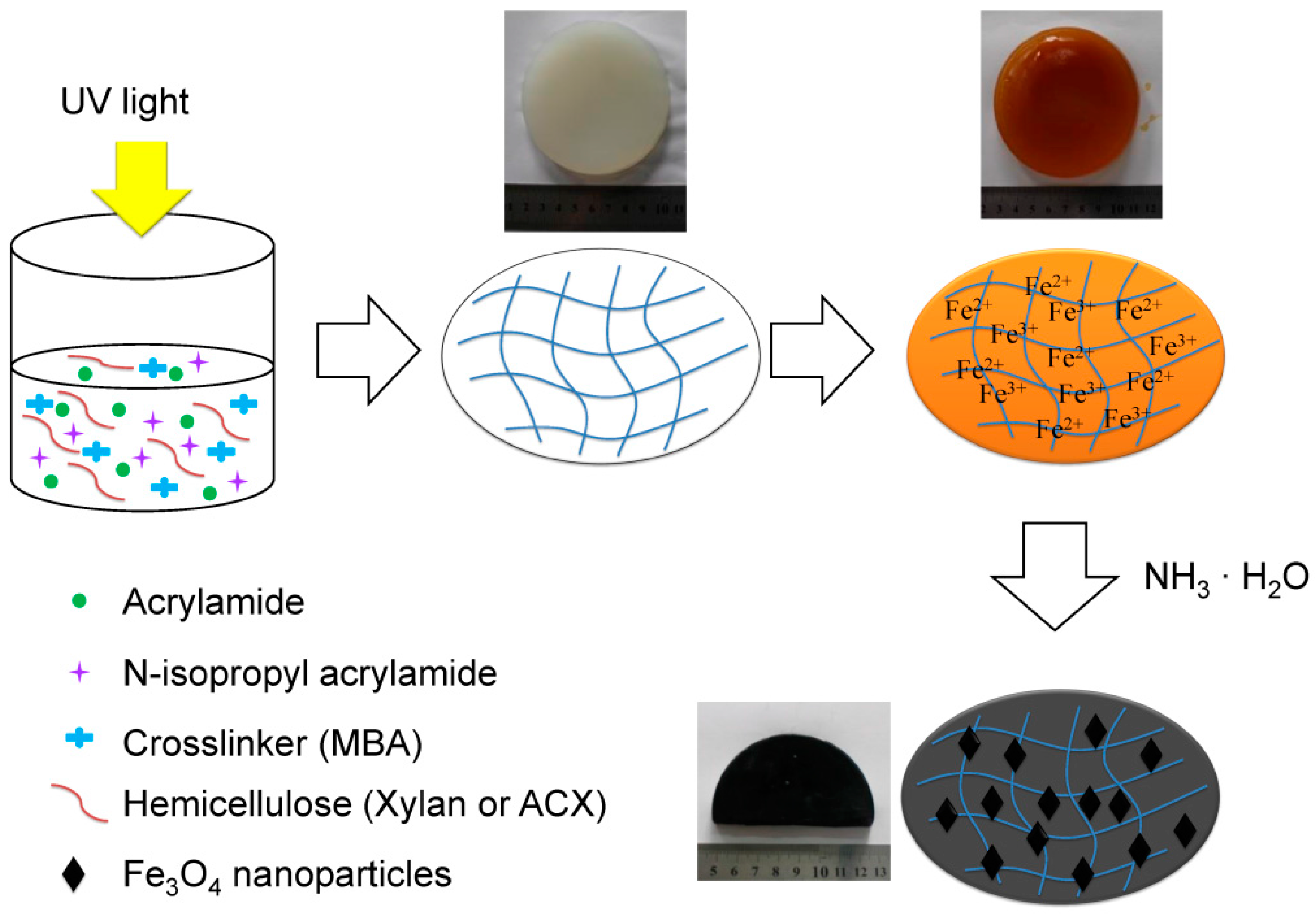
2.2. Preparation of ACX and Xylan or ACX Based Magnetic Gels
2.2.1. Preparation of ACX
2.2.2. Preparation of Xylan or ACX Based Magnetic Hydrogels
2.3. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.4. Morphology of Hydrogels
2.5. X-ray Diffraction (XRD) Analysis
2.6. Raman Spectra
2.7. Magnetic Measurements
2.8. Determination of Swelling Degree of Hydrogels
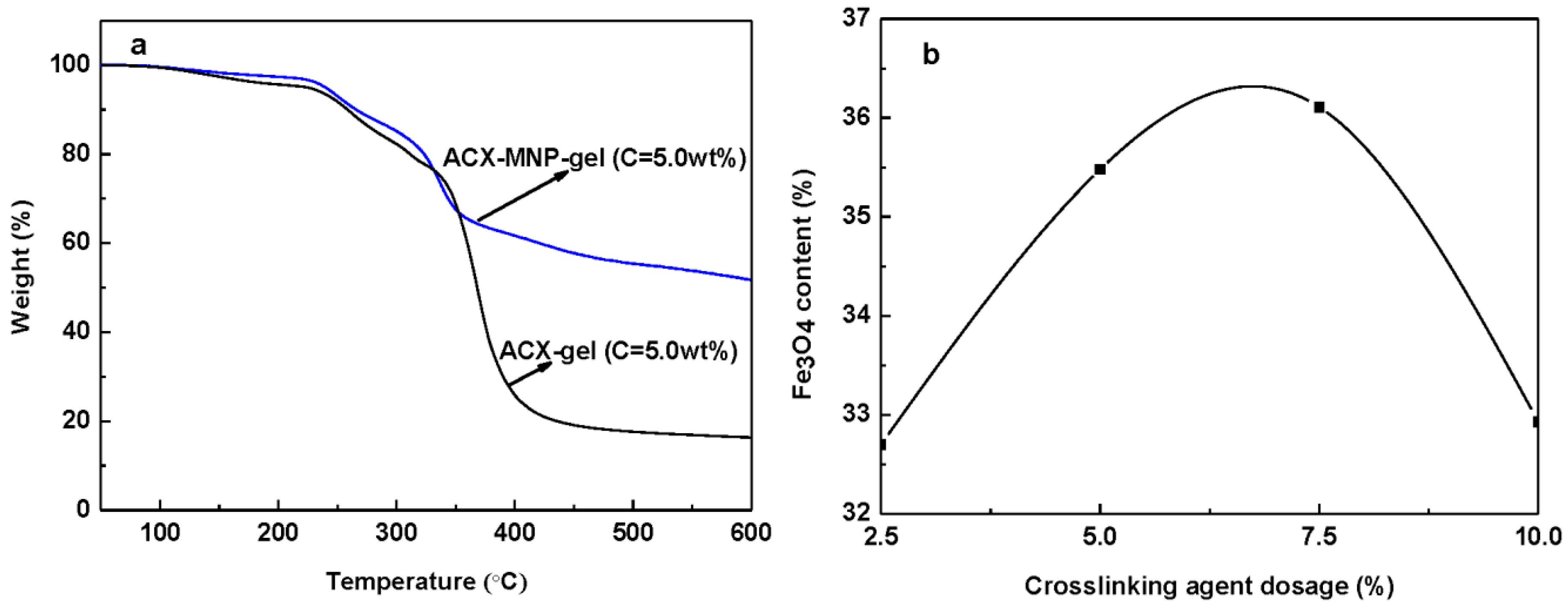
2.9. Thermogravimetric Analysis
2.10. Catalytic Experiment and H2O2 Detection
3. Results and Discussion
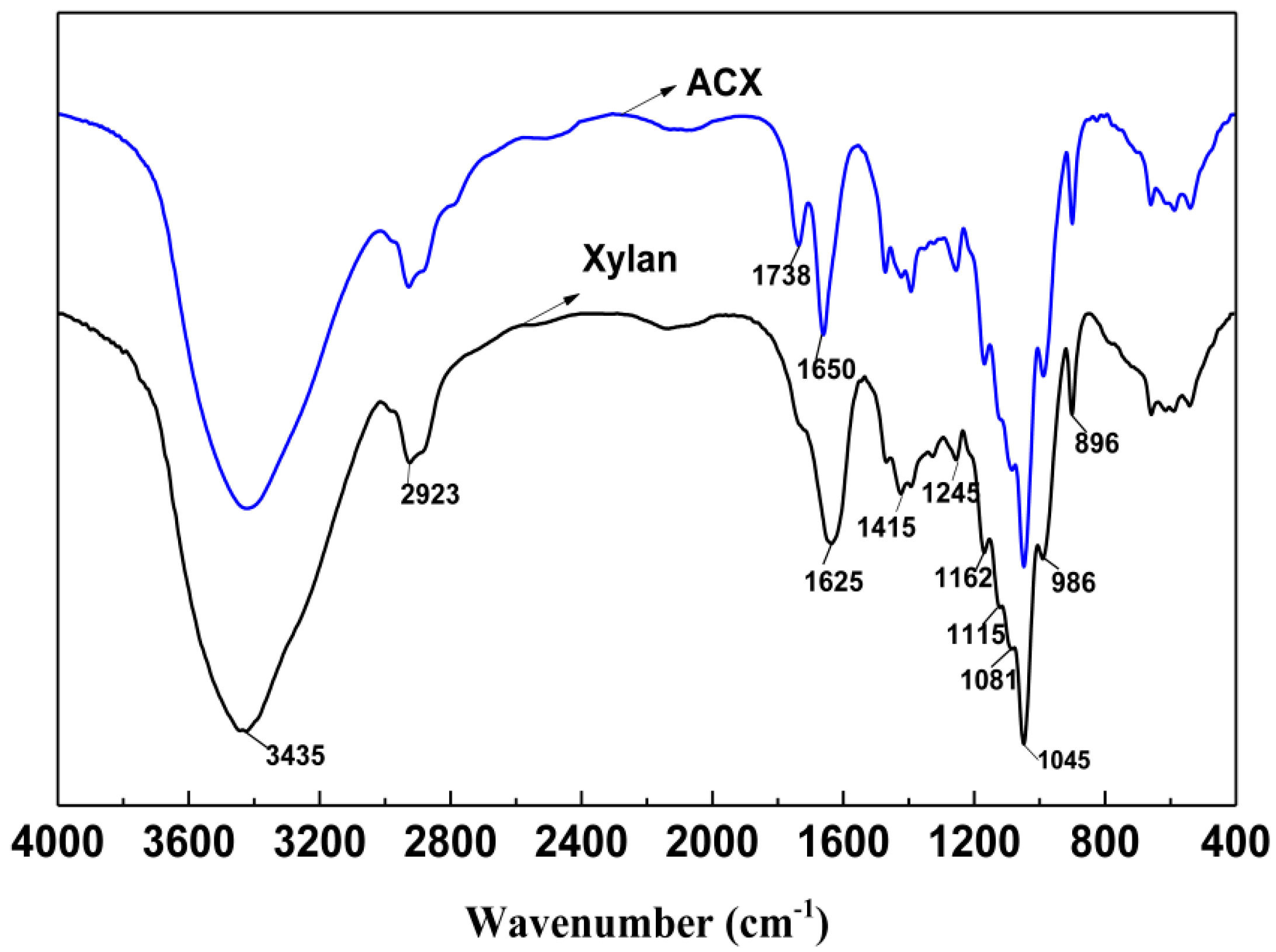
3.1. FTIR Analysis
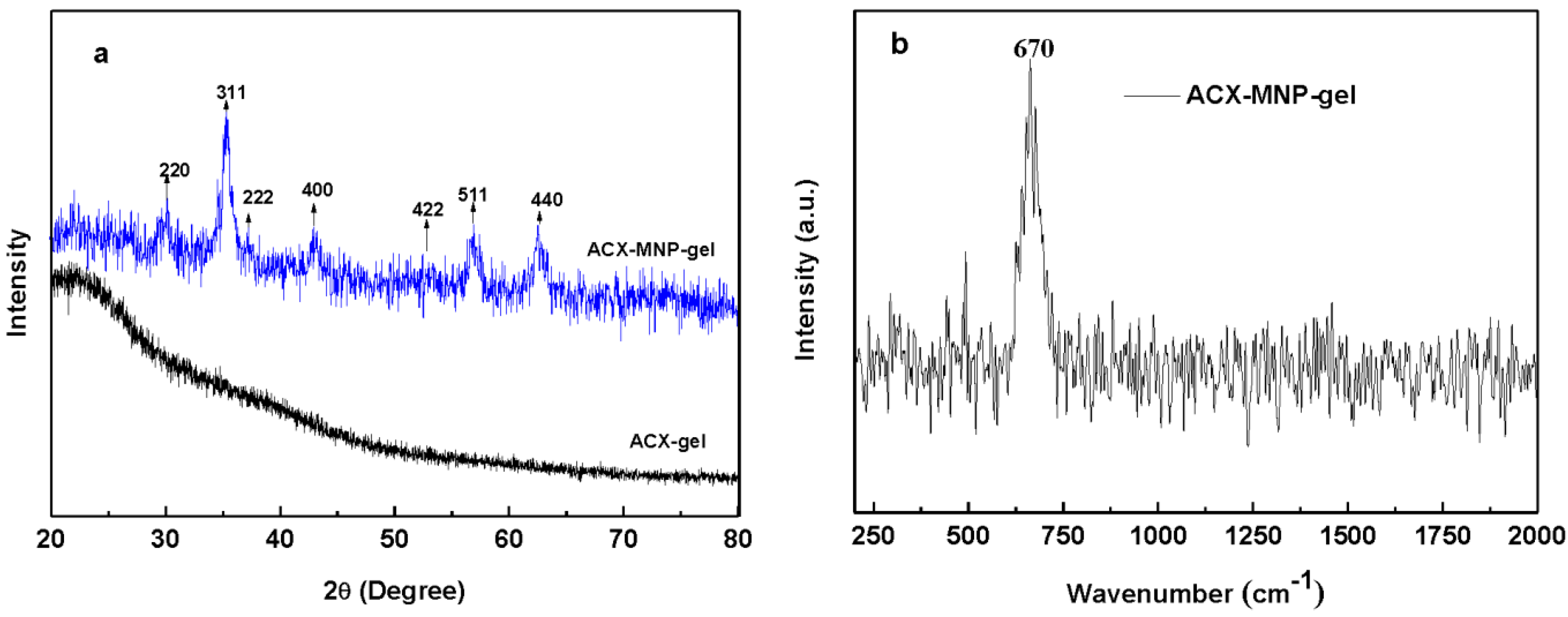
3.2. The Crystal Structure Analysis of Fe3O4
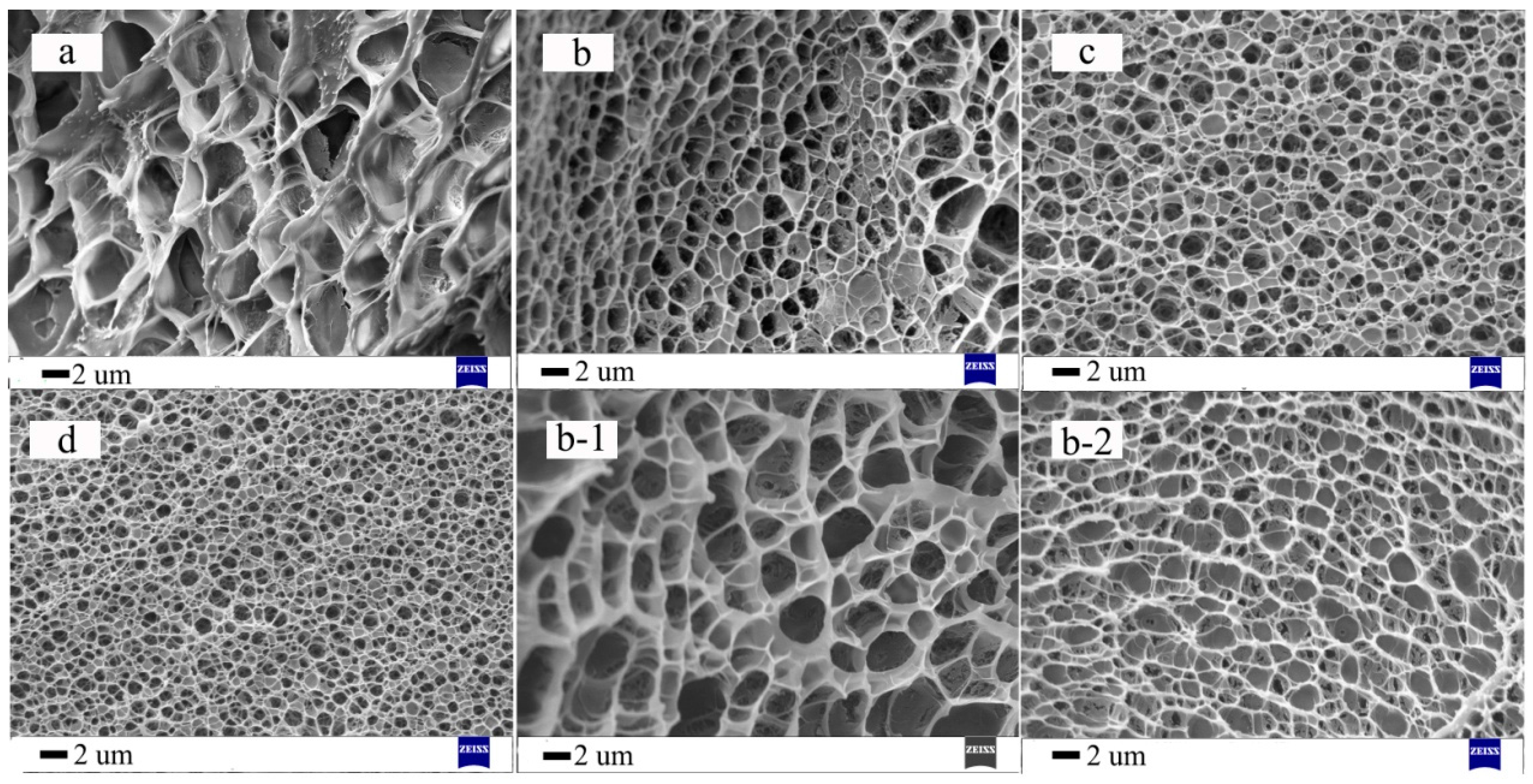
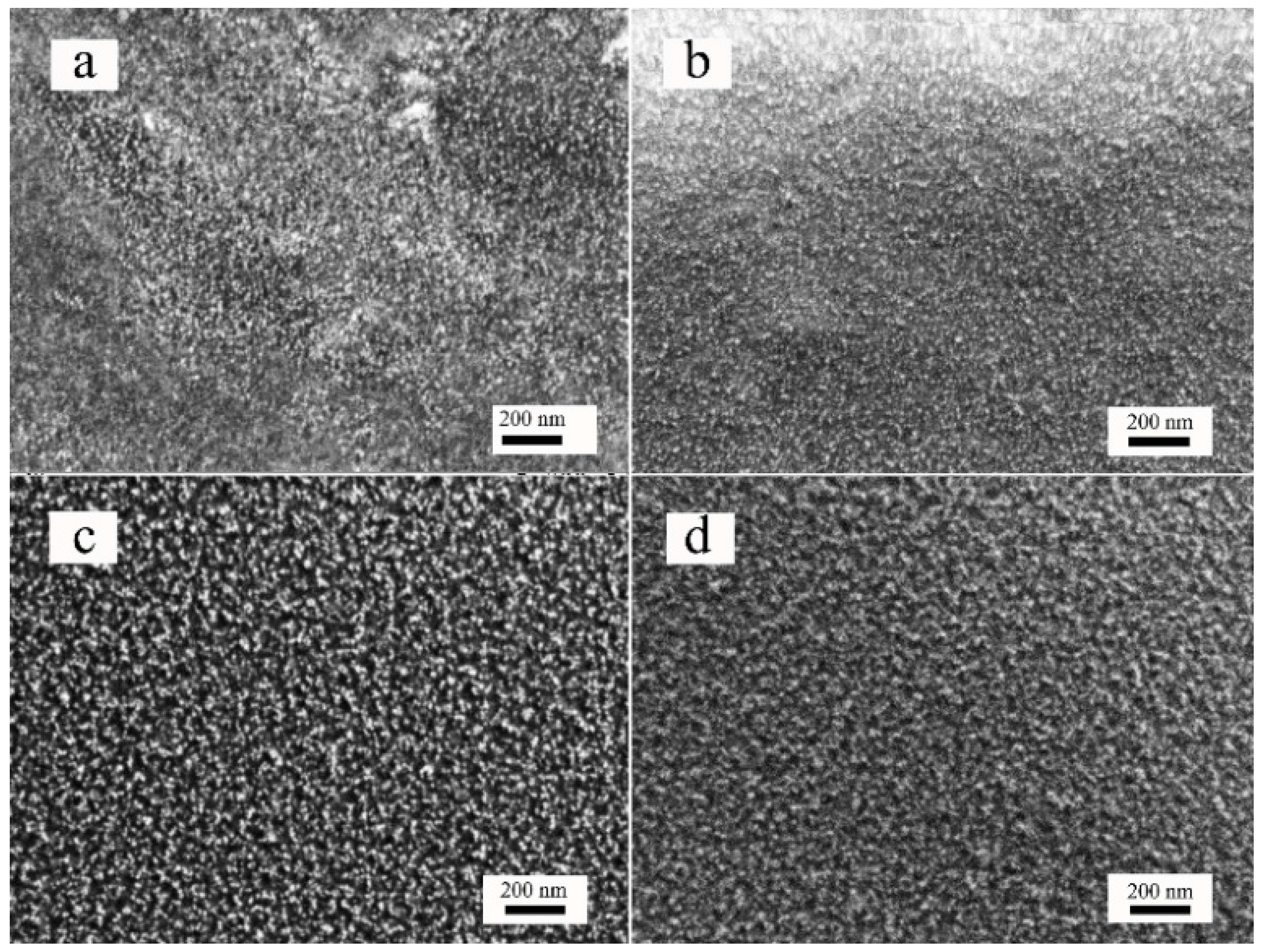
3.3. Morphological Analysis
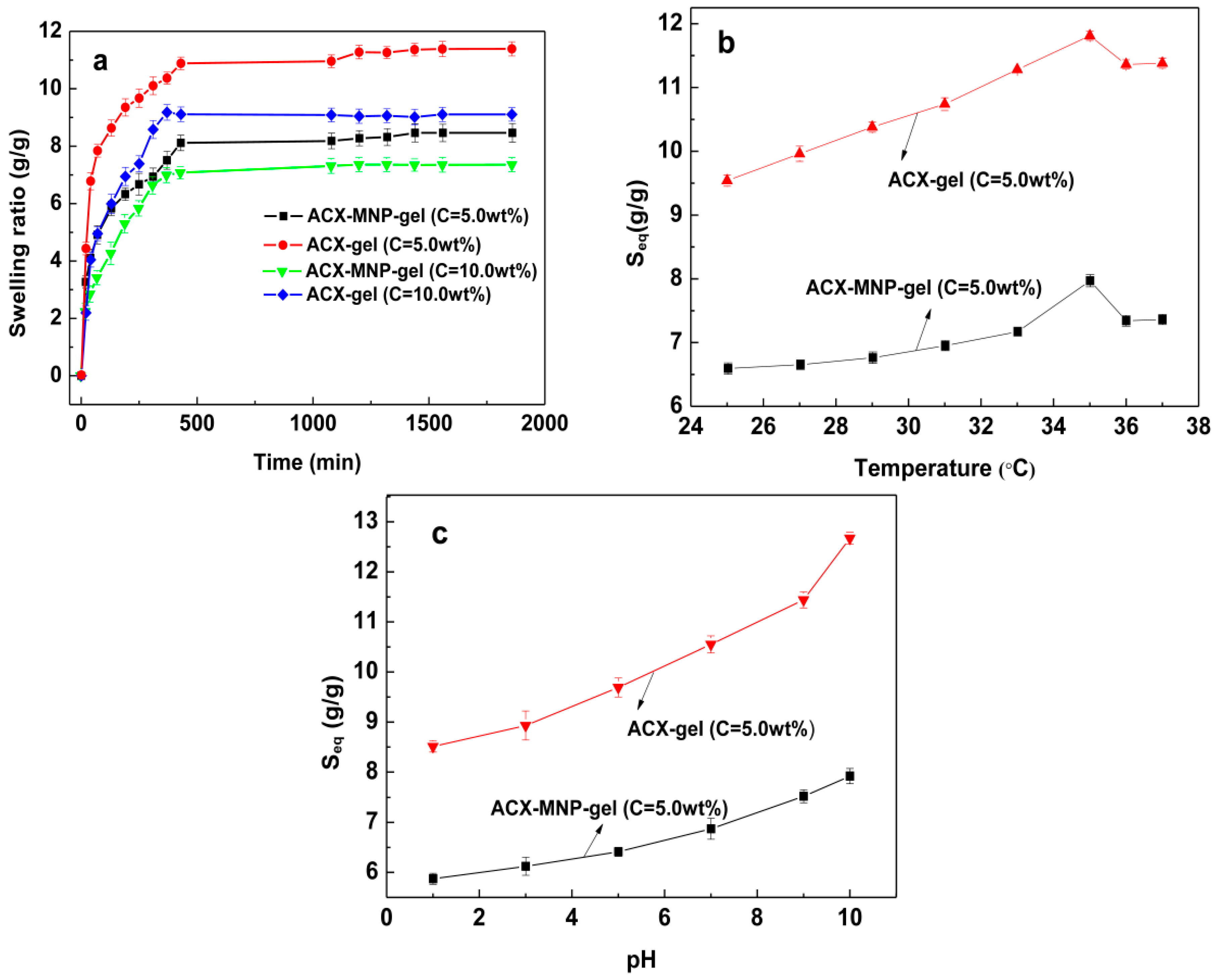
3.4. Swelling Behavior
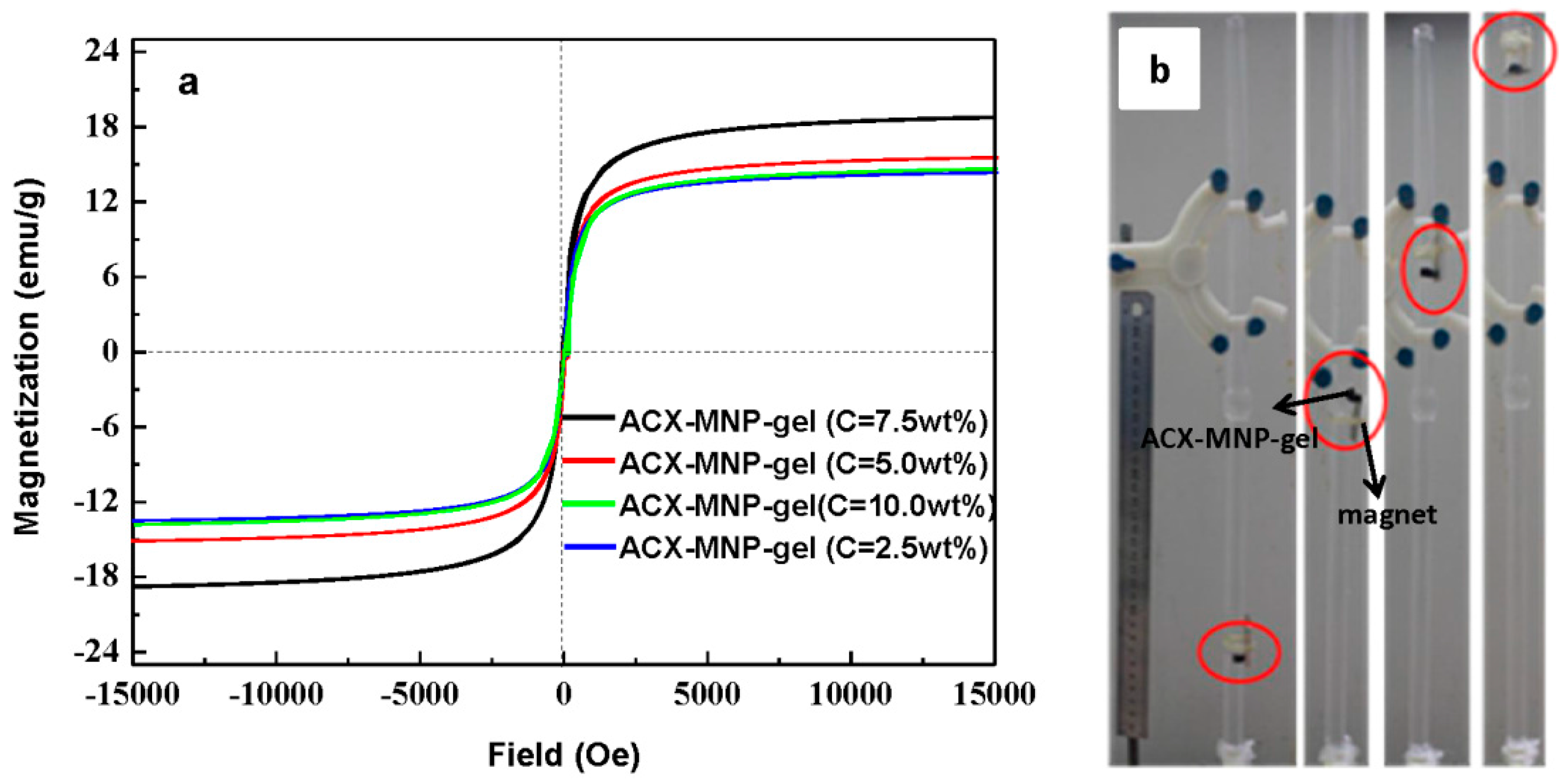
3.5. Magnetic Properties of Hydrogels
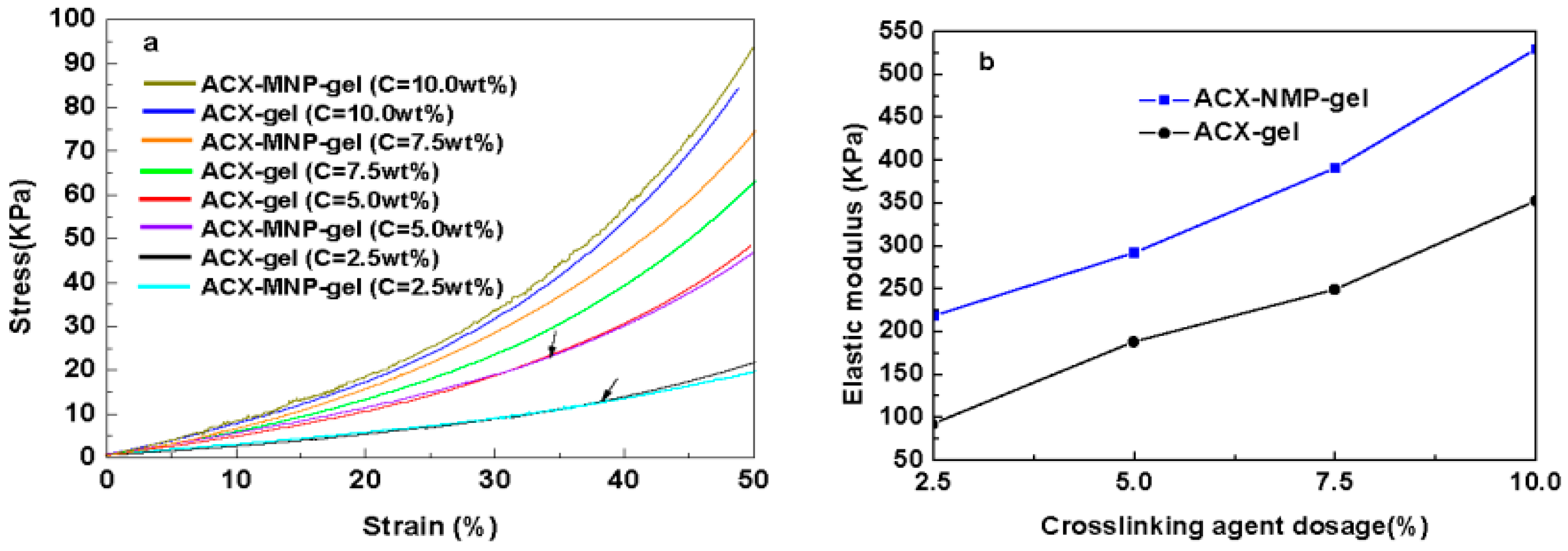
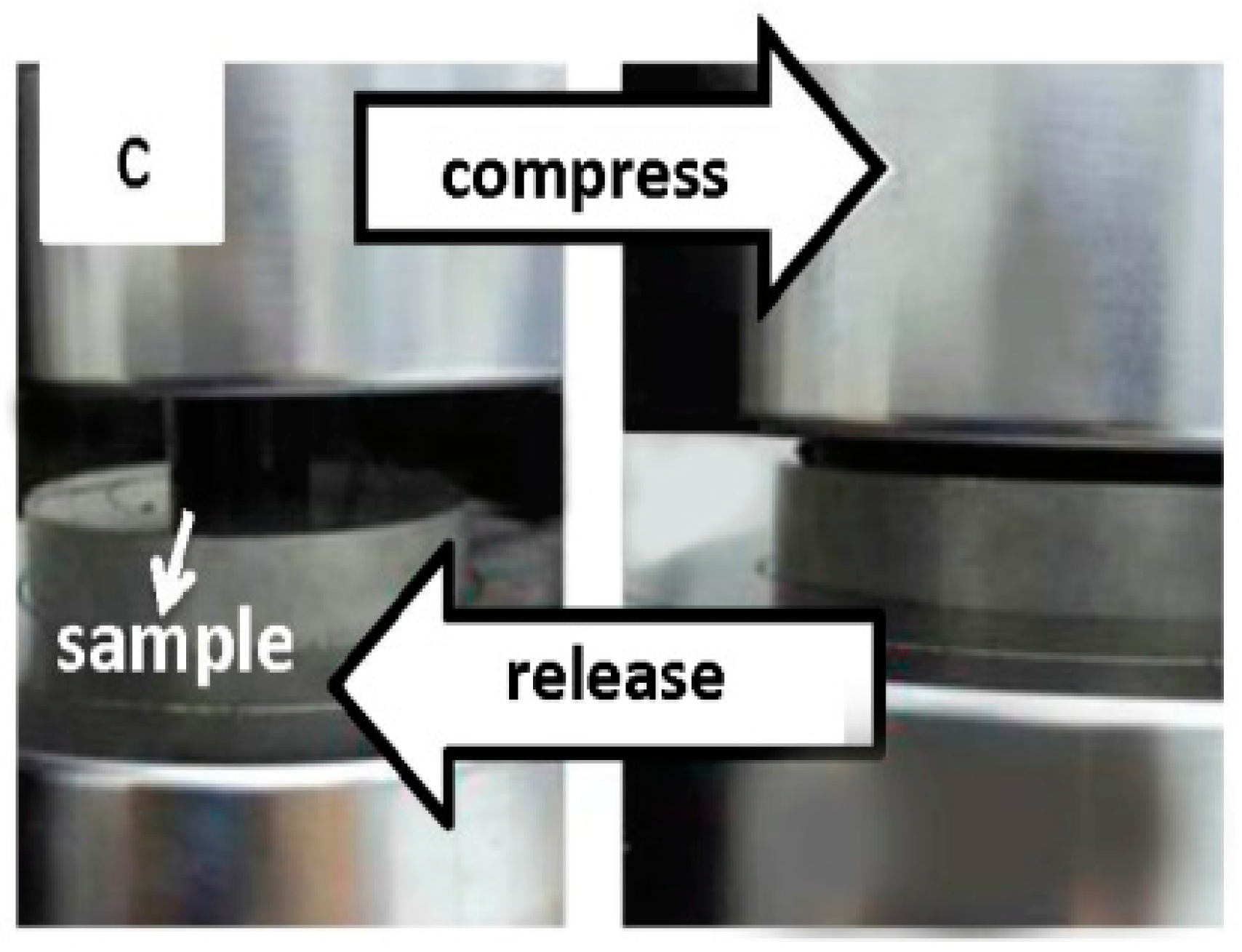
3.6. Mechanical Property Analysis
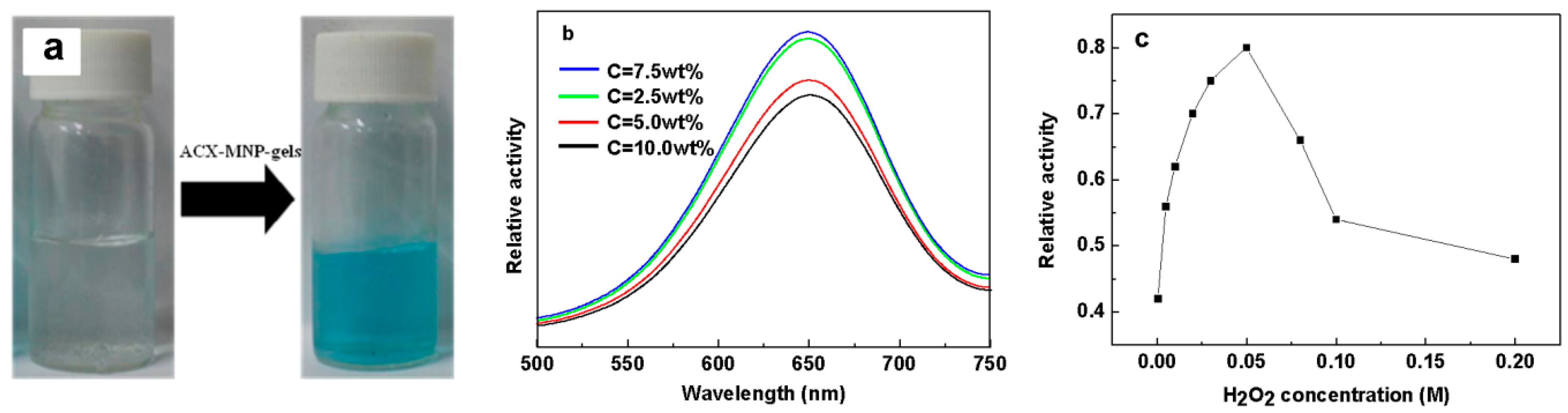
3.7. Catalytic Experiments and H2O2 Detection
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Horkay, F.; Mckenna, G.B. Polymer networks and gels. In Physical Properties of Polymers Handbook; Springer New York: New York, NY, USA, 2007; pp. 497–523. [Google Scholar]
- Sun, X.-F.; Liu, B.-C.; Jing, Z.-X.; Wang, H.-H. Preparation and adsorption property of xylan/poly(acrylic acid) magnetic nanocomposite hydrogel adsorbent. Carbohydr. Polym. 2015, 118, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Charati, M.B.; Lee, I.; Hribar, K.C.; Burdick, J.A. Light-sensitive polypeptide hydrogel and nanorod composites. Small 2010, 6, 1608–1611. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.-F.; Li, M.-J.; Wu, X.-M.; Li, M.; Wu, Y. Preparation of thermoresponsive and pH-sensitivity polymer magnetic hydrogel nanospheres as anticancer drug carriers. Colloids Surf. B 2011, 88, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wei, Z.; Li, F.; Yang, Z.-M.; Chen, Y.-M.; Zrinyi, M.; Osada, Y. Synthesis of a morphology controllable Fe3O4 nanoparticle/hydrogel magnetic nanocomposite inspired by magnetotactic bacteria and its application in H2O2 detection. Green Chem. 2014, 16, 1255–1261. [Google Scholar] [CrossRef]
- Xiang, M.; He, C.-C.; Wang, H.-L. Magnetic polyacrylamide/Fe3O4 nanocomposite hydrogel with high mechanical strength. Acta Phys. Chim. Sin. 2011, 27, 1267–1272. [Google Scholar]
- Hu, S.-H.; Liu, T.-Y.; Liu, D.-M.; Chen, S.-Y. Controlled pulsatile drug release from a ferrogel by a high-frequency magnetic field. Macromolecules 2007, 40, 6786–6788. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, L.; Hu, Y.; Guo, C.-F.; Qian, H.-S.; Zhang, F.-M.; Lou, X.-W. Magnetic-field induced formation of 1D Fe3O4/C/CdS coaxial nanochains as highly efficient and reusable photocatalysts for water treatment. J. Mater. Chem. 2011, 21, 18359–18364. [Google Scholar] [CrossRef]
- Samanta, B.; Yan, H.; Fischer, N.O.; Shi, J.; Jerry, D.J.; Rotello, V.M. Protein-passivated Fe3O4 nanoparticles: Low toxicity and rapid heating for thermal therapy. J. Mater. Chem. 2008, 18, 1204–1208. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.-Z.; Zhuang, J.; Nie, L.; Zhang, J.-B.; Zhang, Y.; Gu, N.; Wang, T.-H.; Feng, J.; Yang, D.-L.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Ellis, W.C.; Tran, C.T.; Denardo, M.A.; Fischer, A.; Ryabov, A.D.; Collins, T.J. Design of more powerful iron-TAML peroxidase enzyme mimics. J. Am. Chem. Soc. 2009, 131, 18052–18053. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, E. Fe3O4 magnetic nanoparticles as peroxidase mimetics and their applications in H2O2 and glucose detection. Anal. Chem. 2008, 80, 2250–2254. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Tang, Y.-L.; Yu, M.-H.; Wang, S.; Li, Y.-L.; Zhu, D.-B. Fluorescence-amplifying detection of hydrogen peroxide with cationic conjugated polymers, and its application to glucose sensing. Adv. Funct. Mater. 2006, 16, 91–94. [Google Scholar] [CrossRef]
- Kong, W.-Q.; Dai, Q.-Q.; Ren, J.-L.; Ma, N.-F. Homogeneous acylation of xylan with 3,5-dinitrobenzoyl in ionic liquid and the adsorption property. Carbohydr. Polym. 2015, 128, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Ebringerova, A.; Heinze, T. Xylan and xylan derivatives—Biopolymers with valuable properties, 1. Naturally occurring xylans structures, procedures and properties. Macromol. Rapid Commun. 2000, 21, 542–556. [Google Scholar] [CrossRef]
- Peng, X.-W.; Ren, J.-L.; Sun, R.-C. Homogeneous esterification of Xylan-Rich hemicelluloses with maleic anhydride in ionic liquid. Biomacromolecules 2010, 11, 3519–3524. [Google Scholar] [CrossRef] [PubMed]
- Vincendon, M. Xylan derivatives: Benzyl ethers, synthesis, and characterization. J. Appl. Polym. Sci. 1998, 67, 455–460. [Google Scholar] [CrossRef]
- Gröndahl, M.; Teleman, A.; Gatenholm, P. Effect of acetylation on the material properties of glucuronoxylan from aspen wood. Carbohydr. Polym. 2003, 52, 359–366. [Google Scholar] [CrossRef]
- Lindblad, M.S.; Ranucci, E.; Albertsson, A.C. Biodegradable polymers from renewable sources. New hemicellulose-based hydrogels. Macromol. Rapid Commun. 2001, 22, 962–967. [Google Scholar] [CrossRef]
- Petzold, K.; Schwikal, K.; Günther, W.; Heinze, T. Carboxymethyl xylan-control of properties by synthesis. Macromol. Symp. 2006, 232, 27–36. [Google Scholar] [CrossRef]
- Ren, J.-L.; Peng, X.-W.; Peng, F.; Sun, R.-C. The preparation and application of the cationic biopolymer based on xylan-rich hemicelluloses from agricultural biomass. Adv. Mater. Res. Switz. 2011, 239, 463–467. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Li, H.-L.; Ren, J.-L.; Liu, C.-F.; Peng, F.; Sun, R.-C. Preparation of xylan citrate—A potential adsorbent for industrial wastewater treatment. Carbohydr. Polym. 2013, 92, 1960–1965. [Google Scholar]
- Cao, X.-F.; Zhong, L.-X.; Peng, X.-W.; Li, S.-M.; Sun, R.-C. Synthesis, characterization, and application of multiresponsive hemicellulose based hydrogels. Am. Chem. Soc. 2014, 247, 224. [Google Scholar]
- Guan, Y.; Zhang, B.; Bian, J.; Peng, F.; Sun, R.-C. Nanoreinforced hemicellulose-based hydrogels prepared by freeze-thaw treatment. Cellulose 2014, 21, 1709–1721. [Google Scholar] [CrossRef]
- Gao, C.-D.; Ren, J.-L.; Kong, W.-Q.; Sun, R.-C.; Chen, Q.-F. Comparative study on temperature/pH sensitive xylan-based hydrogels: Their properties and drug controlled release. RSC Adv. 2015, 5, 90671–90681. [Google Scholar] [CrossRef]
- Peng, X.-W.; Zhong, L.-X.; Ren, J.-L.; Sun, R.-C. Highly effective adsorption of heavy metal ions from aqueous solutions by macroporous xylan-rich hemicelluloses-based hydrogel. J. Agric. Food Chem. 2012, 60, 3909–3916. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.-F.; Peng, X.-W.; Zhong, L.-X.; Sun, R.-C. Multiresponsive hydrogels based on xylan-type hemicelluloses and photoisomerized azobenzene copolymer as drug delivery carrier. J. Agric. Food. Chem. 2014, 62, 10000–10007. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, W.; Plog, A.; Xu, Y.-P.; Buntkowsky, G.; Gutmann, T.; Zhang, K. Multi-responsive cellulose nanocrystal-rhodamine conjugates: An advanced structure study by solid-state dynamic nuclear polarization (DNP) NMR. Phys. Chem. Chem. Phys. 2014, 16, 26322–26329. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.-W.; Ren, J.-L.; Zhong, L.-X.; Sun, R.-C.; Shi, W.-B.; Hu, B.-J. Glycidyl methacrylate derivatized xylan-rich hemicelluloses: Synthesis and characterizations. Cellulose 2012, 19, 1361–1372. [Google Scholar] [CrossRef]
- Sun, R.-C.; Fang, J.-M.; Tomkinson, J.; Geng, Z.-C.; Liu, J.-C. Fractional isolation, physico-chemical characterization and homogeneous esterification of hemicelluloses from fast-growing poplar wood. Carbohydr. Polym. 2001, 44, 29–39. [Google Scholar] [CrossRef]
- Sun, X.-F.; Sun, R.-C.; Fowler, P.; Baird, M.S. Extraction and characterization of original lignin and hemicelluloses from wheat straw. J. Agric. Food Chem. 2005, 53, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-F.; Sun, R.-C.; Tomkinson, J.; Baird, M.S. Preparation of sugarcane bagasse hemicellulosic succinates using NBS as a catalyst. Carbohyd. Polym. 2003, 53, 483–495. [Google Scholar] [CrossRef]
- Schwikal, K.; Heinze, T.; Ebringerova, A.; Petzold, K. Cationic xylan derivatives with high degree of functionalization. Macromol. Symp. 2006, 232, 49–56. [Google Scholar] [CrossRef]
- Amash, A.; Zugenmaier, P. Study on cellulose and xylan filled polypropylene composites. Polym. Bull. 1998, 40, 251–258. [Google Scholar] [CrossRef]
- Amemiya, Y.; Arakaki, A.; Staniland, S.S.; Tanaka, T.; Matsunaga, T. Controlled formation of magnetite crystal by partial oxidation of ferrous hydroxide in the presence of recombinant magnetotactic bacterial protein Mms6. Biomaterials 2007, 28, 5381–5389. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Bian, J.; Peng, F.; Zhang, X.-M.; Sun, R.-C. High strength of hemicelluloses based hydrogels by freeze/thaw technique. Carbohydr. Polym. 2014, 101, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Ai, Z.-H.; Deng, K.-J.; Wan, Q.-F.; Zhang, L.-Z.; Lee, S. Facile microwave-assisted synthesis and magnetic and gas sensing properties of Fe3O4 nanoroses. J. Phys. Chem. C 2010, 114, 6237–6242. [Google Scholar] [CrossRef]
- Zhu, C.-H.; Lu, Y.; Chen, J.-F.; Yu, S.-H. Photothermal poly(N-isopropylacrylamide)/Fe3O4 nanocomposite hydrogel as a movable position heating source under remote control. Small 2014, 10, 2796–2800. [Google Scholar] [CrossRef] [PubMed]
- Shebanova, O.N.; Lazor, P. Raman study of magnetite (Fe3O4): Laser-induced thermal effects and oxidation. J. Raman Spectrosc. 2003, 34, 845–852. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Li, B.-Q.; Zhou, Y.; Jia, D.-C. In situ mineralization of magnetite nanoparticles in chitosan hydrogel. Nanoscale Res. Lett. 2009, 4, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.H.; Zheng, C.M.; Zhang, F.X.; Yang, Y.L.; Wu, G.J.; Yu, A.M.; Guan, N.J. Size-controlled synthesis of magnetite (Fe3O4) nanoparticles coated with glucose and gluconic acid from a single Fe(III) precursor by a sucrose bifunctional hydrothermal method. J. Phys. Chem. C 2009, 113, 16002–16008. [Google Scholar] [CrossRef]
- Jing, Z.-X.; Sun, X.-F.; Ye, Q.; Li, Y.-J. Hemicellulose-based porous hydrogel for methylene blue adsorption. Adv. Mater. Res. Switz. 2012, 560, 482–487. [Google Scholar] [CrossRef]
- Grassmann, O.; Löbmann, P. Biomimetic nucleation and growth of CaCO3 in hydrogels incorporating carboxylate groups. Biomaterials 2004, 25, 277–282. [Google Scholar] [CrossRef]
- Yu, S.-H.; Colfen, H.; Tauer, K.; Antonietti, M. Tectonic arrangement of BaCO3 nanocrystals into helices induced by a racemic block copolymer. Nat. Mater. 2005, 4, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Z.; Wu, D.Q.; Chu, C.C. Synthesis, characterization and controlled drug release of thermosensitive IPN-PNIPAAm hydrogels. Biomaterials 2004, 25, 3793–3805. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Ballauff, M. The volume transition in thermosensitive core-shell latex particles containing charged groups. Colloid Polym. Sci. 1999, 277, 1210–1214. [Google Scholar] [CrossRef]
- Di, X.J.; Peng, X.G.; McKenna, G.B. Dynamics of a thermo-responsive microgel colloid near to the glass transition. J. Chem. Phys. 2014, 140. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Dong, J.; Ding, Y.M.; Han, W.J. Environmental responses of poly(N-isopropylacrylamide-co-glycidyl methacrylate derivatized dextran) hydrogels. J. Appl. Polym. Sci. 2005, 96, 2435–2439. [Google Scholar] [CrossRef]
- Zhou, Y.S.; Yang, D.Z.; Gao, X.Y.; Chen, X.M.; Xu, Q.; Lu, F.M.; Nie, J. Semi-interpenetrating polymer network hydrogels based on water-soluble N-carboxylethyl chitosan and photo polymerized poly (2-hydroxyethyl methacrylate). Carbohydr. Polym. 2009, 75, 293–298. [Google Scholar] [CrossRef]
- Yang, K.; Peng, H.B.; Wen, Y.H.; Li, N. Re-examination of characteristic FTIR spectrum of secondary layer in bilayer oleic acid-coated Fe3O4 nanoparticles. Appl. Surf. Sci. 2010, 256, 3093–3097. [Google Scholar] [CrossRef]
- Grassmann, O.; Müller, G.; Löbmann, P. Organic-inorganic hybrid structure of calcite crystalline assemblies grown in a gelatin hydrogel matrix: Relevance to biomineralization. Chem. Mater. 2002, 14, 4530–4535. [Google Scholar] [CrossRef]
- Gnanaprakash, G.; Mahadevan, S.; Jayakumar, T.; Kalyanasundaram, P.; Philip, J.; Raj, B. Effect of initial pH and temperature of iron salt solutions on formation of magnetite nanoparticles. Mater. Chem. Phys. 2007, 103, 168–175. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, K.; Peng, Z.-M.; Chen, Q.-W. Magnetic properties improvement in Fe3O4 nanoparticles grown under magnetic fields. J. Cryst. Growth 2004, 266, 500–504. [Google Scholar] [CrossRef]
| Xylan-Gels | ACX-Gels | ||
|---|---|---|---|
| NIPAm/xylan (g/g) | 0.1 | NIPAm/ACX (g/g) | 0.1 |
| AM/xylan (g/g) | 5.0 | AM/ACX (g/g) | 5.0 |
| MBA/xylan (g/g) | 2.5–10.0 | MBA/ACX (g/g) | 2.5–10.0 |
| Initiator (%) | 2.5 | Initiator (%) | 2.5 |
| Samples | C | Crystallite Size (nm) |
|---|---|---|
| a | 2.5 wt % | 7.20 |
| b | 5.0 wt % | 9.32 |
| c | 7.5 wt % | 10.24 |
| d | 10.0 wt % | 9.63 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Q.-Q.; Ren, J.-L.; Peng, F.; Chen, X.-F.; Gao, C.-D.; Sun, R.-C. Synthesis of Acylated Xylan-Based Magnetic Fe3O4 Hydrogels and Their Application for H2O2 Detection. Materials 2016, 9, 690. https://doi.org/10.3390/ma9080690
Dai Q-Q, Ren J-L, Peng F, Chen X-F, Gao C-D, Sun R-C. Synthesis of Acylated Xylan-Based Magnetic Fe3O4 Hydrogels and Their Application for H2O2 Detection. Materials. 2016; 9(8):690. https://doi.org/10.3390/ma9080690
Chicago/Turabian StyleDai, Qing-Qing, Jun-Li Ren, Feng Peng, Xiao-Feng Chen, Cun-Dian Gao, and Run-Cang Sun. 2016. "Synthesis of Acylated Xylan-Based Magnetic Fe3O4 Hydrogels and Their Application for H2O2 Detection" Materials 9, no. 8: 690. https://doi.org/10.3390/ma9080690
APA StyleDai, Q.-Q., Ren, J.-L., Peng, F., Chen, X.-F., Gao, C.-D., & Sun, R.-C. (2016). Synthesis of Acylated Xylan-Based Magnetic Fe3O4 Hydrogels and Their Application for H2O2 Detection. Materials, 9(8), 690. https://doi.org/10.3390/ma9080690







