Nitrogen-Rich Energetic Metal-Organic Framework: Synthesis, Structure, Properties, and Thermal Behaviors of Pb(II) Complex Based on N,N-Bis(1H-tetrazole-5-yl)-Amine
Abstract
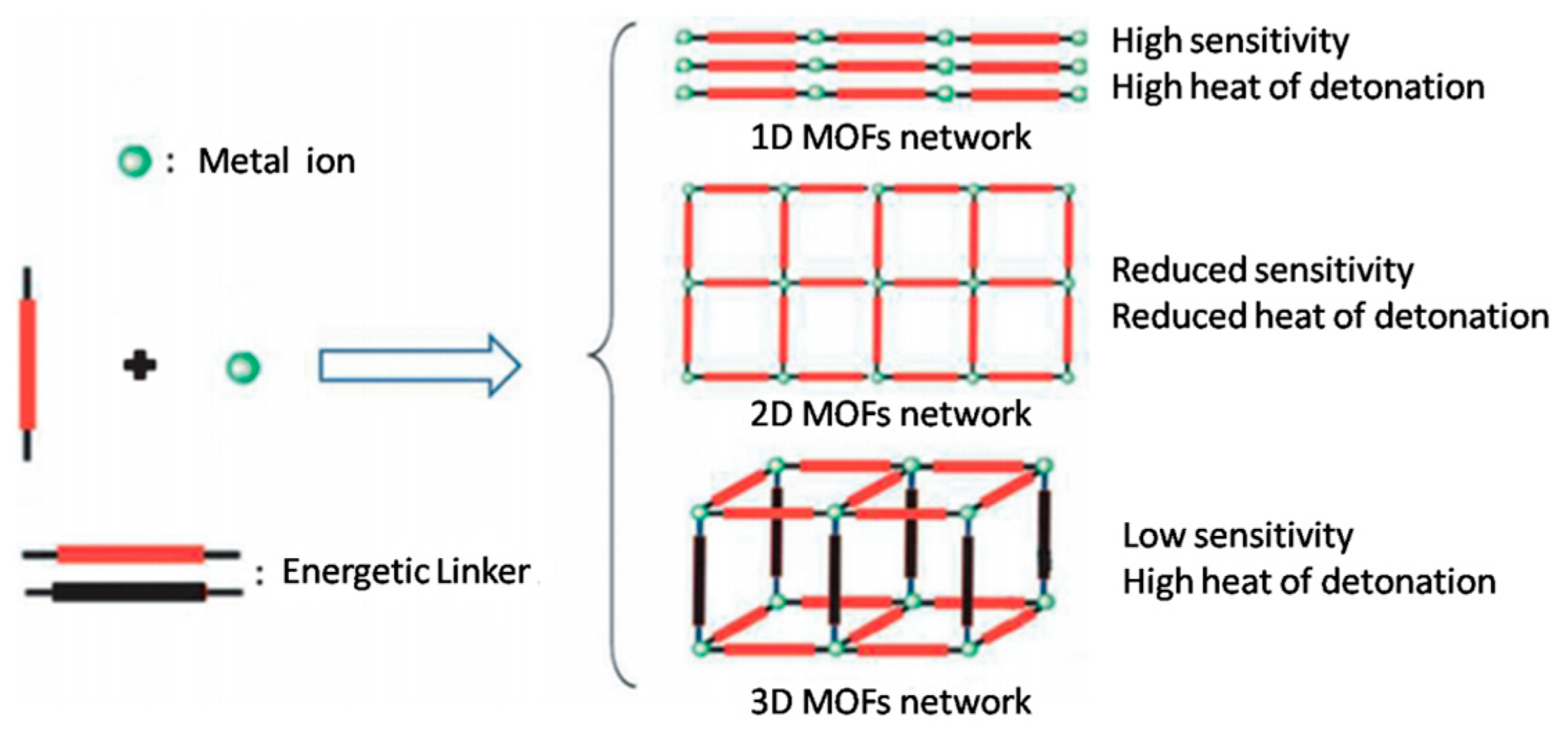
:1. Introduction
2. Results and Discussion
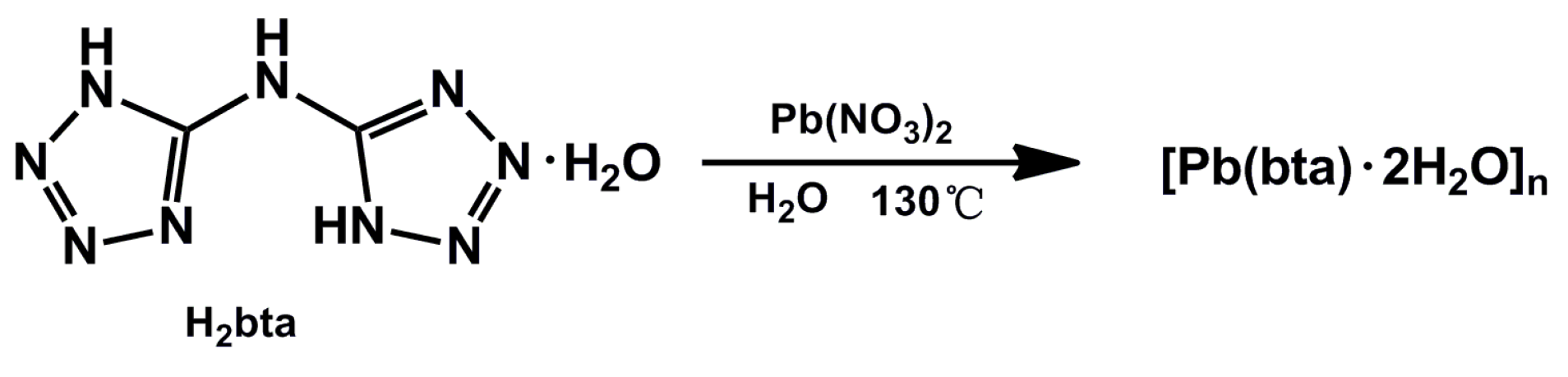
2.1. Synthesis of the Complex
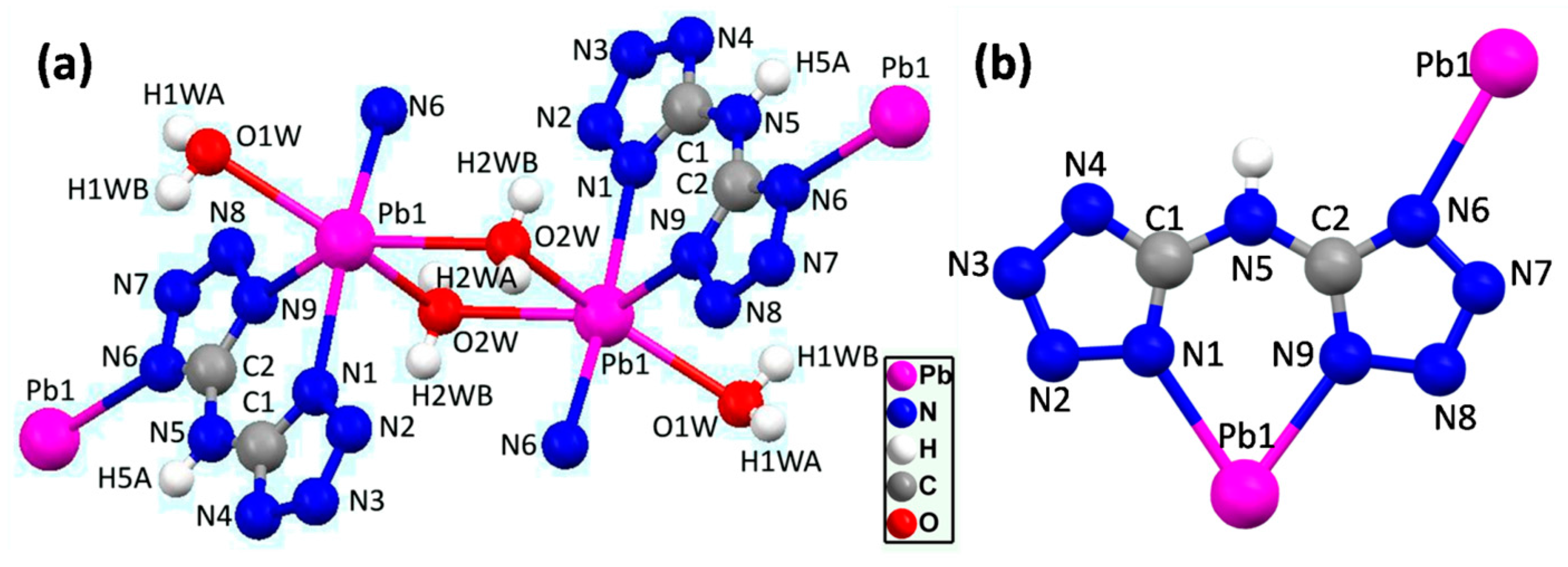
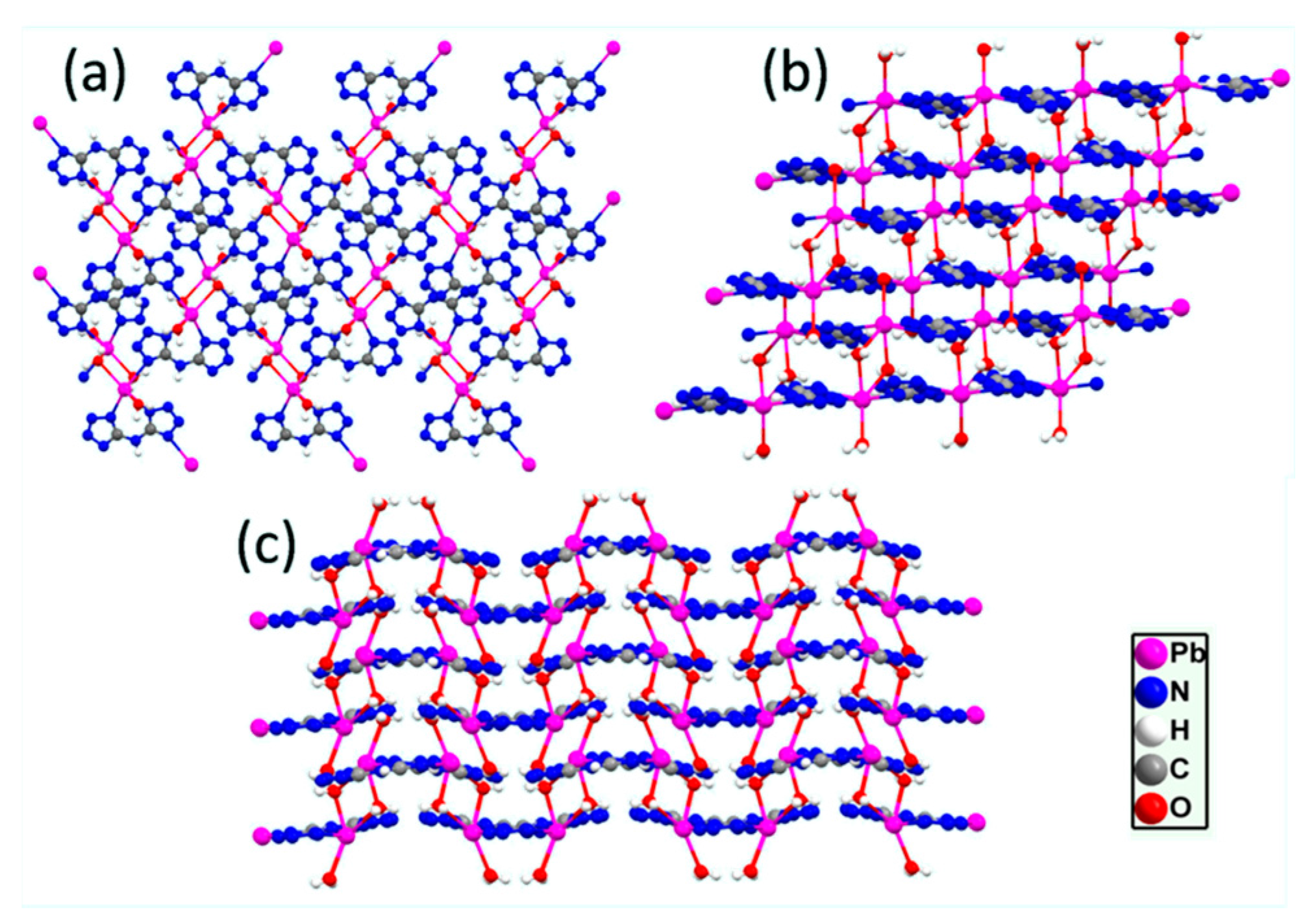
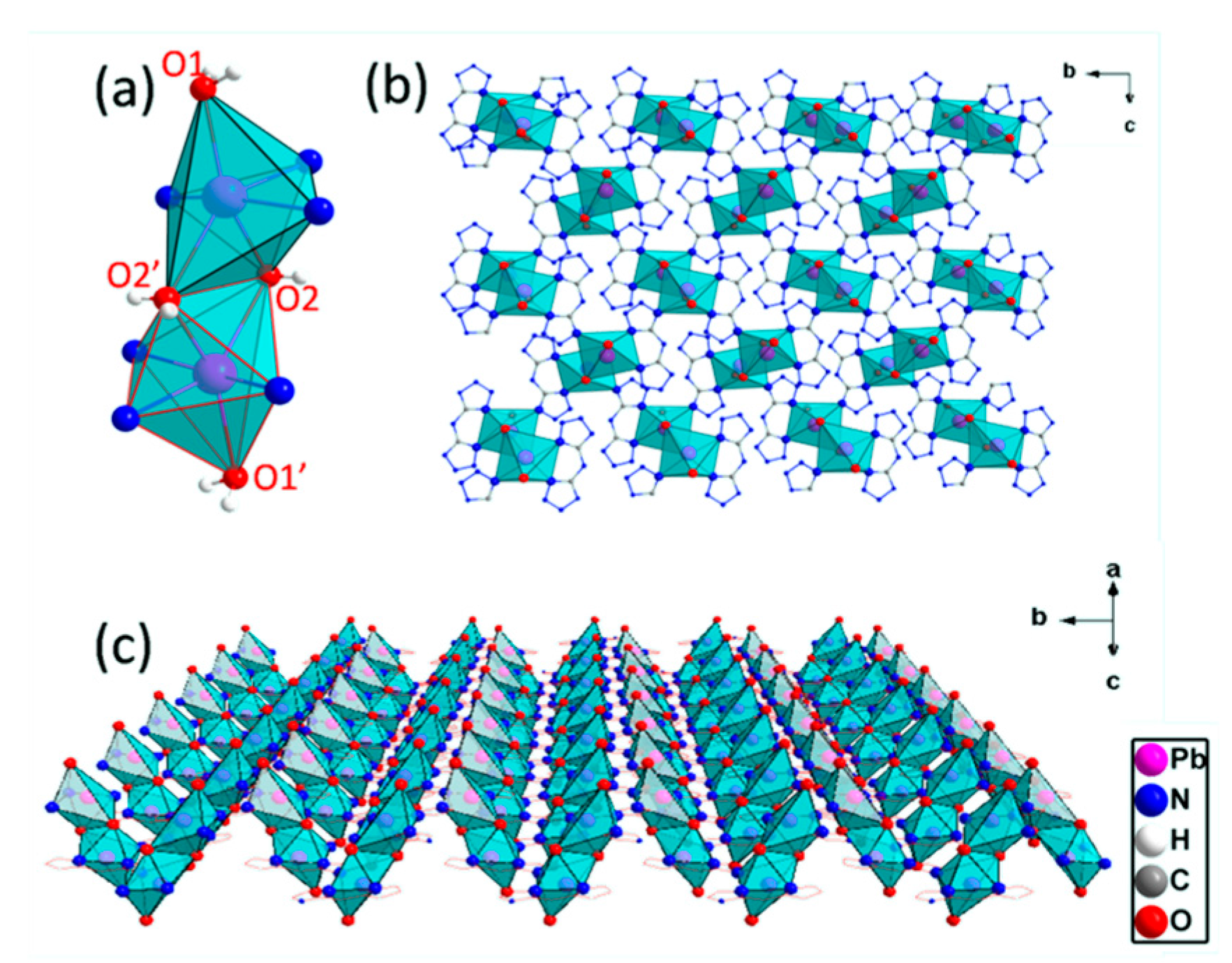
2.2. Crystal Structure of the Complex
2.3. Thermal Decomposition and Non-Isothermal Kinetics Analysis
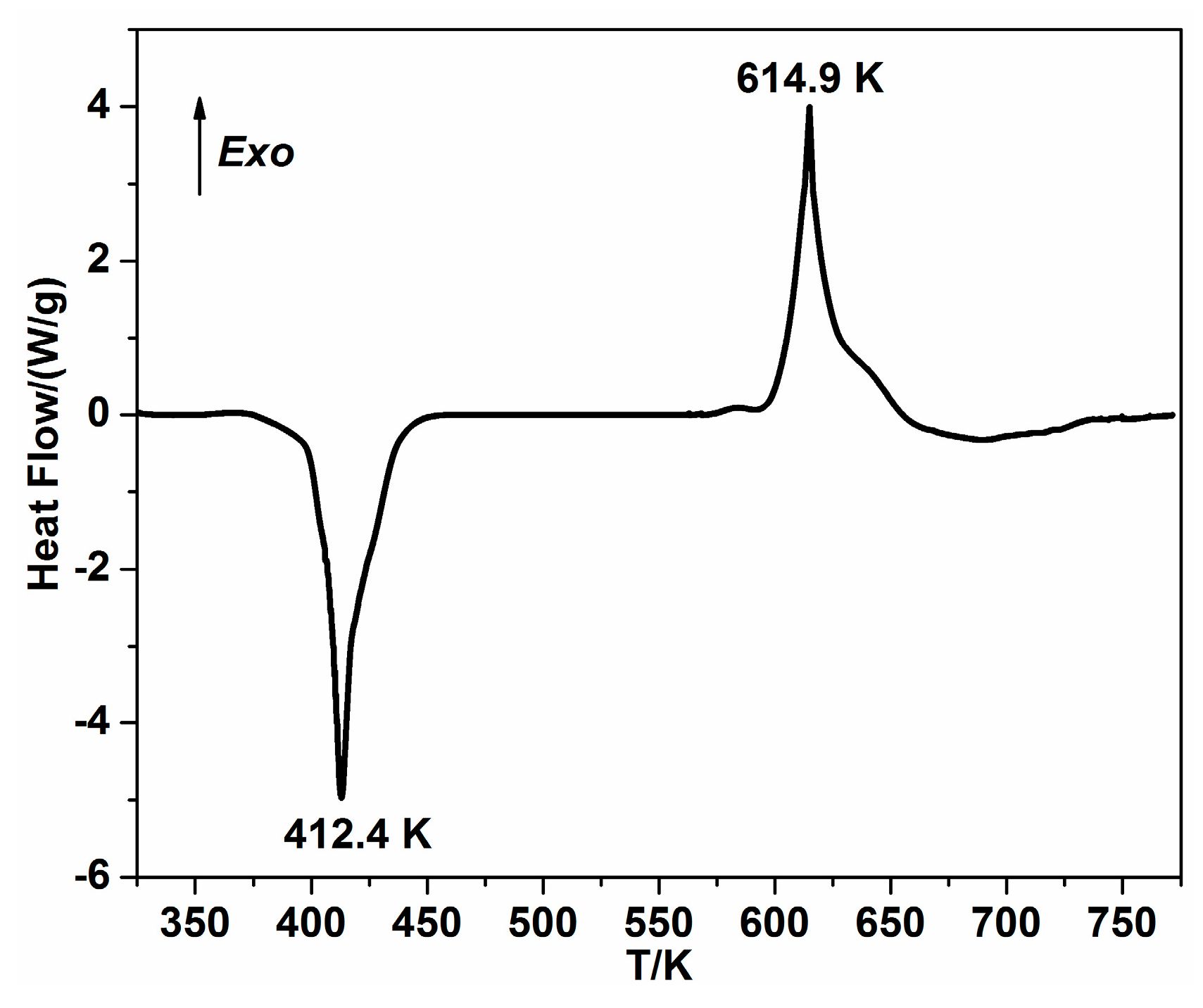
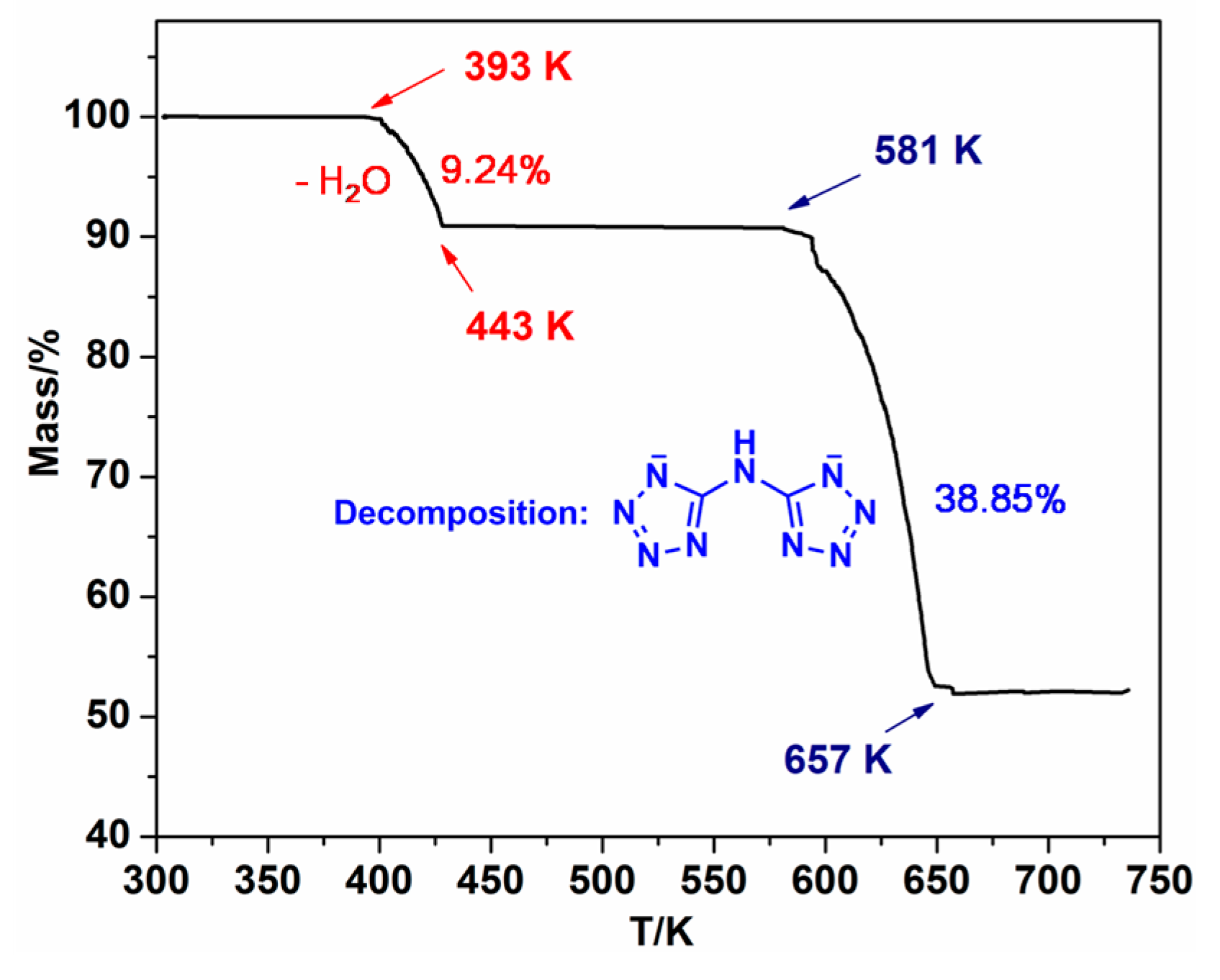
2.3.1. Thermal Decomposition
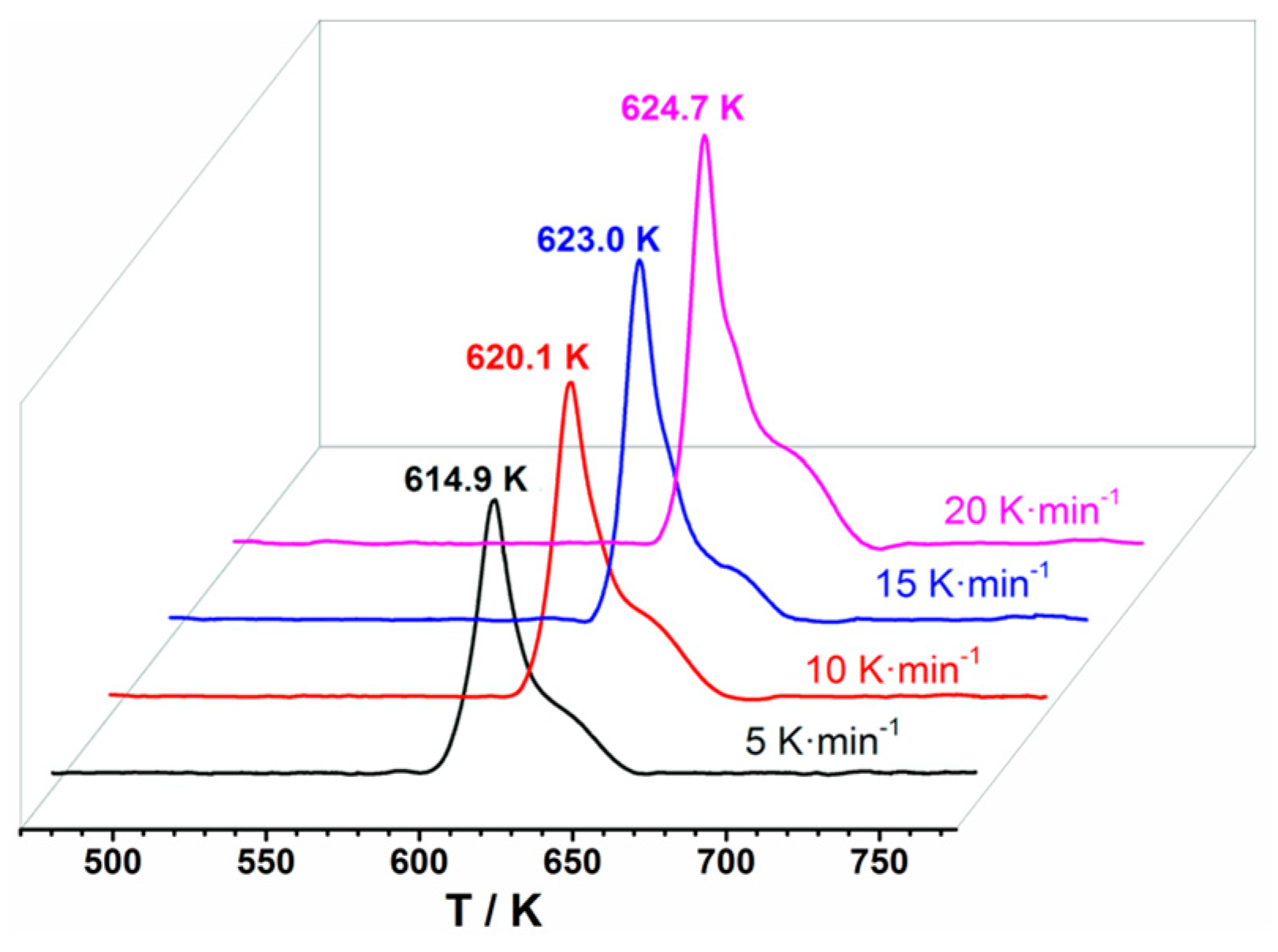
2.3.2. Non-Isothermal Kinetics Analysis
2.4. Energetic Properties
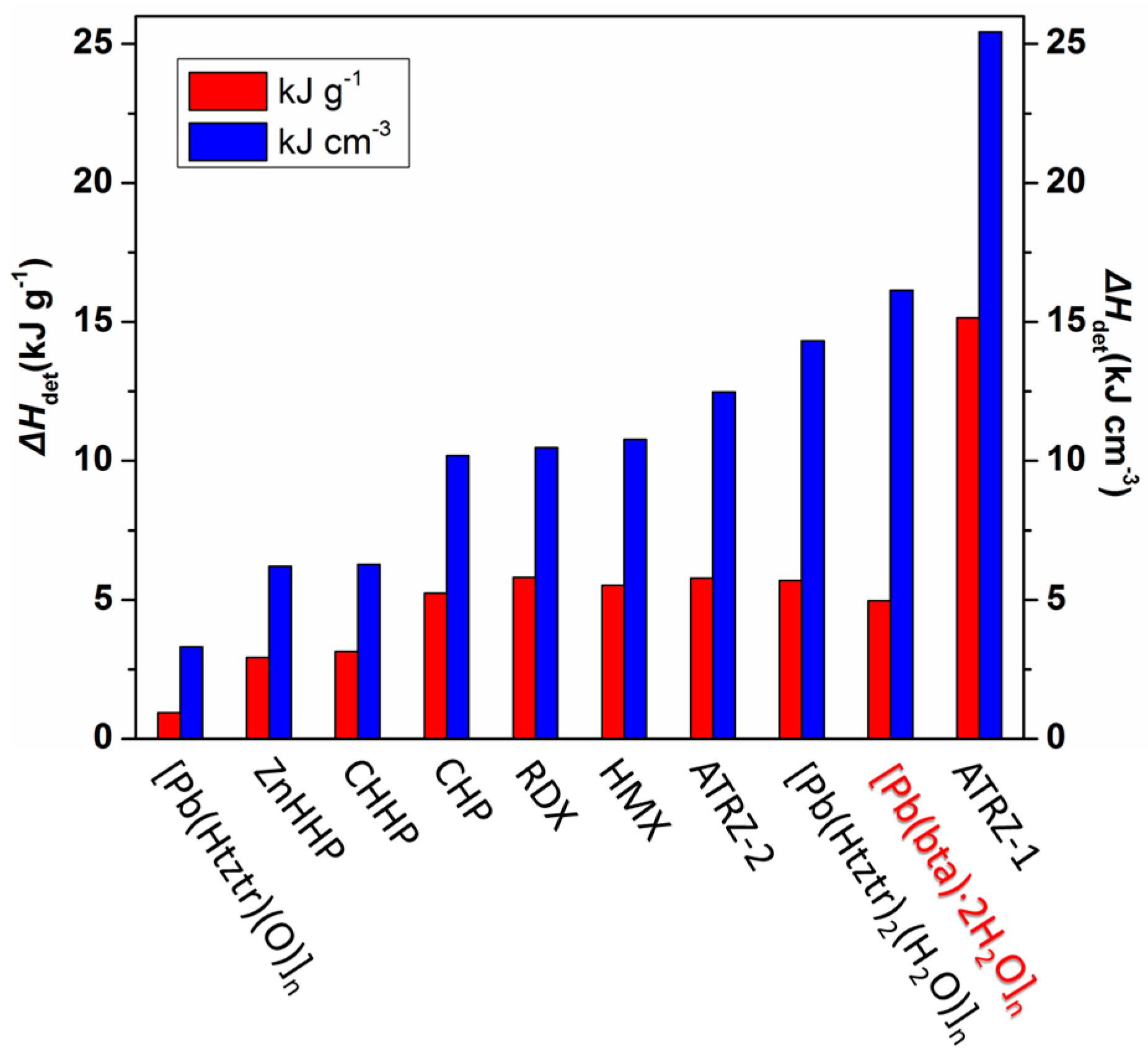
2.4.1. Heat Detonation of Complex
2.4.2. Detonation Properties and Sensitivity
3. Materials and Methods
3.1. Synthesis of [Pb(bta)·2H2O]n
3.2. Single-Crystal X-ray Diffraction Analyses
3.3. Equations for Calculating Non-Isothermal Kinetics
3.4. Calculation for Heat of Detonation
3.5. Calculation for Detonation Velocity and Detonation Pressure
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Eddaoudi, M.; Moler, D.B.; Li, H.; Chen, B.; Reineke, T.M.; O’keeffe, M.; Yaghi, O.M. Modular chemistry: Secondary building units as a basis for the design of highly porous and robust metal-organic carboxylate frameworks. Acc. Chem. Res. 2001, 34, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Phan, A.; Doonan, C.J.; Uribe-Romo, F.J.; Knobler, C.B.; O’keeffe, M.; Yaghi, O.M. Synthesis, structure, and carbon dioxide capture properties of zeoliticimidazolate frameworks. Acc. Chem. Res. 2010, 43, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Das, M.C.; Xiang, S.; Zhang, Z.; Chen, B. Funktionelle gemischtmetall-organische gerüste mit metalloliganden. Angew. Chem. Int. Ed. 2011, 123, 10696–10707. [Google Scholar] [CrossRef]
- Chang, B.K.; Bristowe, N.C.; Bristowe, P.D.; Cheetham, A.K. Van der waals forces in the perfluorinated metal-organic framework zinc 1,2-bis(4-pyridyl)ethane tetrafluoroterephthalate. Phys. Chem. Chem. Phys. 2012, 14, 7059–7064. [Google Scholar] [CrossRef] [PubMed]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal-organic framework materials as chemical sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Li, J.R.; Sculley, J.; Zhou, H.C. Metal-organic frameworks for separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.S.; Pilania, G.; Wang, C.; Ramprasad, R. How critical are the van der Waals interactions in polymer crystals? J. Phys. Chem. A 2012, 116, 9347–9352. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Zhu, C.; Liu, Y.; Cui, Y. Mesoporous metal-organic framework materials. Chem. Soc. Rev. 2012, 41, 1677–1695. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Rosi, N.L.; Eckert, J.; Eddaoudi, M.; Vodak, D.T.; Kim, J.; O’Keeffe, M.; Yaghi, O.M. Hydrogen storage in microporous metal-organic frameworks. Science 2003, 300, 1127–1129. [Google Scholar] [CrossRef] [PubMed]
- Belof, J.L.; Stern, A.C.; Eddaoudi, M.; Space, B. On the mechanism of hydrogen storage in a metal-organic framework material. J. Am. Chem. Soc. 2007, 129, 15202–15210. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.S.; Snurr, R.Q. Development and evaluation of porous materials for carbon dioxide separation and capture. Angew. Chem. Int. Ed. 2011, 50, 11586–11596. [Google Scholar] [CrossRef] [PubMed]
- Suh, M.P.; Park, H.J.; Prasad, T.K.; Lim, D.W. Hydrogen storage in metal-organic frameworks. Chem. Rev. 2012, 112, 782–835. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Liang, C.; Yang, J.; Contreras, D.S.; Clancy, Y.L.; Lobkovsky, E.B.; Yaghi, O.M.; Dai, S. A microporous metal-organic framework for gas-chromatographic separation of alkanes. Angew. Chem. Int. Ed. 2006, 45, 1390–1393. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.S.; Mulfort, K.L.; Frost, H.; Ryan, P.; Punnathanam, S.; Broadbelt, L.J.; Hupp, J.T.; Snurr, R.Q. Separation of CO2 from CH4 using mixed-ligand metal-organic frameworks. Langmuir 2008, 24, 8592–8598. [Google Scholar] [CrossRef] [PubMed]
- Bloch, E.D.; Queen, W.L.; Krishna, R.; Zadrozny, J.M.; Brown, C.M.; Long, J.R. Hydrocarbon separations in a metal-organic framework with open iron(II) coordination sites. Science 2012, 335, 1606–1610. [Google Scholar] [CrossRef] [PubMed]
- Bloch, W.M.; Babarao, R.; Hill, M.R.; Doonan, C.J.; Sumby, C.J. Post-synthetic structural processing in a metal-organic framework material as a mechanism for exceptional CO2/N2 selectivity. J. Am. Chem. Soc. 2013, 135, 10441–10448. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.; Srirambalaji, R.; Kim, K. Homochiral metal-organic frameworks for asymmetric heterogeneous catalysis. Chem. Rev. 2012, 112, 1196–1231. [Google Scholar] [CrossRef] [PubMed]
- Genna, D.T.; Wong-Foy, A.G.; Matzger, A.J.; Sanford, M.S. Heterogenization of homogeneous catalysts in metal-organic frameworks via cation exchange. J. Am. Chem. Soc. 2013, 135, 10586–10589. [Google Scholar] [CrossRef] [PubMed]
- Nepal, B.; Das, S. Sustained water oxidation by a catalyst cage-isolated in a metal-organic framework. Angew. Chem. Int. Ed. 2013, 52, 7224–7227. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.L.; Li, J.; Xu, Q. Immobilizing metal nanoparticles to metal–organic frameworks with size and location control for optimizing catalytic performance. J. Am. Chem. Soc. 2013, 135, 10210–10213. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Serre, C.; Maurin, G.; Ramsahye, N.A.; Balas, F.; Vallet-Regí, M.; Sebban, M.; Taulelle, F.; Férey, G. Flexible porous metal-organic frameworks for a controlled drug delivery. J. Am. Chem. Soc. 2008, 130, 6774–6780. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous metal-organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 2010, 9, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Della Rocca, J.; Liu, D.; Lin, W. Nanoscale metal-organic frameworks for biomedical imaging and drug delivery. Acc. Chem. Res. 2011, 40, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Bushuyev, O.S.; Brown, P.; Maiti, A.; Gee, R.H.; Peterson, G.R.; Weeks, B.L.; Hope-Weeks, L.J. Ionic polymers as a new structural motif for high-energy-density materials. J. Am. Chem. Soc. 2012, 134, 1422–1425. [Google Scholar] [CrossRef] [PubMed]
- Tao, G.H.; Parrish, D.A.; Shreeve, J.M. Nitrogen-rich 5-(1-methylhydrazinyl)tetrazole and its copper and silver complexes. Inorg. Chem. 2012, 51, 5305–5312. [Google Scholar] [CrossRef] [PubMed]
- Bushuyev, O.S.; Peterson, G.R.; Brown, P.; Maiti, A.; Gee, R.H.; Weeks, B.L.; Hope-Weeks, L.J. Metal-organic frameworks (MOFs) as safer, structurally reinforced energetics. Chem. Eur. J. 2013, 19, 1706–1711. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, Y.; Qi, C.; Zhao, X.; Zhang, J.; Zhang, S.; Pang, S. 3D energetic metal-organic frameworks: Synthesis and properties of high energy materials. Angew. Chem. Int. Ed. 2013, 52, 14031–14035. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Zheng, F.K.; Wu, M.F.; Liu, Z.F.; Chen, J.; Guo, G.C.; Wu, A.Q. Hydrothermal syntheses, crystal structures and physical properties of a new family of energetic coordination polymers with nitrogen-rich ligand N-[2-(1H-tetrazol-5-yl)ethyl]glycine. CrystEngComm 2013, 15, 2616–2623. [Google Scholar] [CrossRef]
- Wu, B.D.; Zhang, T.L.; Li, Y.L.; Tong, W.C.; Zhou, Z.N.; Zhang, J.G.; Yang, L. Energetic Compounds Based on 4-Amino-1,2,4-triazole (ATZ) and Picrate (PA):[Zn(H2O)6](PA)2·3H2O and [Zn(ATZ)3](PA)2·2.5H2O]n. Z. Anorg. Allg. Chem. 2013, 639, 2209–2215. [Google Scholar] [CrossRef]
- Wu, B.D.; Zhou, Z.N.; Li, F.G.; Yang, L.; Zhang, T.L.; Zhang, J.G. Preparation, crystal structures, thermal decompositions and explosive properties of two new high-nitrogen azide ethylenediamine energetic compounds. New J. Chem. 2013, 37, 646–653. [Google Scholar] [CrossRef]
- Gao, W.; Liu, X.; Su, Z.; Zhang, S.; Yang, Q.; Wei, Q.; Chen, S.; Xie, G.; Yang, X.; Gao, S. High-energy-density materials with remarkable thermostability and insensitivity: Syntheses, structures and physicochemical properties of Pb(II) compounds with 3-(tetrazol-5-yl) triazole. J. Mater. Chem. A 2014, 2, 11958–11965. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Q.; Su, Z.; Chen, S.; Xie, G.; Wei, Q.; Gao, S. 3D high-energy-density and low sensitivity materials: Synthesis, structure and physicochemical properties of an azide–Cu(II) complex with 3,5-dinitrobenzoic acid. RSC Adv. 2014, 4, 16087–16093. [Google Scholar] [CrossRef]
- Tong, W.C.; Yang, L.; Wu, B.D.; Zhang, T.L. Preparation, crystal structure, and thermal decomposition of a novel nitrogen-rich compound Zn3(ATZ)6(N3)6 (ATZ = 4-amino-1,2,4-triazole, N% = 61.7%). Z. Anorg. Allg. Chem. 2014, 640, 980–985. [Google Scholar] [CrossRef]
- Wu, B.D.; Bi, Y.G.; Li, F.G.; Yang, L.; Zhou, Z.N.; Zhang, J.G.; Zhang, T.L. A novel stable high-nitrogen energetic compound: Copper(II) 1,2-diaminopropane azide. Z. Anorg. Allg. Chem. 2014, 640, 224–228. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Yang, Q.; Su, Z.; Gao, W.; Wei, Q.; Xie, G.; Chen, S.; Gao, S. A new strategy for storage and transportation of sensitive high-energy materials: Guest-dependent energy and sensitivity of 3D metal-organic-framework-based energetic compounds. Chem. Eur. J. 2014, 20, 7906–7910. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Huang, S.; Zhang, Q.; Yu, Q.; Zhou, X.; Li, H.; Li, J. Two nitrogen-rich Ni(II) coordination compounds based on 5,5’-azotetrazole: Synthesis, characterization and effect on thermal decomposition for RDX, HMX and AP. RSC Adv. 2015, 5, 32872–32879. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, X.; Duan, L.; Yang, Q.; Wei, Q.; Xie, G.; Chen, S.; Yang, X.; Gao, S. In situ synthesized 3D heterometallic metal-organic framework (MOF) as a high-energy-density material shows high heat of detonation, good thermostability and insensitivity. Dalton Trans. 2015, 44, 2333–2339. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gao, W.; Sun, P.; Su, Z.; Chen, S.; Wei, Q.; Xie, G.; Gao, S. Environmentally friendly high-energy MOFs: Crystal structures, thermostability, insensitivity and remarkable detonation performances. Green Chem. 2015, 17, 831–836. [Google Scholar] [CrossRef]
- Liu, X.; Qu, X.; Zhang, S.; Ke, H.; Yang, Q.; Shi, Q.; Wei, Q.; Xie, G.; Chen, S. High-performance energetic characteristics and magnetic properties of a three-dimensional cobalt(II) metal-organic framework assembled with azido and triazole. Inorg. Chem. 2015, 54, 11520–11525. [Google Scholar] [CrossRef] [PubMed]
- McDonald, K.A.; Seth, S.; Matzger, A.J. Coordination polymers with high energy density: An emerging class of explosives. Cryst. Growth Des. 2015, 15, 5963–5972. [Google Scholar] [CrossRef]
- Qu, X.; Zhang, S.; Yang, Q.; Su, Z.; Wei, Q.; Xie, G.; Chen, S. Silver(I)-based energetic coordination polymers: Synthesis, structure and energy performance. New J. Chem. 2015, 39, 7849–7857. [Google Scholar] [CrossRef]
- Xu, C.X.; Zhang, J.G.; Yin, X.; Jin, X.; Li, T.; Zhang, T.L.; Zhou, Z.N. Cd(II) complexes with different nuclearity and dimensionality based on 3-hydrazino-4-amino-1,2,4-triazole. J. Solid State Chem. 2015, 226, 59–65. [Google Scholar] [CrossRef]
- Qu, X.N.; Zhang, S.; Wang, B.Z.; Yang, Q.; Han, J.; Wei, Q.; Xie, G.; Chen, S.P. An Ag(I) energetic metal-organic framework assembled with the energetic combination of furazan and tetrazole: Synthesis, structure and energetic performance. Dalton Trans. 2016, 45, 6968–6973. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Song, X.; Ge, J.; Zhao, G.; Zhang, W.; Xie, G.; Chen, S.; Gao, S. A 2D nickel-based energetic MOFs incorporating 3,5-diamino-1,2,4-triazole and malonic acid: Synthesis, crystal structure and thermochemical study. J. Chem. Thermodyn. 2016, 92, 132–138. [Google Scholar] [CrossRef]
- Yue, Q.Y.; Lu, Y.M.; Chuan, F.X.; Yuan, D.; Chen, D.Y.; Yang, G.W.; Li, Q.Y. Synthesis, crystal structure, luminescence and thermal behavior of a new energetic zinc(II) compound. Inorg. Chem. Commun. 2016, 68, 68–71. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, M.; Lin, P.; Malgras, V.; Tang, J.; Alshehri, S.M.; Yamauchi, Y.; Du, S.; Zhang, J. A highly energetic N-rich metal-organic framework as a new high-energy-density material. Chem. Eur. J. 2016, 22, 1141–1145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Du, Y.; Dong, K.; Su, H.; Zhang, S.; Li, S.; Pang, S. Taming dinitramide anions within an energetic metal–organic framework: A new strategy for synthesis and tunable properties of high energy materials. Chem. Mater. 2016, 28, 1472–1480. [Google Scholar] [CrossRef]
- Zhang, Q.; Shreeve, J.M. Metal-organic frameworks as high explosives: A new concept for energetic materials. Angew. Chem. Int. Ed. 2014, 53, 2540–2542. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, X.; Liu, B.; Xia, Z.; Wang, W.; Yang, Q.; Ke, H.; Wei, Q.; Xie, G.; Chen, S. 3D Co(II, III) mixed-valence metal-organic framework affording field-induced slow magnetic relaxation. Sci. China Chem. 2015, 58, 1032–1038. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Q.; Liu, X.; Qu, X.; Wei, Q.; Xie, G.; Chen, S.; Gao, S. High-energy metal–organic frameworks (HE-MOFs): Synthesis, structure and energetic performance. Coord. Chem. Rev. 2016, 307, 292–312. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Su, H.; Li, S.; Zhang, S.; Pang, S. A simple method for the prediction of the detonation performances of metal-containing explosives. J. Phys. Chem. A 2014, 118, 4575–4581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shreeve, J.M. 3D nitrogen-rich metal-organic frameworks: Opportunities for safer energetics. Dalton Trans. 2016, 45, 2363–2368. [Google Scholar] [CrossRef] [PubMed]
- Deblitz, R.; Hrib, C.G.; Blaurock, S.; Jones, P.G.; Plenikowski, G.; Edelmann, F.T. Explosive werner-type cobalt(III) complexes. Inorg. Chem. Front. 2014, 1, 621–640. [Google Scholar] [CrossRef]
- Evers, J.; Gospodinov, I.; Joas, M.; Klapotke, T.M.; Stierstorfer, J. Cocrystallization of photosensitive energetic copper(II) perchlorate complexes with the nitrogen-rich ligand 1,2-di(1H-tetrazol-5-yl)ethane. Inorg. Chem. 2014, 53, 11749–11756. [Google Scholar] [CrossRef] [PubMed]
- Blair, L.H.; Colakel, A.; Vrcelj, R.M.; Sinclair, I.; Coles, S.J. Metal-organic fireworks: MOFs as integrated structural scaffolds for pyrotechnic materials. Chem. Commun. 2015, 51, 12185–12188. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.; Gálvez-Ruiz, J.C.; Klapötke, T.M.; Mayer, P.; Weber, B.; Weigand, J.J. BTA copper complexes. Inorg. Chem. 2005, 44, 8044–8052. [Google Scholar] [CrossRef] [PubMed]
- Klapötke, T.M.; Mayer, P.; Stierstorfer, J.; Weigand, J.J. Bistetrazolylamines—Synthesis and characterization. J. Mater. Chem. 2008, 18, 5248. [Google Scholar] [CrossRef]
- Guo, Y.; Gao, H.; Twamley, B.; Shreeve, J.M. Energetic nitrogen rich salts of N,N-bis[1(2)H-tetrazol-5-yl]amine. Adv. Mater. 2007, 19, 2884–2888. [Google Scholar] [CrossRef]
- Guo, Y.; Tao, G.H.; Zeng, Z.; Gao, H.; Parrish, D.A.; Shreeve, J.M. Energetic salts based on monoanions of N,N-bis[1(2)H-tetrazol-5-yl]amine. Chem. Eur. J. 2010, 16, 3753–3762. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.L.; He, L.; Liu, H.Y.; Tao, G.H.; Nie, F.D.; Huang, M.; Hu, C.W. Nitrogen-rich energetic ionic liquids based on the N,N-bis[1H-tetrazol-5-yl]amine anion—Syntheses, structures, and properties. Eur. J. Inorg. Chem. 2013, 5009–5019. [Google Scholar] [CrossRef]
- Xue, B.D.; Yang, Q.; Chen, S.P.; Gao, S.L. Synthesis, crystal structure, and thermodynamics of a high-nitrogen copper complex with N,N-bis[1(2)H-tetrazol-5-yl] amine. J. Therm. Anal. Calorim. 2010, 101, 997–1002. [Google Scholar] [CrossRef]
- Li, N.; Chen, W.B.; Guan, Y.F.; OuYang, Z.J.; Dong, W. Chlorine anion–π and π−–π− interactions in two tetrazolyl derivative based Cu2+ complexes and quantum chemical calculations. Inorg. Chim. Acta 2014, 409, 349–352. [Google Scholar] [CrossRef]
- Qu, B.; Lu, X.; Wu, Y.; You, X.; Xu, X. Synthesis of copper micro-rods with layered nano-structure by thermal decomposition of the coordination complex Cu(BTA)2. Nanoscale Res. Lett. 2015, 10, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.; Habib, F.; Aharen, T.; Clerac, R.; Hu, A.; Murugesu, M. High-temperature spin crossover behavior in a nitrogen-rich Fe(III)-based system. Inorg. Chem. 2013, 52, 1825–1831. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Yue, Q.; Wang, Y.Q.; Cheng, A.L.; Gao, E.Q. Coordination compounds of bis(5-tetrazolyl)amine with manganese(II), zinc(II) and cadmium(II): Synthesis, structure and magnetic properties. Dalton Trans. 2008, 34, 4621–4629. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.B.; Wang, M.S.; Zhou, W.W.; Xu, G.; Guo, G.C.; Huang, J.S. Novel 3-D PtS-like tetrazolate-bridged manganese(II) complex exhibiting spin-canted antiferromagnetism and field-induced spin-flop transition. Inorg. Chem. 2008, 47, 8935–8942. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Zhang, X.M. Temperature tuned synthesis of zinc N,N-bis[1(2)H-tetrazol-5-yl]-amine complexes: From Zn3O cluster-based 3-connected 63-hcb and (3,6)-connected (43)2(46)-kgd layers to Zn5(OH)4 chain-based 3D open framework. Cryst. Growth Des. 2008, 8, 3077–3083. [Google Scholar] [CrossRef]
- Li, F.; Bi, Y.; Zhao, W.; Zhang, T.; Zhou, Z.; Yang, L. Nitrogen-rich salts based on the energetic [monoaquabis N,N-bis[1(2)H-tetrazol-5-yl] amine)-zinc(II)] anion: A promising design in the development of new energetic materials. Inorg. Chem. 2015, 54, 2050–2057. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.M.; Guan, Y.F.; Wang, D.Y.; Dong, W.; Wang, X.T.; Gao, S. Syntheses, structures and properties of seven isomorphous 1D Ln3+ complexes Ln(BTA)(HCOO)(H2O)3 (H2BTA = bis(tetrazoly)amine, Ln = Pr, Gd, Eu, Tb, Dy, Er, Yb) and two 3D Ln3+ complexes Ln(HCOO)3 (Ln = Pr, Nd). Dalton Trans. 2008, 44, 6165–6169. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, S.; Gao, S. Syntheses and characterization of lead(II) N,N-bis[1(2)H-tetrazol-5-yl] amine compounds and effects on thermal decomposition of ammonium perchlorate. Eur. J. Inorg. Chem. 2009, 2009, 3475–3480. [Google Scholar] [CrossRef]
- Wu, J.T.; Zhang, J.G.; Yin, X.; He, P.; Zhang, T.L. Synthesis and characterization of the nitrophenol energetic ionic salts of 5,6,7,8-tetrahydrotetrazolo[1,5-b][1,2,4]triazine. Eur. J. Inorg. Chem. 2014, 2014, 4690–4695. [Google Scholar] [CrossRef]
- Liu, Q.; Jin, B.; Peng, R.; Guo, Z.; Zhao, J.; Zhang, Q.; Shang, Y. Synthesis, characterization and properties of nitrogen-rich compounds based on cyanuric acid: A promising design in the development of new energetic materials. J. Mater. Chem. A 2016, 4, 4971–4981. [Google Scholar] [CrossRef]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Tables of bond lengths determined by X-ray and neutron diffraction. Part I. Bond lengths in organic compounds. J. Chem. Soc. Perkin Trans. 2 1987, S1–S19. [Google Scholar] [CrossRef]
- Li, F.; Zhao, W.; Chen, S.; Zhang, T.; Zhou, Z.; Yang, L. Nitrogen-rich alkali metal salts (Na and K) of [bis(N,N-bis[1H-tetrazol-5-yl] amine)-zinc(II)] anion: Syntheses, crystal structures, and energetic properties. Z. Anorg. Allg. Chem. 2015, 641, 911–916. [Google Scholar] [CrossRef]
- Cacho-Bailo, F.; Téllez, C.; Coronas, J. Interactive Thermal Effects on Metal-Organic Framework Polymer Composite Membranes. Chem. Eur. J. 2016, 22, 9533–9536. [Google Scholar] [CrossRef] [PubMed]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Doyle, C.D. Kinetic analysis of thermogravimetric data. J. Appl. Polym. Sci. 1961, 5, 285–292. [Google Scholar] [CrossRef]
- Ozawa, T. A new method of analyzing thermogravimetric data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Geetha, M.; Nair, U.R.; Sarwade, D.B.; Gore, G.M.; Asthana, S.N.; Singh, H.J. Studies on CL-20: The most powerful high energy material. J. Therm. Anal. Calorim. 2003, 73, 913–922. [Google Scholar] [CrossRef]
- Turcotte, R.; Vachon, M.; Kwok, Q.S.; Wang, R.; Jones, D.E. Thermal study of HNIW (CL-20). Thermochim. Acta 2005, 433, 105–115. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXS-97, Program for Crystal Structure Solution; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXS-97, Program for Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Delley, B. An all-electron numerical method for solving the local density functional for polyatomicmolecules. J. Chem. Phys. 1990, 92, 508–517. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]

| Empirical Formula | C2H5N9O2Pb |
|---|---|
| Formula weight | 394.34 |
| Crystal Color | colorless |
| Crystal size (mm3) | 0.21 × 0.20 × 0.19 mm |
| Crystal system | Monoclinic |
| Space group | P21/n |
| a (Å) | 6.592(5) |
| b (Å) | 11.987(9) |
| c (Å) | 10.552(8) |
| α (°) | 90 |
| β (°) | 104.856(12) |
| γ (°) | 90 |
| V (Å3) | 806.0(10) |
| Z | 4 |
| ρcalcd (g·cm−3) | 3.250 |
| T (K) | 150(2) |
| F (000) | 712 |
| θ (°) | 2.62 to 25.00 |
| R int. | 0.0425 |
| Data | 1423 |
| Restraints | 4 |
| parameters | 127 |
| GOF a on F2 | 1.051 |
| R1 b (I > 2σ (I)) | 0.0241 |
| ωR2 c (I > 2σ (I)) | 0.0655 |
| R1 (all data) | 0.0261 |
| β/(K·min−1) | Tp/(K) | Kissinger | Ozawa-Doyle | |||
|---|---|---|---|---|---|---|
| E/(kJ·mol−1) | ln A | r | E/(kJ·mol−1) | r | ||
| 5 | 614.9 | 436.1 | 84.93 | 0.9984 | 424.6 | 0.9984 |
| 10 | 620.1 | |||||
| 15 | 623.0 | |||||
| 20 | 624.7 | |||||
| Td a | p b | N c | ΔHdet d | D e | P f | IS g | |
|---|---|---|---|---|---|---|---|
| [Pb(bta)·2H2O]n | 342 | 3.250 | 31.98 | 4.97 | 8.963 | 47.47 | >40.0 |
| CHP h [25] | 194 | 1.948 | 14.71 | 5.23 | 8.225 | 31.73 | 0.5 |
| CHHP h [27] | 231 | 2.000 | 28.25 | 3.14 | 6.205 | 17.96 | 0.8 |
| ZnHHP h [27] | 293 | 2.117 | 23.61 | 2.93 | 7.016 | 23.58 | 2.5 |
| ATRZ-1 h [29] | 243 | 1.680 | 53.35 | 15.14 | 9.160 | 35.68 | 22.5 |
| ATRZ-2 h [29] | 257 | 2.160 | 43.76 | 5.78 | 7.773 | 29.70 | 30.0 |
| [Pb(Htztr)2 h (H2O)]n [32] | 340 | 2.519 | 39.40 | 5.69 | 7.715 | 31.57 | >40.0 |
| [Pb(Htztr)(O)]n h [32] | 318 | 3.511 | 27.20 | 0.94 | 8.122 | 40.12 | >40.0 |
| HMX [25] | 287 | 1.950 | 37.84 | 5.52 | 8.900 | 38.39 | 7.4 |
| RDX [25] | 210 | 1.806 | 37.80 | 5.80 | 8.600 | 33.92 | 7.4 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Jin, B.; Zhang, Q.; Shang, Y.; Guo, Z.; Tan, B.; Peng, R. Nitrogen-Rich Energetic Metal-Organic Framework: Synthesis, Structure, Properties, and Thermal Behaviors of Pb(II) Complex Based on N,N-Bis(1H-tetrazole-5-yl)-Amine. Materials 2016, 9, 681. https://doi.org/10.3390/ma9080681
Liu Q, Jin B, Zhang Q, Shang Y, Guo Z, Tan B, Peng R. Nitrogen-Rich Energetic Metal-Organic Framework: Synthesis, Structure, Properties, and Thermal Behaviors of Pb(II) Complex Based on N,N-Bis(1H-tetrazole-5-yl)-Amine. Materials. 2016; 9(8):681. https://doi.org/10.3390/ma9080681
Chicago/Turabian StyleLiu, Qiangqiang, Bo Jin, Qingchun Zhang, Yu Shang, Zhicheng Guo, Bisheng Tan, and Rufang Peng. 2016. "Nitrogen-Rich Energetic Metal-Organic Framework: Synthesis, Structure, Properties, and Thermal Behaviors of Pb(II) Complex Based on N,N-Bis(1H-tetrazole-5-yl)-Amine" Materials 9, no. 8: 681. https://doi.org/10.3390/ma9080681
APA StyleLiu, Q., Jin, B., Zhang, Q., Shang, Y., Guo, Z., Tan, B., & Peng, R. (2016). Nitrogen-Rich Energetic Metal-Organic Framework: Synthesis, Structure, Properties, and Thermal Behaviors of Pb(II) Complex Based on N,N-Bis(1H-tetrazole-5-yl)-Amine. Materials, 9(8), 681. https://doi.org/10.3390/ma9080681





