Chemical and Structural Characterization of Several Mid-Term Explanted Breast Prostheses
Abstract
:1. Introduction
2. Results
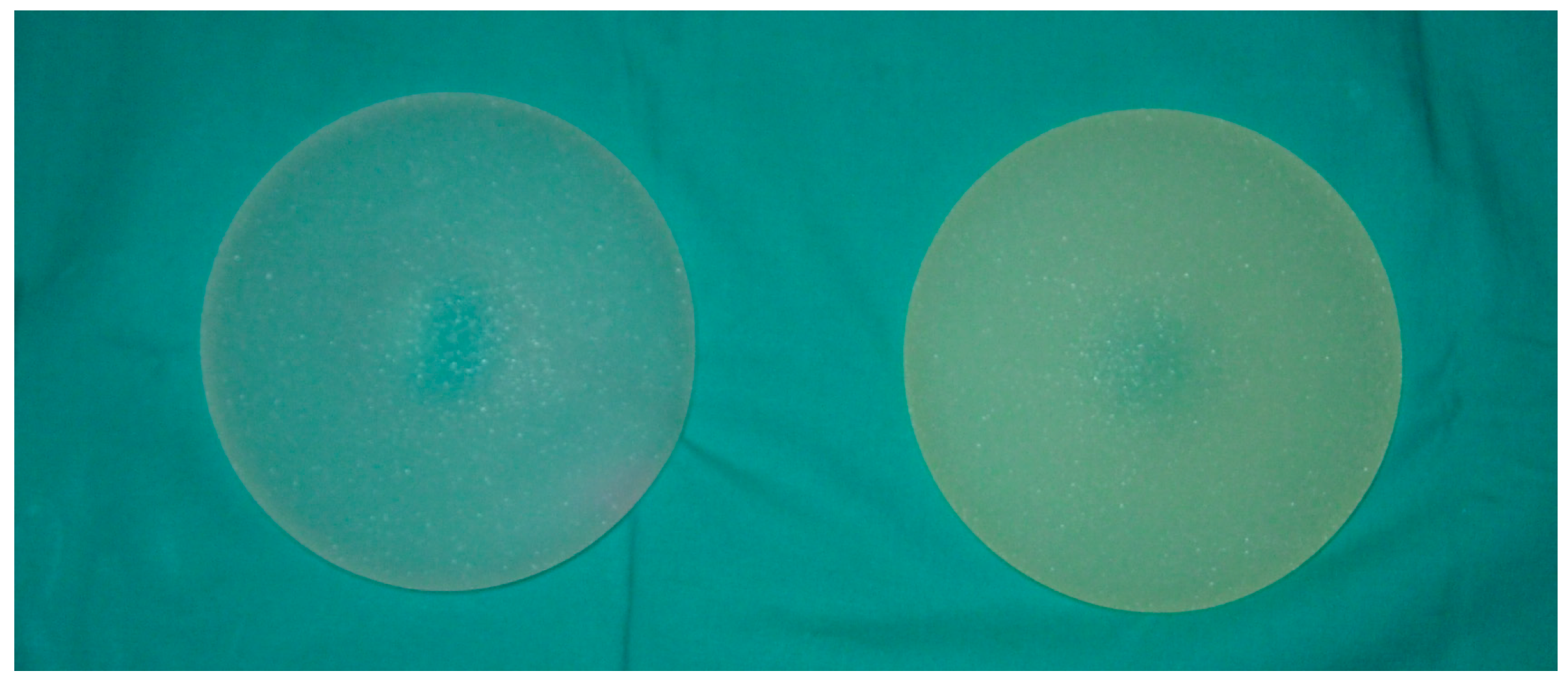
2.1. Naked Eye and Photographic Images
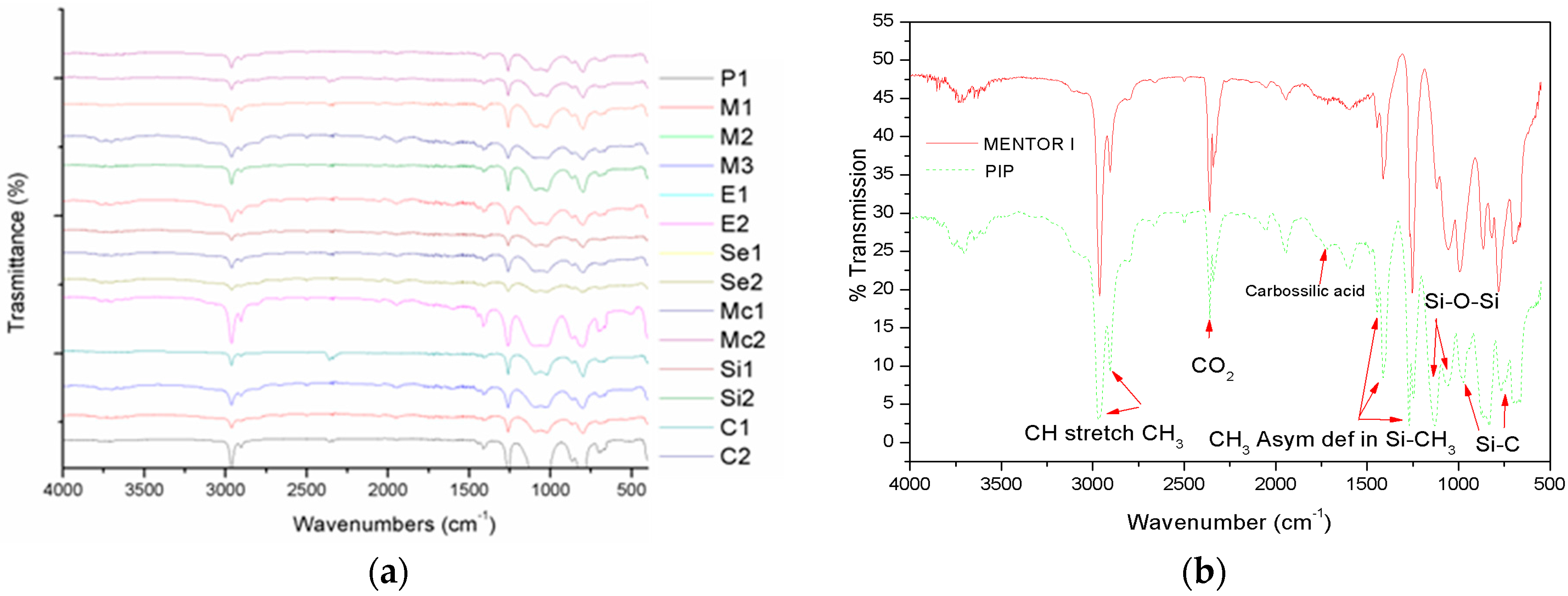
2.2. FT-IR Characterization
2.3. ICP-MS Analysis
2.4. GC-MS Analysis
2.5. Proteomic Analysis
2.6. Mechanical Characterization
3. Discussion
4. Materials and Methods
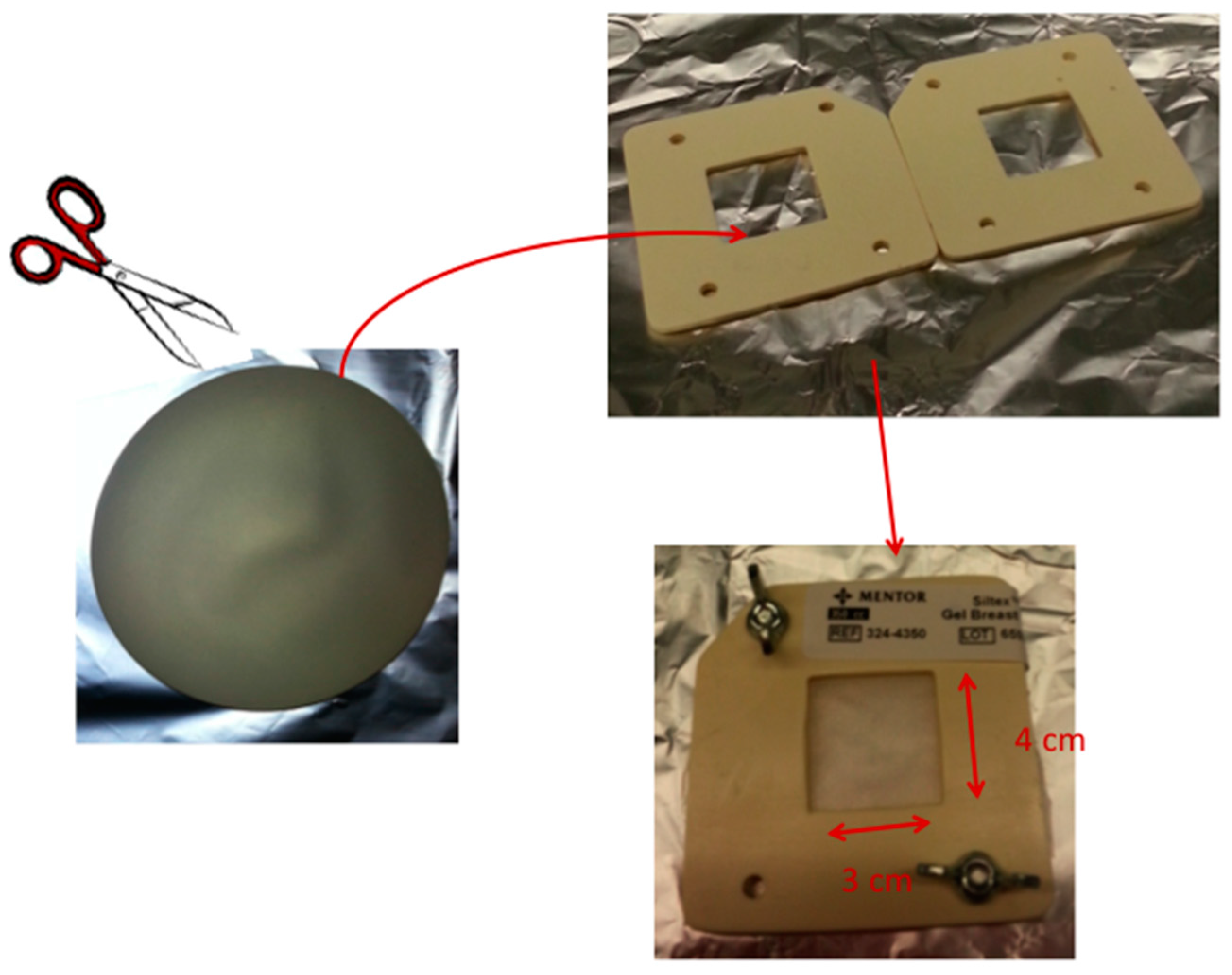
4.1. Breast Implant Samples
4.2. FT-IR Characterization
4.3. ICP-MS Analysis
4.4. GC-MS Analysis
4.5. Proteomic Analyses
4.6. Mechanical Characterization
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Swarts, E.; Kop, A.M.; Nilasaroya, A.; Keogh, C.V.; Cooper, T. Rupture of Poly Implant Prothese SiliconeBreast Implants: An Implant Retrieval Study. Plast. Reconstr. Surg. 2013, 4, 480e–489e. [Google Scholar] [CrossRef]
- Reyal, F.; Feron, J.-G. The Impact of Poly Implant Prothese Fraud on Breast Cancer Patients: A Report by the Institut Curie. Plast. Reconstr. Surg. 2013, 4, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Birkefeld, A.B.; Eckert, H.; Pfleiderer, B. A study of the aging of silicone breast implants using 29Si, 1H relaxtion and DSC measurements. Biomaterials 2004, 25, 4405–4413. [Google Scholar] [CrossRef] [PubMed]
- Flassbeck, D.; Pfleiderer, B.; Klemens, P.; Heumann, K.G.; Eltze, E.; Hirner, A.V. Determination of siloxanes, silicon, and platinum in tissues of women with silicone gel-filled implants. Anal. Bioanal. Chem. 2003, 375, 356–362. [Google Scholar] [PubMed]
- Adams, W.P.J.; Robinson, J.B.J.; Rohrich, R.J. Lipid infiltration as a possible biologic cause of silicone gel breast implant aging. Plast. Reconstr. Surg. 1998, 101, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Monstrey, S.; Christophe, A.; Delanghe, J.; De Vriese, S.; Hamdi, M.; Van Landuyt, K.; Blondeel, P. What exactly was wrong with the Trilucent breast implants? A unifying hypothesis. Plast. Reconstr. Surg. 2004, 113, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Adams, W.P.; Kenkel, J.M.; Rohrich, R.D.; Beran, S.; Conner, W.C. A prospective analysis of patients undergoing silicone breast implant explantation. Plast. Reconstr. Surg. 2000, 105, 2538–2543. [Google Scholar]
- Birkefeld, A.B.; Bertermann, R.; Eckert, H.; Pfleiderer, B. Liquid- and solid-state high-resolution NMR methods for the investigation of aging processes of silicone breast implants. Biomaterials 2003, 24, 35–46. [Google Scholar] [CrossRef]
- Garrido, L.; Young, V.L. Analysis of periprosthetic capsular tissue from women with silicone breast implants by magic-angle spinning NMR. Magn. Reson. Med. 1999, 42, 436–441. [Google Scholar] [PubMed]
- Philips, J.W.; de Camera, D.L.; Lockwood, M.D.; Grebner, W.C.C. Strength of silicone breast implants. Plast. Reconstr. Surg. 1996, 97, 1215–1225. [Google Scholar] [CrossRef]
- Picard, F.; Alikacem, N.; Guidoin, R.; Auger, M. Multinuclear solid-state NMR spectroscopy of envelopes from virgin and explanted silicone breast prostheses: An exploratory study. Magn. Reson. Med. 1997, 37, 11–17. [Google Scholar] [CrossRef] [PubMed]
- SCENIHR (Scientific Committee on Emerging and Newly Identified Health Risks). Scientific Opinion on the Safety of Poly Implant Prothèse (PIP) Silicone Breast Implants. Available online: http://ec.europa.eu/health/scientific_committees/emerging/docs/scenihr_o_043.pdf (accessed on 12 May 2014).
- Aladily, T.N.; Medeiros, L.; Jeffrey, A.; Mitual, B. Anaplastic Large Cell Lymphoma Associated with Breast Implants: A Report of 13 Cases. Am. J. Surg. Pathol. 2012, 36, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Miranda, R.N. Breast Implant–Associated Anaplastic Large-Cell Lymphoma: Long-Term Follow-Up of 60 Patients. J. Clin. Oncol. 2014, 32, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Brody, G.S.; Deapen, D.; Taylor, C.R. Anaplastic Large Cell Lymphoma Occurring in Women with Breast Implants: Analysis of 173 Cases. Plast. Reconstr. Surg. 2015, 135, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Kadin, M.E. Biomarkers Provide Clues to Early Events in the Pathogenesis of Breast Implant-Associated Anaplastic Large Cell Lymphoma. Aesthet. Surg. J. 2016, 36, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Kidder, L.H.; Kalasinsky, V.F.; Luke, J.L.; Levin, I.W.; Lewis, E.N. Visualization of silicone gel in human breast tissue using new infrared imaging spectroscopy. Nat. Med. 1997, 3, 235–237. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.C.; Whitesides, G.M. Poly(dimethylsiloxane) as a Material for Fabricating Microfluidic Devices. Acc. Chem. Res. 2002, 35, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Leo, G.; Cartechini, L.; Pucci, P.; Sgamellotti, A.; Marino, G.; Birolo, L. Proteomic strategies for the identification of proteinaceous binders in paintings. Anal. Bioanal. Chem. 2009, 395, 2269–2280. [Google Scholar] [CrossRef] [PubMed]
| Breast Implant | Li (μg/L) | Mn (μg/L) | Fe (μg/L) | Co (μg/L) | Mo (μg/L) | Sb (μg/L) | Pt (μg/L) |
|---|---|---|---|---|---|---|---|
| Mentor 1 | <1.0 | 2.9 ± 0.3 | 15 ± 5 | 0.67 ± 0.06 | 0.6 ± 0.3 | 1.70 ± 0.1 | 9 ± 4 |
| Mentor 2 | <1.0 | 2.1 ± 0.8 | <0.716 | 0.55 ± 0.10 | 0.53 ± 0.04 | 1.55 ± 0.03 | 55 ± 7 |
| Mentor 3 | 3 ± 1 | 2.1 ± 0.7 | <0.716 | 0.63 ± 0.02 | 0.56 ± 0.02 | 1.62 ± 0.15 | 23 ± 10 |
| Mentor 4 | <1.0 | 2.7 ± 0.7 | 4 ± 2 | 0.66 ± 0.06 | 0.57 ± 0.14 | 1.88 ± 0.13 | 4.7 ± 0.8 |
| Pip 1 | 5 ± 3 | 2.6 ± 0.6 | 11 ± 7 | 0.64 ± 0.07 | 0.54 ± 0.09 | 1.78 ± 0.16 | 0.8 ± 0.3 |
| Pip 2 | 6.6 ± 0.5 | 1.9 ± 0.3 | <0.716 | 0.55 ± 0.17 | 0.64 ± 0.12 | 1.48 ± 0.04 | 1.2 ± 0.2 |
| Mcghan 1 | 6 ± 3 | 1.6 ± 0.2 | <0.716 | 0.51 ± 0.15 | 0.30 ± 0.02 | 1.52 ± 0.16 | 50 ± 9 |
| Mcghan 2 | 5 ± 3 | 2.9 ± 0.3 | 8 ± 4 | 0.67 ± 0.01 | 0.55 ± 0.16 | 1.9 ± 0.3 | 22 ± 8 |
| Eurosilicon 1 | 9.8 ± 0.1 | 2.1 ± 0.4 | <0.716 | 0.53 ± 0.11 | 0.59 ± 0.16 | 1.5 ± 0.2 | 32 ± 5 |
| Eurosilicon 2 | 5.8 ± 0.1 | 2.6 ± 0.4 | <0.716 | 0.83 ± 0.18 | 0.51 ± 0.10 | 1.6 ± 0.2 | 21 ± 1 |
| Silimed 1 | 6 ± 3 | 1.7 ± 0.5 | <0.716 | 0.68 ± 0.08 | 0.55 ± 0.05 | 1.54 ± 0.19 | 1.4 ± 0.2 |
| Silimed 2 | 7.1 ± 0.3 | 2.7 ± 0.5 | <0.716 | 0.75 ± 0.04 | 0.50 ± 0.02 | 1.61 ± 0.11 | 2.4 ± 0.4 |
| Sebin 1 | 14 ± 9 | 2.7 ± 0.8 | <0.716 | 0.54 ± 0.03 | 0.5 ± 0.2 | 1.9 ± 0.2 | <0.406 |
| Sebin 2 | 8.7 ± 0.1 | 3.1 ± 0.3 | <0.716 | 0.44 ± 0.10 | 0.69 ± 0.13 | 1.5 ± 0.2 | 27 ± 9 |
| Cox 1 | 2.3 ± 0.1 | 2.4 ± 0.7 | <0.716 | 0.62 ± 0.06 | 0.6 ± 0.2 | 1.63 ± 0.12 | 1.3 ± 0.2 |
| Cox 2 | <1.0 | 1.6 ± 0.2 | <0.716 | 0.61 ± 0.09 | 0.43 ± 0.11 | 1.2 ± 0.2 | 3.3 ± 0.2 |
| AVG | 6.6 | 2.3 | 9.5 | 0.62 | 0.54 | 1.6 | 17 |
| Breast Implant | B (μg/L) | Si (μg/L) | P (μg/L) | Cr (μg/L) | Fe (μg/L) | Ni (μg/L) | Zn (μg/L) | Ge (μg/L) |
|---|---|---|---|---|---|---|---|---|
| Mentor 1 | 41 ± 10 | 441 ± 23 | 31 ± 2 | 97 ± 3 | 15 ± 5 | 45 ± 1 | 46 ± 2 | 11 ± 6 |
| Mentor 2 | 0.95 ± 0.50 | 397 ± 13 | 27 ± 5 | 85 ± 3 | <0.716 | 38.7 ± 0.7 | 47 ± 2 | 6.0 ± 0.9 |
| Mentor 3 | <0.5 | 285 ± 8 | 31 ± 1 | 86.0 ± 0.9 | <0.716 | 37.6 ± 1.7 | 45.7 ± 0.9 | <0.652 |
| Mentor 4 | <0.5 | 238 ± 8 | 27 ± 4 | 93 ± 2 | 4 ± 2 | 43.4 ± 1.4 | 46.1 ± 0.8 | <0.652 |
| Pip 1 | 19 ± 9 | 539 ± 10 | 31 ± 7 | 96 ± 3 | 11 ± 7 | 42.8 ± 1.5 | 48 ± 2 | 18 ± 6 |
| Pip 2 | 23 ± 6 | 529 ± 8 | 25 ± 4 | 85 ± 5 | <0.716 | 38 ± 2 | 48.5 ± 0.7 | 28 ± 5 |
| Mcghan 1 | <0.5 | 319 ± 7 | 27 ± 4 | 80 ± 2 | <0.716 | 37.2 ± 1.0 | 45 ± 2 | <0.652 |
| Mcghan 2 | <0.5 | 294 ± 2 | 35 ± 2 | 94 ± 2 | 8 ± 4 | 40.4 ± 1.7 | 46.4 ± 1.2 | <0.652 |
| Eurosilicon 1 | <0.5 | 280 ± 3 | 24 ± 3 | 82 ± 4 | <0.716 | 37.1 ± 1.7 | 45 ± 2 | <0.652 |
| Eurosilicon 2 | <0.5 | 298 ± 3 | 21 ± 3 | 88 ± 4 | <0.716 | 43.1 ± 1.1 | 49 ± 2 | <0.652 |
| Silimed 1 | <0.5 | 208 ± 4 | 28 ± 5 | 85 ± 1 | <0.716 | 36.9 ± 1.1 | 43.6 ± 1.3 | <0.652 |
| Silimed 2 | <0.5 | 267 ± 5 | 19 ± 3 | 91 ± 2 | <0.716 | 35.1 ± 0.7 | 41 ± 1 | <0.652 |
| Sebin 1 | <0.5 | 346 ± 4 | 24 ± 4 | 89 ± 3 | <0.716 | 40 ± 2 | 47 ± 2 | <0.652 |
| Sebin 2 | <0.5 | 406 ± 2 | 32 ± 1 | 82 ± 4 | <0.716 | 44 ± 1 | 49 ± 3 | <0.652 |
| Cox 1 | <0.5 | 209 ± 1 | 31 ± 4 | 90.0 ± 0.4 | <0.716 | 39 ± 2 | 44.5 ± 0.2 | <0.652 |
| Cox 2 | <0.5 | 216 ± 5 | 25 ± 4 | 74 ± 2 | <0.716 | 32.1 ± 0.8 | 42.9 ± 1.6 | <0.652 |
| AVG | 21 | 329 | 27 | 87 | 9 | 39 | 46 | 16 |
| Organic Substances | M 1 | M 2 | Mc 1 | Mc 2 | E 1 | C 1 | P 2 | S 1 |
|---|---|---|---|---|---|---|---|---|
| 1-Chlorodecane (%) | – | – | 0.1 | 0.4 | 1.1 | – | – | – |
| 1-Chlorotetradecane (%) | – | – | – | – | 0.2 | – | – | |
| Octadecenoic Acid (%) | – | – | – | – | – | – | 0.01 | 0.04 |
| Palmitic acid (%) | 0.5 | – | – | – | – | – | – | – |
| Oleic acid (%) | – | 0.1 | – | – | – | – | 0.1 | – |
| Stearic acid (%) | – | – | – | – | – | 0.3 | 1.7 | 0.1 |
| Cyclic Siloxanes | M1 | M2 | M3 | M4 | P1 | P2 | Mc1 | Mc2 | E1 | E2 | Si1 | Si2 | Se1 | Se2 | C1 | C2 | AVG |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C6 | 20.2 | 50.8 | 50.6 | – | – | 53.5 | 47.5 | 11.8 | 15.2 | 14.5 | – | – | – | – | – | – | 33 |
| C8 | 24.3 | 23.8 | 22.1 | – | – | 24.1 | 20.9 | 26.8 | 25.1 | 21.6 | – | – | 18.8 | 21.8 | – | – | 23 |
| C10 | 9.6 | 6.9 | 6.4 | – | 12.3 | 7.3 | 6.1 | 12.5 | 11.3 | 14.1 | – | – | 19.2 | 12.2 | – | – | 11 |
| C12 | 2.7 | 1.6 | 1.4 | – | 7.2 | 1.7 | 1.4 | 7.2 | 7.3 | 9.3 | – | – | 8.8 | 7.8 | – | – | 5 |
| C14 | 1.5 | 0.6 | 0.5 | 6.9 | 3.4 | 0.6 | 0.6 | 1.6 | 1.5 | 1.8 | 3.1 | 4.2 | 2.8 | 3.8 | 7.5 | 2.5 | 2.7 |
| C16 | 0.6 | 0.3 | 0.2 | 2.9 | 2.1 | 0.2 | 0.1 | 0.5 | 0.2 | 0.4 | 0.7 | 0.5 | 1.6 | 1.9 | 4.6 | 1.4 | 1.1 |
| C18 | 0.6 | – | – | 2.1 | 1.4 | – | 0.1 | – | – | – | 0.2 | 0.6 | 0.9 | 0.6 | 2.4 | 1.2 | 1 |
| C20 | 0.4 | – | – | 1.8 | 0.9 | – | 0.4 | – | – | – | 0.2 | 0.4 | 0.5 | 0.7 | 2.2 | 0.9 | 0.8 |
| C22 | 0.4 | – | – | 0.5 | 0.7 | – | – | – | – | – | 0.1 | 0.2 | 0.2 | 0.8 | 1.7 | 0.6 | 0.6 |
| C24 | – | – | – | 0.9 | 0.6 | – | – | – | – | – | 0.05 | 0.1 | – | – | 1.2 | 0.3 | 0.52 |
| C26 | – | – | – | 0.5 | 0.5 | – | – | – | – | – | 0.1 | 0.3 | – | – | 0.8 | 0.03 | 0.37 |
| C28 | – | – | – | 0.1 | 0.4 | – | – | – | – | – | 0.02 | 0.07 | – | – | 0.7 | 0.02 | 0.22 |
| C30 | – | – | – | 0.5 | 0.3 | – | – | – | – | – | – | – | – | – | 0.4 | 0.01 | 0.3 |
| C32 | – | – | – | 0.03 | 0.2 | – | – | – | – | – | – | – | – | – | 0.3 | 0.02 | 0.14 |
| C34 | – | – | – | – | 0.1 | – | – | – | – | – | – | – | – | – | – | – | 0.1 |
| C36 | – | – | – | – | 0.1 | – | – | – | – | – | – | – | – | – | – | – | 0.1 |
| C38 | – | – | – | – | 0.1 | – | – | – | – | – | – | – | – | – | – | – | 0.1 |
| Linear Siloxanes | M1 | M2 | M3 | M4 | P1 | P2 | Mc1 | Mc2 | E1 | E2 | Si1 | Si2 | Se1 | Se2 | C1 | C2 | AVG |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C10 | 2.0 | 1.3 | 1.4 | – | – | 0.8 | 1.9 | 2.2 | 1.9 | 1.7 | – | – | – | – | – | – | 1.7 |
| C12 | 4.3 | 2.3 | 2.3 | 1.4 | – | 1.6 | 3.1 | 5.4 | 5.3 | 4.3 | 23.1 | 19.1 | 4.1 | 3.8 | 0.6 | 21.7 | 7.2 |
| C14 | 5.3 | 2.5 | 2.8 | 2.3 | 7.3 | 1.9 | 3.4 | 3.3 | 3.1 | 5.1 | 22.6 | 20.3 | 6.7 | 9.7 | 5.1 | 20.9 | 7.7 |
| C16 | 5.4 | 2.1 | 2.5 | 2.8 | 7.9 | 1.8 | 3.2 | 6.9 | 6.5 | 7.5 | 17.1 | 15.2 | 8.3 | 7.3 | 8.8 | 15.5 | 7.4 |
| C18 | 5.3 | 1.7 | 2.2 | 2.5 | 7.4 | 1.6 | 2.7 | 5.9 | 5.6 | 6.5 | 11.3 | 10.4 | 7.6 | 5.6 | 11.7 | 10.1 | 6.1 |
| C20 | 4.6 | 1.1 | 1.4 | 2.2 | 5.9 | 1.1 | 1.8 | 3.9 | 4.2 | 3.3 | 8.2 | 6.2 | 6.1 | 3.1 | 13.2 | 7.2 | 4.6 |
| C22 | 3.2 | 0.6 | 0.8 | 1.4 | 4.3 | 0.6 | 1.0 | 2.4 | 2.7 | 1.7 | 5.6 | 6.4 | 4.1 | 5.1 | 11.3 | 4.9 | 3.6 |
| C24 | 2.2 | 0.3 | 0.4 | 0.8 | 3.5 | 0.4 | 0.5 | 1.3 | 1.6 | 1.1 | 3.2 | 2.1 | 2.8 | 1.8 | 7.4 | 3.1 | 2.0 |
| C26 | 1.5 | 0.2 | 0.2 | 0.4 | 3.2 | 0.2 | 0.3 | 0.7 | 0.9 | 0.7 | 1.5 | 2.5 | 2.1 | 2.6 | 4.1 | 1.6 | 1.4 |
| C28 | 0.8 | – | 0.1 | 0.2 | 2.7 | 0.1 | 0.2 | 0.4 | 0.5 | 0.5 | 0.6 | 1.6 | 1.1 | 1.9 | 2.1 | 0.7 | 0.9 |
| C30 | 0.4 | – | – | 0.1 | 2.2 | – | 0.1 | 0.2 | 0.3 | 0.3 | 0.2 | 1.2 | 0.6 | 1.6 | 1.1 | 0.2 | 0.6 |
| C32 | – | – | – | 1.4 | 1.6 | – | – | – | – | – | 0.2 | 0.4 | – | – | 0.7 | 0.2 | 0.7 |
| C34 | – | – | – | 2.3 | 1.3 | – | – | – | – | – | 0.05 | 0.05 | – | – | 0.5 | 0.04 | 0.7 |
| C36 | – | – | – | 2.8 | 0.9 | – | – | – | – | – | – | – | – | – | 0.6 | 0.1 | 1.1 |
| C38 | – | – | – | – | 0.7 | – | – | – | – | – | – | – | – | – | – | – | 0.7 |
| C40 | – | – | – | – | 0.6 | – | – | – | – | – | – | – | – | – | – | – | 0.6 |
| C42 | – | – | – | – | 0.5 | – | – | – | – | – | – | – | – | – | – | – | 0.5 |
| C44 | – | – | – | – | 0.4 | – | – | – | – | – | – | – | – | – | – | – | 0.4 |
| C46 | – | – | – | – | 0.3 | – | – | – | – | – | – | – | – | – | – | – | 0.3 |
| Breast Sample | MODULUS (MPa) | STRAIN AT BREAK (%) | STRESS AT BREAK (MPa) |
|---|---|---|---|
| Mentor 1 | 1.07 | 465 | 2.7 |
| Mentor 2 | 1.26 | 686 | 7.3 |
| Mentor 3 | 1.15 | 545 | 4.5 |
| Mentor 4 | 1.37 | 776 | 8.75 |
| Pip 1 | 0.94 | 282 | 2.08 |
| Pip 2 | 0.7 | 204 | 1.6 |
| Mcghan 1 | 1.12 | 510 | 5.12 |
| Mcghan 2 | 0.97 | 347 | 2.8 |
| Eurosilicon 1 | 0.77 | 292 | 1.97 |
| Eurosilicon 2 | 1.03 | 313 | 4.2 |
| Silimed 1 | 0.94 | 531 | 4.41 |
| Silimed 2 | 1.36 | 404 | 3.6 |
| Sebin 1 | 0.95 | 320 | 2.45 |
| Sebin 2 | 1.12 | 431 | 3.18 |
| Cox 1 | 1.06 | 435 | 2.18 |
| Cox 2 | 0.86 | 395 | 1.08 |
| AVG | 1.04 | 433 | 3.6 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amoresano, A.; De Stefano, L.; Rea, I.; Pane, F.; Birolo, L.; Schonauer, F. Chemical and Structural Characterization of Several Mid-Term Explanted Breast Prostheses. Materials 2016, 9, 678. https://doi.org/10.3390/ma9080678
Amoresano A, De Stefano L, Rea I, Pane F, Birolo L, Schonauer F. Chemical and Structural Characterization of Several Mid-Term Explanted Breast Prostheses. Materials. 2016; 9(8):678. https://doi.org/10.3390/ma9080678
Chicago/Turabian StyleAmoresano, Angela, Luca De Stefano, Ilaria Rea, Federica Pane, Leila Birolo, and Fabrizio Schonauer. 2016. "Chemical and Structural Characterization of Several Mid-Term Explanted Breast Prostheses" Materials 9, no. 8: 678. https://doi.org/10.3390/ma9080678
APA StyleAmoresano, A., De Stefano, L., Rea, I., Pane, F., Birolo, L., & Schonauer, F. (2016). Chemical and Structural Characterization of Several Mid-Term Explanted Breast Prostheses. Materials, 9(8), 678. https://doi.org/10.3390/ma9080678








