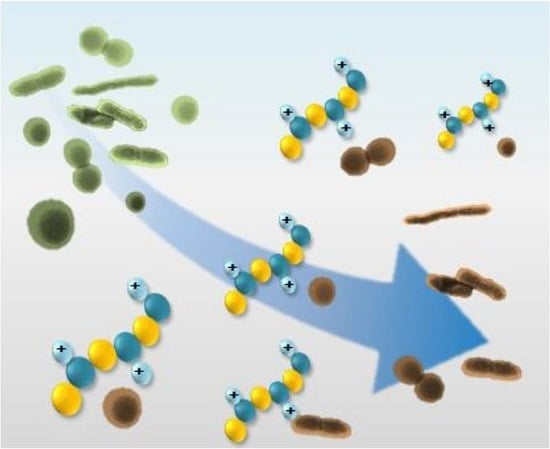
Recent Developments in Antimicrobial Polymers: A Review
Abstract
:1. Introduction
2. Most Relevant Microbial Threats to Health and Antimicrobial Evaluation Methods
2.1. Antimicrobial Resistance
2.2. Bacteria and the ESKAPE Group Concept
2.3. Fungi
2.4. Mechanisms of Microbial Cell Death Induced by Antimicrobials
2.4.1. Reactive Oxygen Species-Induced Cytotoxicity
2.4.2. Microbial Cell Envelope Disruption
2.4.3. Inhibition of Nucleic Acid and Protein Synthesis
2.5. Evaluation of Antimicrobial Activity
2.5.1. Indicator Organisms for Antimicrobial Characterization
2.5.2. In Vitro Antimicrobial Activity Assay
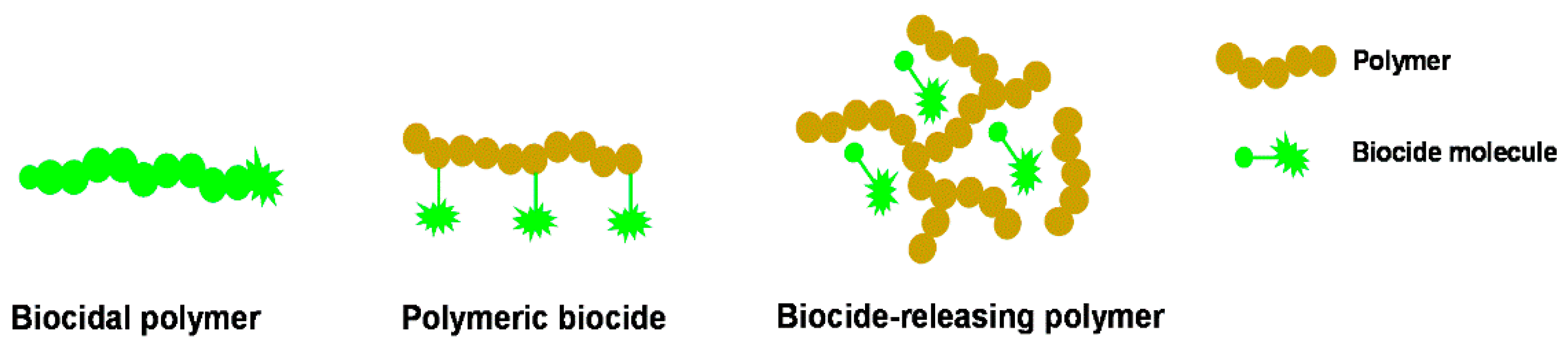
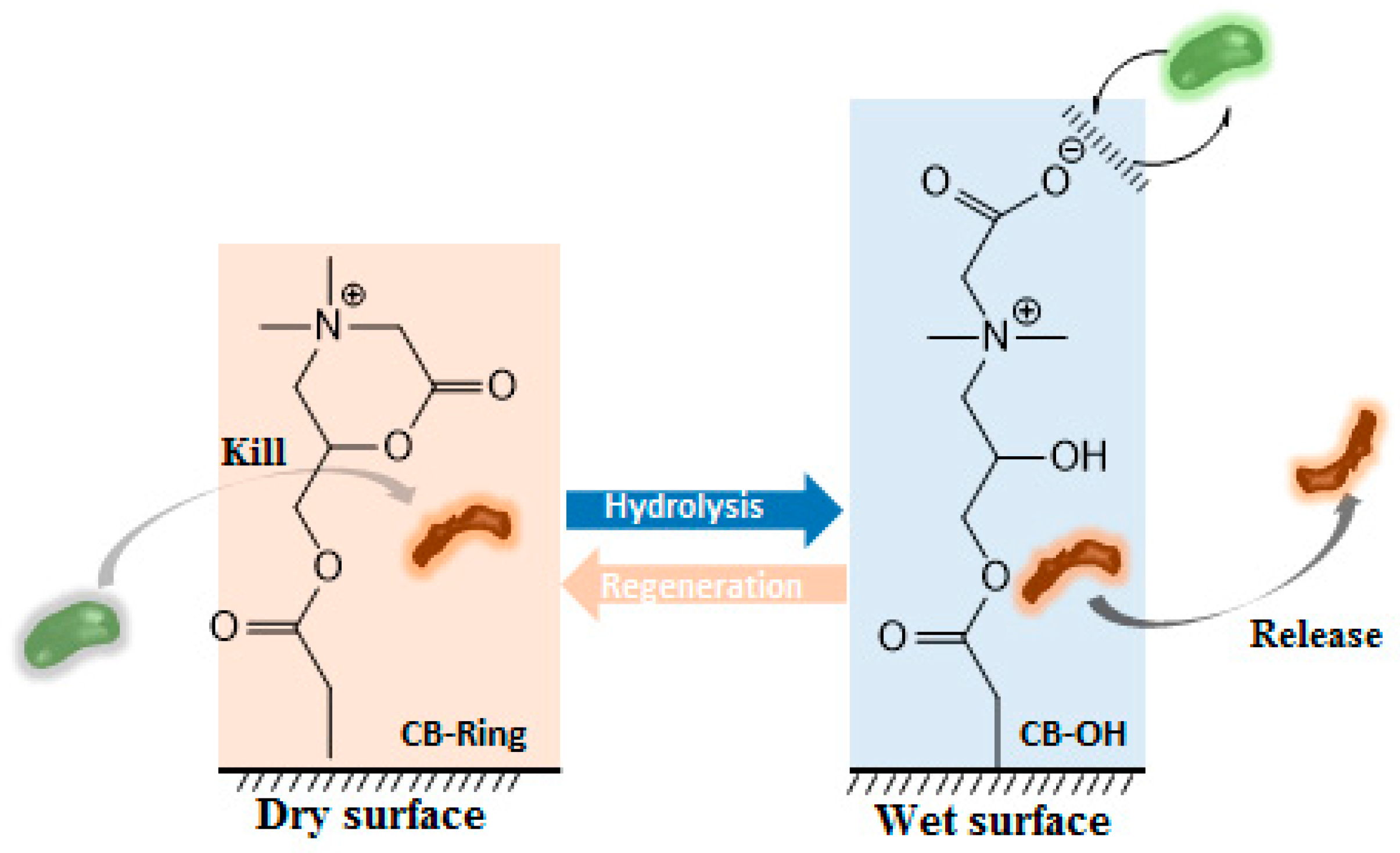
3. Biocidal Polymers
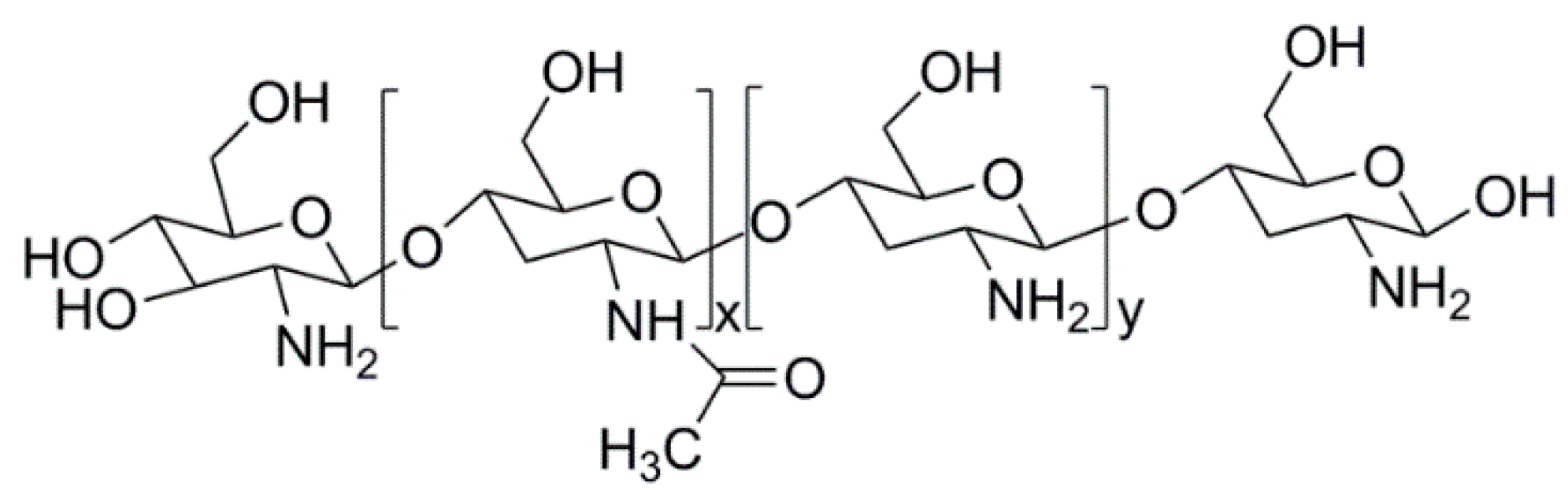
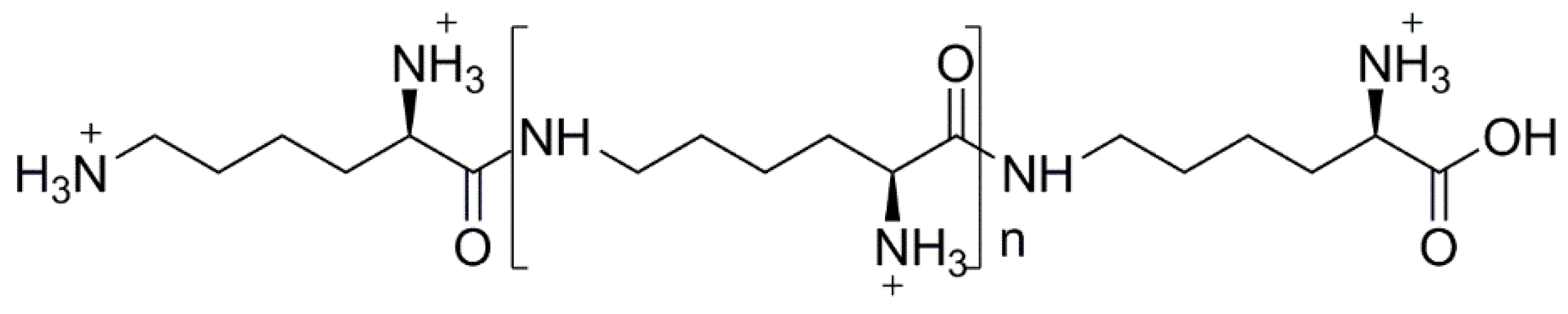
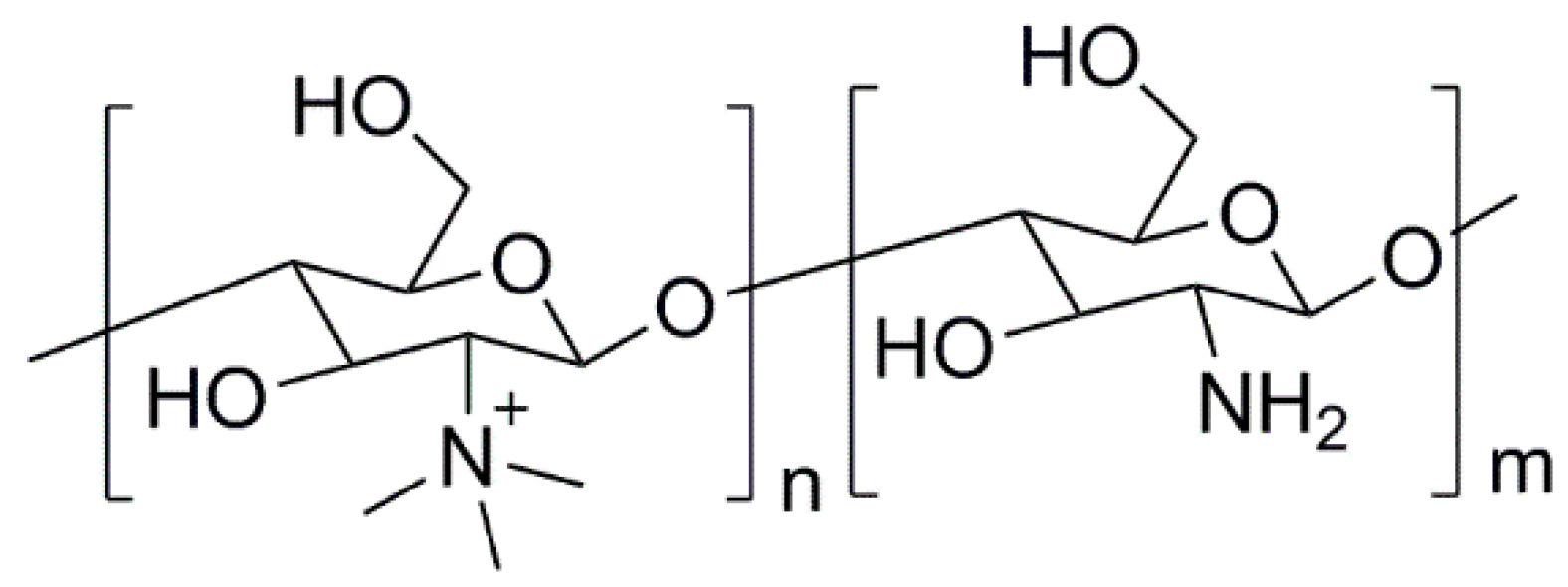
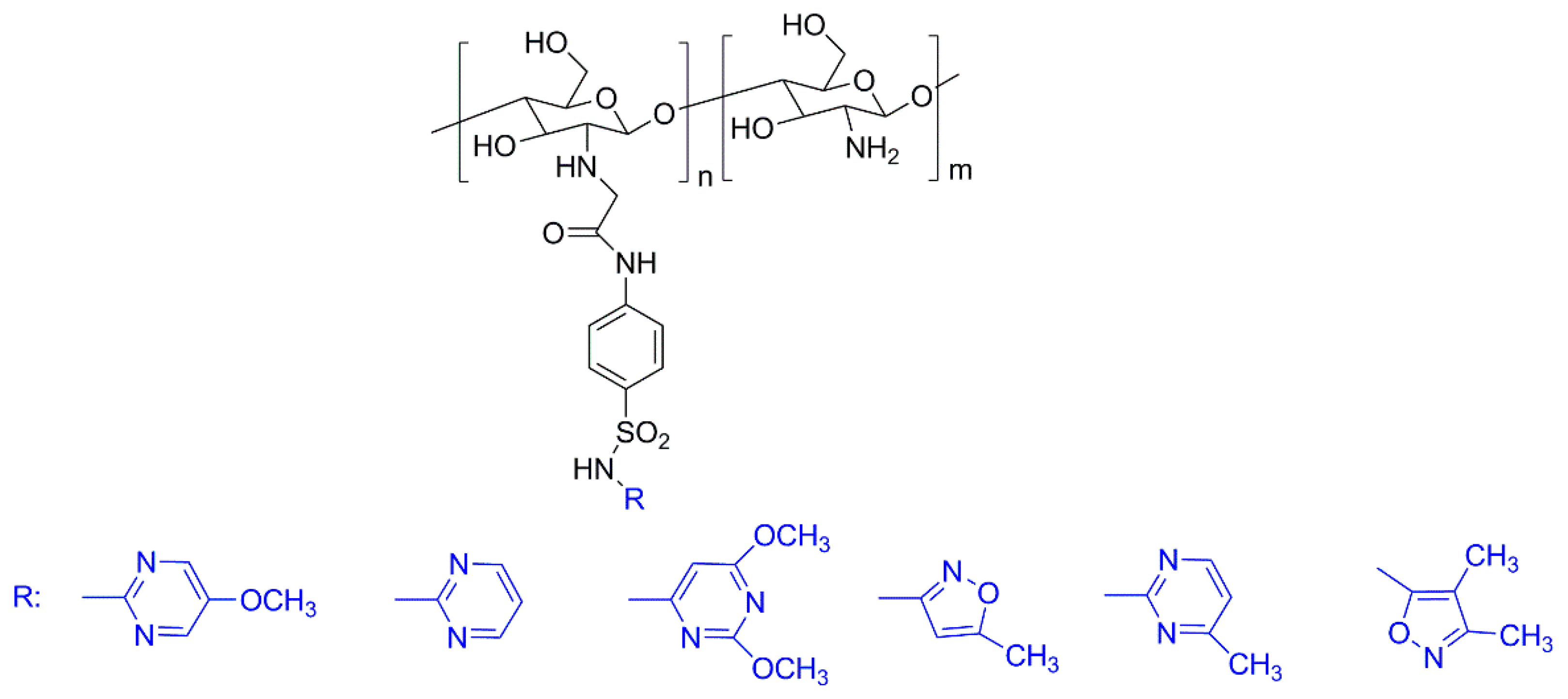
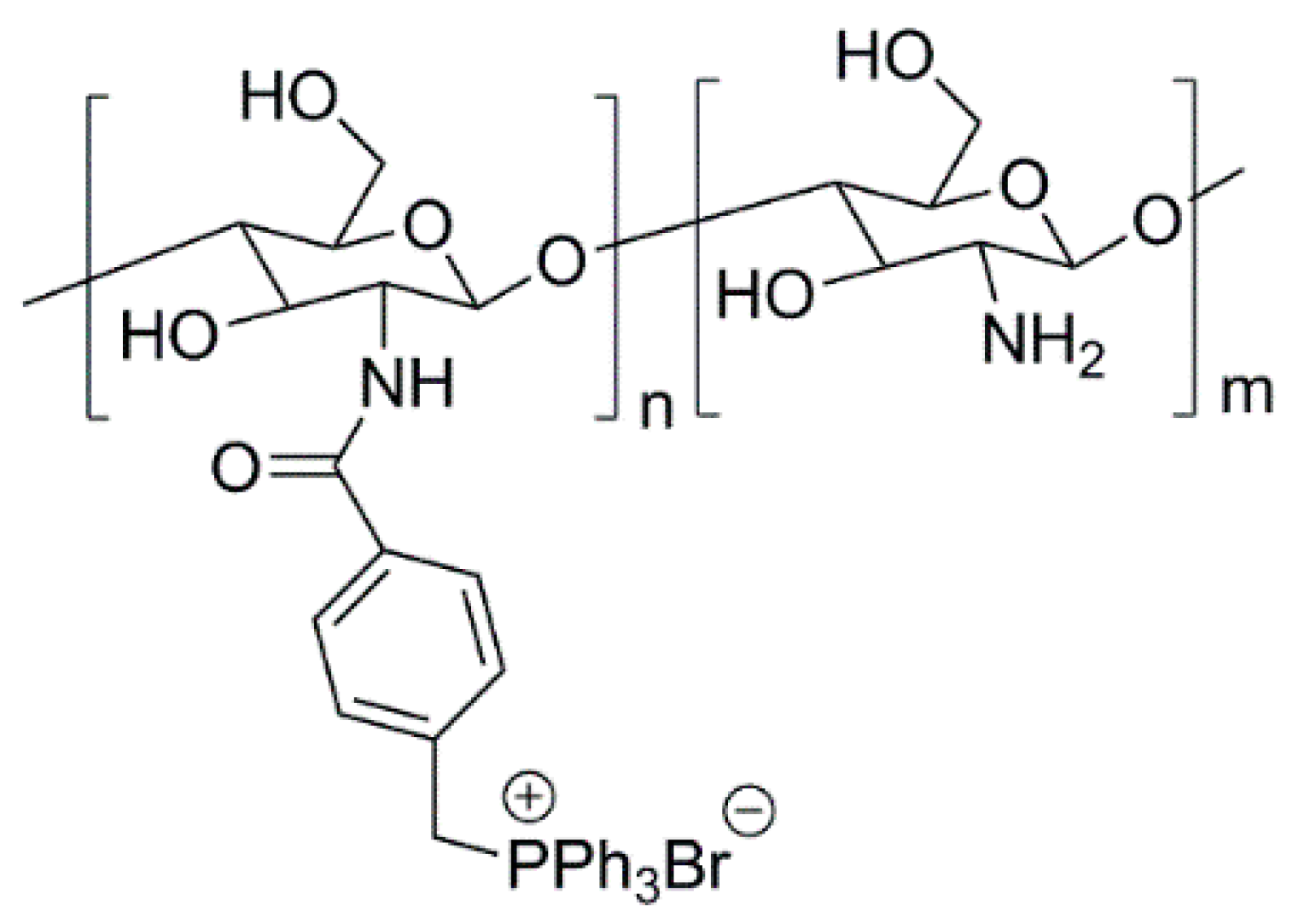
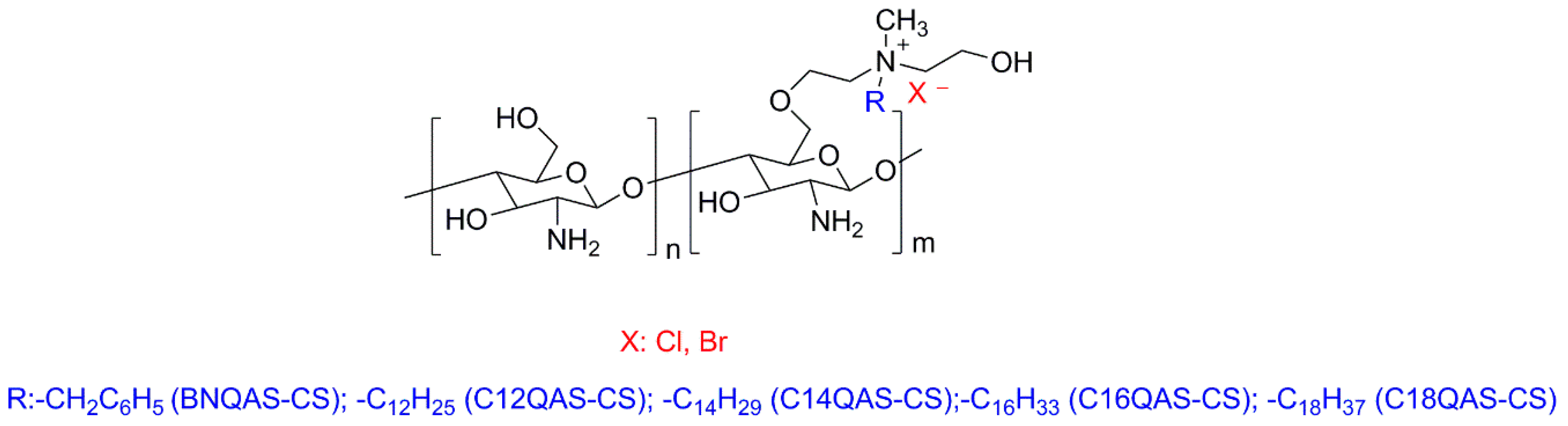
3.1. Natural Polymers and Derivatives
3.2. Synthetic Polymers
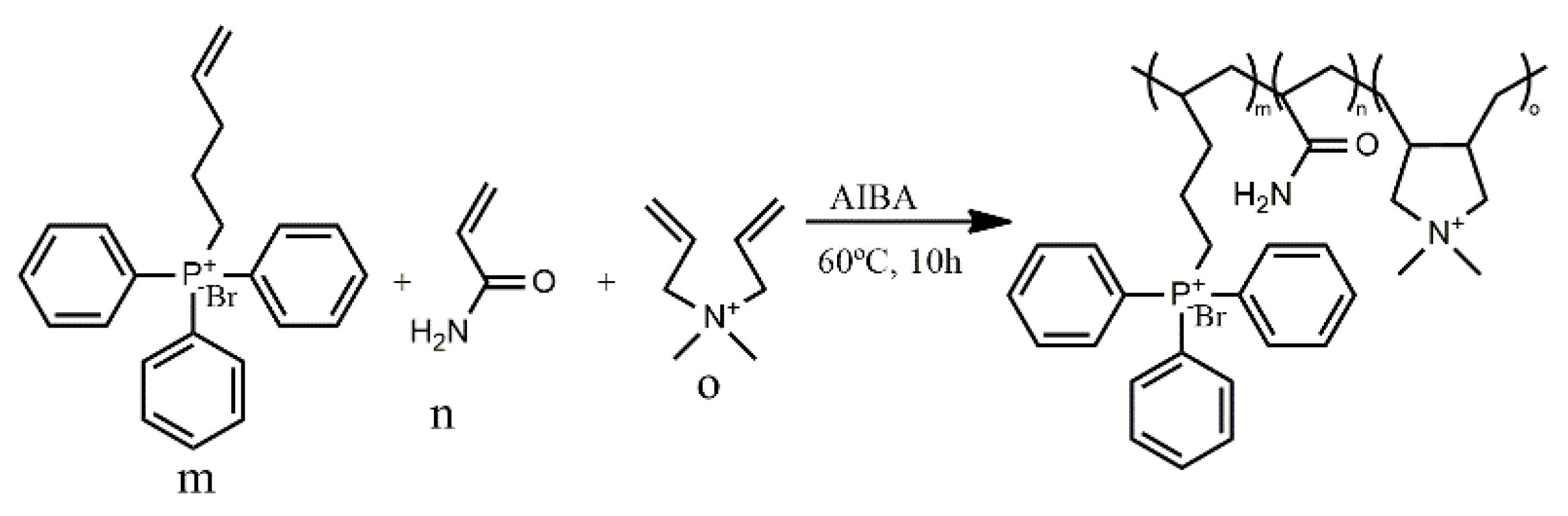
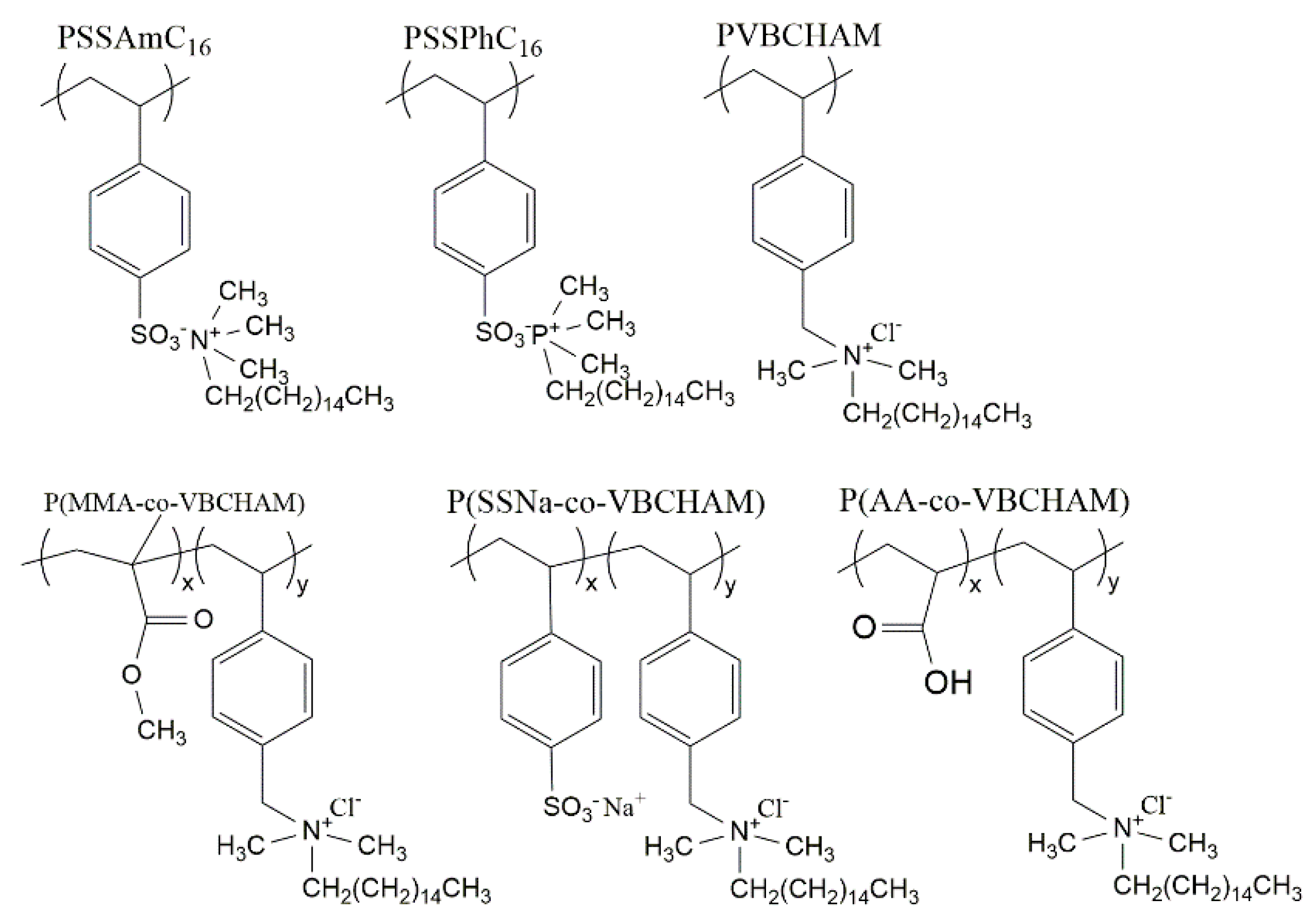
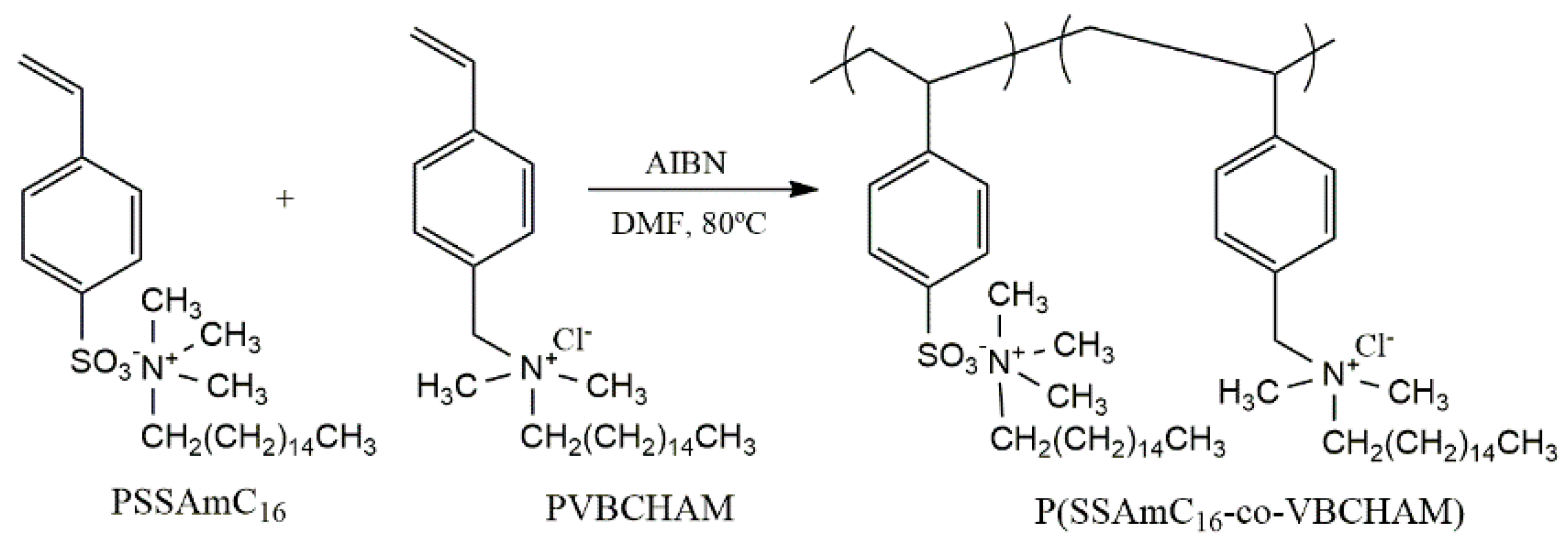
4. Polymeric Biocides
5. Biocide-Releasing Polymers
5.1. Nanoparticles Release
5.2. Antibiotics Release
6. Polymers Containing Photosensitizers: A New Class of Biocidal Polymers
7. Conclusions and Outlook
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Acrylic acid |
| ADV | Non-enveloped adenovirus |
| AEMPs | Primary ammonium ethyl methacrylate homopolymers |
| AgNPs | Silver nanoparticles |
| AIBA | 2,20-azobis(2-methylpropionamidine)dihydrochloride |
| AM | Acrylamide |
| AMPs | Antimicrobial peptides |
| APMA | Methacrylamide hydrochloride |
| ATC | 2-(acryloyloxy)ethyltrimethylammonium chloride |
| bla | β-lactamase |
| BSI | Bloodstream infection |
| CFU | Colony forming units |
| CTA | Macro chain transfer agent |
| DADMAC | Diallyl dimethyl ammonium chloride |
| DS | Degree of substitution |
| EPO | Eudragit EPO |
| ESBL | Spectrum β-Lactamase |
| FRP | Free radical polymerization |
| GPMA | 3-guanadinopropyl methacrylamide |
| HEMA | 2-hydroxyethyl methacrylate |
| LB | Lysogeny broth |
| LbL | Layer-by-layer |
| LMW | Low molecular weight |
| LPS | Lipopolysaccharide |
| LVF | Levofloxacin |
| MBC | Minimum bactericidal concentration |
| MDR | Multidrug-resistant |
| MFC | Minimal fungicidal concentration |
| MIC | Minimum inhibitory concentration assay |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| MSN | Mesoporous silica nanoparticles |
| NPCu | Polymer/Cu nanoparticles |
| OD | Optical density |
| OPF/SMA | Oligo(poly(ethylene glycol)fumarate) and sodium methacrylate |
| PCL | Poly(ε-caprolactone) |
| PDMAEMA | Poly(2-dimethylamino)ethyl methacrylate |
| PDMS | Poly(dimethylsoloxane) |
| PE | Polyethylene |
| PEG | Poly(ethylene glycol) |
| PFPQ | Polyfluorene derivative |
| PGA | Penicillin G amidase |
| PHEMA | Poly(2-hydroxyethyl methacrylate) |
| PHMB | Polyhexamethylene biguanide |
| PP | Polypropylene |
| PS | Photosensitizer |
| PTPB | (4-penten-1-yl) triphenylphosphonium bromide |
| PU | Polyurethane |
| PVAms | Poly(vinyl amines) |
| PVP | Poly(vinyl pyrrolidone) |
| QAS | Quaternary ammonium salts |
| QPM | Tributyl(4-vinylbenzyl)phosphonium |
| QPS | Quaternary phosphonium salts |
| RAFT | Reversible addition-fragmentation chain transfer |
| RDRP | Reversible deactivation radical polymerization |
| ROS | Reactive oxygen species |
| SSNa | Sodium 4-styrene sulfonate |
| TMC | N,N,N-trimethyl chitosan |
| VBCHAM | Vinyl benzyl dimethylhexadecylammonium chloride |
| WHO | World Health Organization |
References
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. P T 2015, 40, 277–283. [Google Scholar] [PubMed]
- Siedenbiedel, F.; Tiller, J.C. Antimicrobial polymers in solution and on surfaces: Overview and functional principles. Polymers 2012, 4, 46–71. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Fernández-García, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Worley, S.D.; Broughton, R. The chemistry and applications of antimicrobial polymers: A state-of-the-art review. Biomacromolecules 2007, 8, 1359–1384. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Duvvuri, L.S.; Farah, S.; Beyth, N.; Domb, A.J.; Khan, W. Antimicrobial polymers. Adv. Healthc. Mater. 2014, 3, 1969–1985. [Google Scholar] [CrossRef] [PubMed]
- Bowersock, T.L.; Woodyard, L.; Hamilton, A.J.; DeFord, J.A. Inhibition of staphylococci by vancomycin absorbed on triidodecylmethyl ammonium chloride-coated intravenous catheter. J. Control. Release 1994, 31, 237–243. [Google Scholar] [CrossRef]
- Xue, Y.; Xiao, H.; Zhang, Y. Antimicrobial polymeric materials with quaternary ammonium and phosphonium salts. Int. J. Mol. Sci. 2015, 16, 3626–3655. [Google Scholar] [CrossRef] [PubMed]
- Barzic, A.I.; Ioan, S. Antibacterial drugs—From basic concepts to complex therapeutic mechanisms of polymer systems. In Concepts, Compounds and the Alternatives of Antibacterials; Bobbarala, V., Ed.; Science, Technology and Medicine: London, UK, 2015. [Google Scholar]
- Kenawy, E.-R.; Kandil, S. Synthesis, antimicrobial activity and applications of polymers with ammonium and phosphonium groups. In Polymeric Materials with Antimicrobial Activity: From Synthesis to Applications; The Royal Society of Chemistry: Cambridge, UK, 2014; Chapter 3; pp. 54–74. [Google Scholar]
- Ganewatta, M.S.; Tang, C. Controlling macromolecular structures towards effective antimicrobial polymers. Polymer 2015, 63, A1–A29. [Google Scholar] [CrossRef]
- Panarin, E.F.; Solovskii, M.V.; Ekzemplyarov, O.N. Synthesis and antimicrobial properties of polymers containing quaternary ammonimum groups. Pharm. Chem. J. 1972, 5, 406–408. [Google Scholar] [CrossRef]
- Jämsä, S.; Mahlberg, R.; Holopainen, U.; Ropponen, J.; Savolainen, A.; Ritschkoff, A.C. Slow release of a biocidal agent from polymeric microcapsules for preventing biodeterioration. Prog. Org. Coat. 2013, 76, 269–276. [Google Scholar] [CrossRef]
- Li, Y.; Liu, G.; Wang, X.; Hu, J.; Liu, S. Enzyme-responsive polymeric vesicles for bacterial-strain-selective delivery of antimicrobial agents. Angew. Chem. Int. Ed. Engl. 2016, 55, 1760–1764. [Google Scholar] [CrossRef] [PubMed]
- Bshena, O.; Heunis, T.; Dicks, L.M.; Klumperman, B. Antimicrobial fibers: Therapeutic possibilities and recent advances. Future Med. Chem. 2011, 3, 1821–1847. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Nie, X.; Zou, M.; Shi, Y.; Cheng, G. Recent advances in materials for extended-release antibiotic delivery system. J. Antibiot. 2011, 64, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Dallas, P.; Sharma, V.K.; Zboril, R. Silver polymeric nanocomposites as advanced antimicrobial agents: Classification, synthetic paths, applications, and perspectives. Adv. Colloid Interface Sci. 2011, 166, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.; Fatima, J.; Shakil, S.; Rizvi, S.M.D.; Kamal, M.A. Antibiotic resistance and extended spectrum beta-lactamases: Types, epidemiology and treatment. Saudi J. Biol. Sci. 2015, 22, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Pendleton, J.N.; Gorman, S.P.; Gilmore, B.F. Clinical relevance of the eskape pathogens. Expert Rev. Anti Infect. Ther. 2013, 11, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Rice, L.; Xa, B. Progress and challenges in implementing the research on eskape pathogens. Infect. Control Hosp. Epidemiol. 2010, 31, S7–S10. [Google Scholar] [CrossRef] [PubMed]
- Tommasi, R.; Brown, D.G.; Walkup, G.K.; Manchester, J.I.; Miller, A.A. Eskapeing the labyrinth of antibacterial discovery. Nat. Rev. Drug Discov. 2015, 14, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Farias, P.; Espírito Santo, C.; Branco, R.; Francisco, R.; Santos, S.; Hansen, L.; Sorensen, S.; Morais, P.V. Natural hotspots for multiple resistances gain: Arsenic and antibiotic resistance in heterotrophic aerobic bacteria from marine hydrothermal vent fields. Appl. Environ. Microbiol. 2015, 81, 2534–2543. [Google Scholar] [CrossRef] [PubMed]
- Morar, M.; Wright, G.D. The genomic enzymology of antibiotic resistance. Annu. Rev. Genet. 2010, 44, 25–51. [Google Scholar] [CrossRef] [PubMed]
- Penchovsky, R.; Traykovska, M. Designing drugs that overcome antibacterial resistance: Where do we stand and what should we do? Exp. Opin. Drug Disc. 2015, 10, 631–650. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance, Fact Sheet N°194. Available online: http://www.who.int/mediacentre/factsheets/fs194/en/# (accessed on 6 April 2016).
- Witte, W. International dissemination of antibiotic resistant strains of bacterial pathogens. Infect. Genet. Evol. 2004, 4, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Brumfitt, W.; Perccival, A.; Louvois, J.D. The role of penicillinase in determining natural and acquired resistance of gram-negative bacteria to penicillins. Microbiology 1963, 32, 77–89. [Google Scholar]
- Chen, C.-W.; Hsu, C.-Y.; Lai, S.-M.; Syu, W.-J.; Wang, T.-Y.; Lai, P.-S. Metal nanobullets for multidrug resistant bacteria and biofilms. Adv. Drug Deliv. Rev. 2014, 78, 88–104. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.A.; Rice, L.B. Antifungal agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 1999, 12, 501–517. [Google Scholar] [PubMed]
- Rice, L.B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No eskape. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef] [PubMed]
- Gardete, S.; Tomasz, A. Mechanisms of vancomycin resistance in staphylococcus aureus. J. Clin. Investig. 2014, 124, 2836–2840. [Google Scholar] [CrossRef] [PubMed]
- Billington, E.O.; Phang, S.H.; Gregson, D.B.; Pitout, J.D.D.; Ross, T.; Church, D.L.; Laupland, K.B.; Parkins, M.D. Incidence, risk factors, and outcomes for enterococcus spp. Blood stream infections: A population-based study. Int. J. Infect. Dis. 2014, 26, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Goff, D.A.; Bauer, K.A.; Mangino, J.E. Bad bugs need old drugs: A stewardship program’s evaluation of minocycline for multidrug-resistant acinetobacter baumannii infections. Clin. Infect. Dis. 2014, 59, S381–S387. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, E.B.; Tam, V.H. Impact of multidrug-resistant pseudomonas aeruginosa infection on patient outcomes. Exp. Rev. Pharmacoecon. Outcomes Res. 2010, 10, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Haleem Khan, A.A.; Mohan Karuppayil, S. Fungal pollution of indoor environments and its management. Saudi J. Biol. Sci. 2012, 19, 405–426. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, O.V.; Leong, S.-L.L.; Lantz, H.; Rice, T.; Dijksterhuis, J.; Houbraken, J.; Samson, R.A.; Schnürer, J. Phylogeny and intraspecific variation of the extreme xerophile, xeromyces bisporus. Fungal Biol. 2011, 115, 1100–1111. [Google Scholar] [CrossRef] [PubMed]
- Madan, M.; Thin, K.S. Physiology of Fungi; APH Publishing Corporation: New Delhi, India, 1998. [Google Scholar]
- Medicine, I.O. Emerging Infections: Microbial Threats to Health in the United States; The National Academies Press: Washington, DC, USA, 1992; p. 312. [Google Scholar]
- Warnock, D.W. Fungal diseases: An evolving public health challenge. Med. Mycol. 2006, 44, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Benndorf, D.; Muller, A.; Bock, K.; Manuwald, O.; Herbarth, O.; von Bergen, M. Identification of spore allergens from the indoor mould aspergillus versicolor. Allergy 2008, 63, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Hamid, A.S.; Tesfamariam, I.G.; Zhang, Y.; Zhang, Z.G. Aflatoxin b1-induced hepatocellular carcinoma in developing countries: Geographical distribution, mechanism of action and prevention. Oncol. Lett. 2013, 5, 1087–1092. [Google Scholar] [PubMed]
- Imlay, J. Oxidative stress. EcoSal Plus 2009. [Google Scholar] [CrossRef] [PubMed]
- Anjem, A.; Varghese, S.; Imlay, J.A. Manganese import is a key element of the oxyr response to hydrogen peroxide in Escherichia coli. Mol. Microbiol. 2009, 72, 844–858. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Wagner, J.R. DNA base damage by reactive oxygen species, oxidizing agents, and uv radiation. Cold Spring Harb. Perspect. Biol. 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Cabiscol, E.; Tamarit, J.; Ros, J. Oxidative stress in bacteria and protein damage by reactive oxygen species. Int. Microbiol. 2000, 3, 3–8. [Google Scholar] [PubMed]
- Slauch, J.M. How does the oxidative burst of macrophages kill bacteria? Still an open question. Mol. Microbiol. 2011, 80, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Xu, L.; Porter, N.A. Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef] [PubMed]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 44, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; He, C.; Xiao, C.; Chen, X. Reactive oxygen species (ros) responsive polymers for biomedical applications. Macromol. Biosci. 2016, 16, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Parachin, N.S.; Franco, O.L. New edge of antibiotic development: Antimicrobial peptides and corresponding resistance. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Hoque, J.; Akkapeddi, P.; Yadav, V.; Manjunath, G.B.; Uppu, D.S.S.M.; Konai, M.M.; Yarlagadda, V.; Sanyal, K.; Haldar, J. Broad spectrum antibacterial and antifungal polymeric paint materials: Synthesis, structure-activity relationship, and membrane-active mode of action. ACS Appl. Mater. Interfaces 2015, 7, 1804–1815. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Morales, J.; Amariei, G.; Leton, P.; Rosal, R. Antimicrobial activity of poly(vinyl alcohol)-poly(acrylic acid) electrospun nanofibers. Colloids Surf. B Biointerfaces 2016, 146, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Munch, D.; Sahl, H.G. Structural variations of the cell wall precursor lipid ii in gram-positive bacteria—Impact on binding and efficacy of antimicrobial peptides. Biochim. Biophys. Acta 2015, 1848, 3062–3071. [Google Scholar] [CrossRef] [PubMed]
- Guilhelmelli, F.; Vilela, N.; Albuquerque, P.; Derengowski, L.; Silva-Pereira, I.; Kyaw, C. Antimicrobial development challenges: The various mechanisms of action of antimicrobial peptides and of bacterial resistance. Front. Microbiol. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Bahar, A.A.; Ren, D. Antimicrobial peptides. Pharmaceuticals 2013, 6, 1543–1575. [Google Scholar] [CrossRef] [PubMed]
- López, S.N.; Castelli, M.V.; Zacchino, S.A.; Domínguez, J.N.; Lobo, G.; Charris-Charris, J.; Cortés, J.C.; Ribas, J.C.; Devia, C.; Rodríguez, A.M.; et al. In vitro antifungal evaluation and structure-activity relationships of a new series of chalcone derivatives and synthetic analogues, with inhibitory properties against polymers of the fungal cell wall. Bioorg. Med. Chem. 2001, 9, 1999–2013. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Subbalakshmi, C.; Sitaram, N. Mechanism of antimicrobial action of indolicidin. FEMS Microbiol. Lett. 1998, 160, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Nan, Y.H.; Bang, J.K.; Shin, S.Y. Design of novel indolicidin-derived antimicrobial peptides with enhanced cell specificity and potent anti-inflammatory activity. Peptides 2009, 30, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Chindera, K.; Mahato, M.; Kumar Sharma, A.; Horsley, H.; Kloc-Muniak, K.; Kamaruzzaman, N.F.; Kumar, S.; McFarlane, A.; Stach, J.; Bentin, T.; et al. The antimicrobial polymer phmb enters cells and selectively condenses bacterial chromosomes. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Matejuk, A.; Leng, Q.; Begum, M.D.; Woodle, M.C.; Scaria, P.; Chou, S.T.; Mixson, A.J. Peptide-based antifungal therapies against emerging infections. Drugs Future 2010, 35, 197. Available online: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2873032/ (accessed on 18 July 2016). [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Effectiveness Test; USP 51; The United States Pharmacopeial Convention, Inc.: Rockville, MD, USA, 2013; pp. 54–55.
- Standard Test Method for Determining the Activity of Incorporated Antimicrobial Agent(s) in Polymeric or Hydrophobic Materials; ASTM E2180-07; ASTM International: West Conshohocken, PA, USA, 2012.
- Method 964.02—Pseudomonas aeruginosa; AOAC Official Methods of Analysis; AOAC International: Gaithersburg, MD, USA, 2013.
- Standard Test Method for Determining the Antimicrobial Activity of Antimicrobial Agents under Dynamic Contact Conditions; ASTM E2149-13a; ASTM International: West Conshohocken, PA, USA, 2013.
- Antimicrobial Activity Assessment of New Carpets (2011); developed in 1991 by AATCC Committee RA31 (revised 2011 with title change); AATCC TM174; American Association of Textile Chemists and Colorists: Research Triangle Park, NC, USA, 2015.
- Antibacterial Activity Assessment of Textile Materials: Parallel Streak Method (2011); developed in 1976 by AATCC Committee RA31 (revised 2011); AATCC TM147; American Association of Textile Chemists and Colorists: Research Triangle Park, NC, USA, 2015.
- Antibacterial Finishes on Textile Materials: Assessment of (2012); developed in 1961 by AATCC Committee RA31 (revised 2012); AATCC TM100; American Association of Textile Chemists and Colorists: Research Triangle Park, NC, USA, 2014.
- Method 955.14—Salmonella enterica; AOAC Official Methods of Analysis; AOAC International: Gaithersburg, MD, USA, 2013.
- Methods 955.15—Staphylococcus aureus; AOAC Official Methods of Analysis; AOAC International: Gaithersburg, MD, USA, 2013.
- Antifungal Activity, Assessment on Textile Materials: Mildew and Rot Resistance of Textile Materials (2013); developed in 1946 by AATCC Committee RA31 (reaffirmed 2013); AATCC TM30-2013; American Association of Textile Chemists and Colorists: Research Triangle Park, NC, USA, 2014.
- Standard Practice for Determining Resistance of Synthetic Polymeric Materials to Fungi; ASTM G21-15; ASTM International: West Conshohocken, PA, USA, 2015.
- Standard Test Methods for Ability of Adhesive Films to Support or Resist the Growth of Fungi; ASTM D4300-01; ASTM International: West Conshohocken, PA, USA, 2013.
- Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces; ISO 22196:2011; ISO: Geneva, Switzerland, 2011.
- Cubayachi, C.; Couto, R.O.; de Gaitani, C.M.; Pedrazzi, V.; Freitas, O.; Lopez, R.F. Needle-free buccal anesthesia using iontophoresis and amino amide salts combined in a mucoadhesive formulation. Colloids Surf. B Biointerfaces 2015, 136, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Performance Standards for Antimicrobial Susceptibility Testing; Seventeenth Information Supplement; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2007.
- Sanches, L.M.; Petri, D.F.S.; de Melo Carrasco, L.D.; Carmona-Ribeiro, A.M. The antimicrobial activity of free and immobilized poly (diallyldimethylammonium) chloride in nanoparticles of poly (methylmethacrylate). J. Nanobiotechnol. 2015, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Performance Standards for Antimicrobial Disk Susceptibility Tests—Approved Standard; Mo2-A12; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012.
- Cavalieri, S.J. Manual of Antimicrobial Susceptibility Testing; American Society for Microbiology: Washington, DC, USA, 2005. [Google Scholar]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (mic) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef] [PubMed]
- Sam, S.T.; Nuradibah, M.A.; Chin, K.M.; Hani, N. Current application and challenges on packaging industry based on natural polymer blending. In Natural Polymers: Industry Tecnhiques and Applications; Olatunji, O., Ed.; Springer: Heidelberg, Germany, 2016. [Google Scholar]
- Maia, J.; Carvalho, R.A.; Coelho, J.F.J.; Simões, P.N.; Gil, M.H. Insight on the periodate oxidation of dextran and its structural vicissitudes. Polymer 2011, 52, 258–265. [Google Scholar] [CrossRef]
- Sayed, S.; Jardine, M.A. Antimicrobial biopolymers. In Advanced Functional Materials; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 493–533. [Google Scholar]
- Elsabee, M.Z.; Abdou, E.S. Chitosan based edible films and coatings: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1819–1841. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Camacho, A.P.; Cortez-Rocha, M.O.; Castillo-Ortega, M.M.; Burgos-Hernández, A.; Ezquerra-Brauer, J.M.; Plascencia-Jatomea, M. Antimicrobial activity of chitosan nanofibers obtained by electrospinning. Polym. Int. 2011, 60, 1663–1669. [Google Scholar] [CrossRef]
- Hamman, J.H. Chitosan based polyelectrolyte complexes as potential carrier materials in drug delivery systems. Mar. Drugs 2010, 8, 1305–1322. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, K.; Singh, S.K.; Mishra, D.N. Chitosan nanoparticles: A promising system in novel drug delivery. Chem. Pharm. Bull. 2010, 58, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Goy, R.C.; de Britto, D.; Assis, O.B.G. A review of the antimicrobial activity of chitosan. Polímeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Allan, C.R.; Hadwiger, L.A. The fungicidal effect of chitosan on fungi of varying cell wall composition. Exp. Mycol. 1979, 3, 285–287. [Google Scholar] [CrossRef]
- Kendra, D.F.; Hadwiger, L.A. Characterization of the smallest chitosan oligomer that is maximally antifungal to fusarium-solani and elicits pisatin formation in pisum-sativum. Exp. Mycol. 1984, 8, 276–281. [Google Scholar] [CrossRef]
- Muzzarelli, R.; Tarsi, R.; Filippini, O.; Giovanetti, E.; Biagini, G.; Varaldo, P.E. Antimicrobial properties of n-carboxybutyl chitosan. Antimicrob. Agents Chemother. 1990, 34, 2019–2023. [Google Scholar] [CrossRef] [PubMed]
- Savard, T.; Beaulieu, C.; Boucher, I.; Champagne, C.P. Antimicrobial action of hydrolyzed chitosan against spoilage yeasts and lactic acid bacteria of fermented vegetables. J. Food Prot. 2002, 65, 828–833. [Google Scholar] [PubMed]
- Badawy, M.E.I.; Rabea, E.I. A biopolymer chitosan and its derivatives as promising antimicrobial agents against plant pathogens and their applications in crop protection. Int. J. Carbohydr. Chem. 2011, 2011. [Google Scholar] [CrossRef]
- Dutta, P.K.; Tripathi, S.; Mehrotra, G.K.; Dutta, J. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 2009, 114, 1173–1182. [Google Scholar] [CrossRef]
- Lim, S.H.; Hudson, S.M. Review of chitosan and its derivatives as antimicrobial agents and their uses as textile chemicals. J. Macromol. Sci. Polym. Rev. 2003, C43, 223–269. [Google Scholar] [CrossRef]
- Radulescu, M.; Ficai, D.; Oprea, O.; Ficai, A.; Andronescu, E.; Holban, A.M. Antimicrobial chitosan based formulations with impact on different biomedical applications. Curr. Pharm. Biotechnol. 2015, 16, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.F.; Facchi, S.P.; Follmann, H.D.M.; Pereira, A.G.B.; Rubira, A.F.; Muniz, E.C. Antimicrobial activity of chitosan derivatives containing n-quaternized moieties in its backbone: A review. Int. J. Mol. Sci. 2014, 15, 20800–20832. [Google Scholar] [CrossRef] [PubMed]
- Hafdani, F.N.; Sadeghinia, N. A review on application of chitosan as a natural antimicrobial. WASET 2011, 2011, 46–50. [Google Scholar]
- Harish Prashanth, K.V.; Tharanathan, R.N. Chitin/chitosan: Modifications and their unlimited application potential—An overview. Trends Food Sci. Technol. 2007, 18, 117–131. [Google Scholar] [CrossRef]
- Helander, I.M.; Nurmiaho-Lassila, E.L.; Ahvenainen, R.; Rhoades, J.; Roller, S. Chitosan disrupts the barrier properties of the outer membrane of gram-negative bacteria. Int. J. Food Microbiol. 2001, 71, 235–244. [Google Scholar] [CrossRef]
- Je, J.-Y.; Kim, S.-K. Chitosan derivatives killed bacteria by disrupting the outer and inner membrane. J. Agric. Food. Chem. 2006, 54, 6629–6633. [Google Scholar] [CrossRef] [PubMed]
- Raafat, D.; von Bargen, K.; Haas, A.; Sahl, H.-G. Insights into the mode of action of chitosan as an antibacterial compound. Appl. Environ. Microbiol. 2008, 74, 3764–3773. [Google Scholar] [CrossRef] [PubMed]
- Raafat, D.; Sahl, H.-G. Chitosan and its antimicrobial potential—A critical literature survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef] [PubMed]
- Cuero, R.G.; Osuji, G.; Washington, A. N-carboxymethylchitosan inhibition of aflatoxin production: Role of zinc. Biotechnol. Lett. 1991, 13, 441–444. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Montelongo, J.; Nascimento, V.F.; Murillo, D.; Taketa, T.B.; Sahoo, P.; de Souza, A.A.; Beppu, M.M.; Cotta, M.A. Nanofilms of hyaluronan/chitosan assembled layer-by-layer: An antibacterial surface for xylella fastidiosa. Carbohydr. Polym. 2016, 136, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.P.; Mano, J.F.; Queiroz, J.A.; Gouveia, I.C. Layer-by-layer deposition of antimicrobial polymers on cellulosic fibers: A new strategy to develop bioactive textiles. Polym. Adv. Technol. 2013, 24, 1005–1010. [Google Scholar] [CrossRef]
- Shima, S.; Sakai, H. Polylysine produced by strptomyces. Agric. Biol. Chem. 1977, 41, 1807–1809. [Google Scholar]
- Maeda, S.; Kunimoto, K.-K.; Sasaki, C.; Kuwae, A.; Hanai, K. Characterization of microbial poly (ε-l-lysine) by FT-IR, raman and solid state 13c NMR spectroscopies. J. Mol. Struct. 2003, 655, 149–155. [Google Scholar] [CrossRef]
- Geornaras, I.; Yoon, Y.; Belk, K.E.; Smith, G.C.; Sofos, J.N. Antimicrobial activity of ε-polylysine against Escherichia coli O157:H7, Salmonella typhimurium, and Listeria monocytogenes in various food extracts. J. Food Sci. 2007, 72, M330–M334. [Google Scholar] [CrossRef] [PubMed]
- Najjar, M.B.; Kashtanov, D.; Chikindas, M.L. ε-poly-l-lysine and nisin a act synergistically against Gram-positive food-borne pathogens bacillus cereus and listeria monocytogenes. Lett. Appl. Microbiol. 2007, 45, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Shima, S.; Matsuoka, H.; Iwamoto, T.; Sakai, H. Antimicrobial action of epsilon-poly-l-lysine. J. Antibiot. 1984, 37, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; McLandsborough, L.; McClements, D.J. Antimicrobial delivery systems based on electrostatic complexes of cationic ε-polylysine and anionic gum arabic. Food Hydrocoll. 2014, 35, 137–143. [Google Scholar] [CrossRef]
- Ye, R.; Xu, H.; Wan, C.; Peng, S.; Wang, L.; Xu, H.; Aguilar, Z.P.; Xiong, Y.; Zeng, Z.; Wei, H. Antibacterial activity and mechanism of action of ε-poly-l-lysine. Biochem. Biophys. Res. Commun. 2013, 439, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Moschonas, G.; Geornaras, I.; Stopforth, J.D.; Wach, D.; Woerner, D.R.; Belk, K.E.; Smith, G.C.; Sofos, J.N. Activity of caprylic acid, carvacrol, ε-polylysine and their combinations against salmonella in not-ready-to-eat surface-browned, frozen, breaded chicken products. J. Food Sci. 2012, 77, M405–M411. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Li, P.; Qi, X.; Sharif, A.R.M.; Poon, Y.F.; Cao, Y.; Chang, M.W.; Leong, S.S.J.; Chan-Park, M.B. A photopolymerized antimicrobial hydrogel coating derived from epsilon-poly-l-lysine. Biomaterials 2011, 32, 2704–2712. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.C. Review of the environmental toxicity of quaternary ammonium halides. Ecotoxicol. Environ. Saf. 1988, 16, 65–71. [Google Scholar] [CrossRef]
- Ikeda, T.; Tazuke, S. Synthesis and antimicrobial activity of poly(trialkylvinylbenzylammonium chloride)s. Makromol. Chem. 1984, 185, 869–876. [Google Scholar] [CrossRef]
- Kanazawa, A.; Ikeda, T.; Endo, T. Novel polycationic biocides: Synthesis and antibacterial activity of polymeric phosphonium salts. J. Polym. Sci. A Polym. Chem. 1993, 31, 335–343. [Google Scholar] [CrossRef]
- Kanazawa, A.; Ikeda, T.; Endo, T. Antibacterial activity of polymeric sulfonium salts. J. Polym. Sci. A Polym. Chem. 1993, 31, 2873–2876. [Google Scholar] [CrossRef]
- El-Newehy, M.H.; Kenawy, E.-R.; Al-Deyab, S.S. Biocidal polymers: Preparation and antimicrobial assessment of immobilized onium salts onto modified chitosan. Int. J. Polym. Mater. Polym. Biomater. 2014, 63, 758–766. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Abdel-Hay, F.I.; El-Shanshoury, A.E.-R.R.; El-Newehy, M.H. Biologically active polymers. V. Synthesis and antimicrobial activity of modified poly(glycidyl methacrylate-co-2-hydroxyethyl methacrylate) derivatives with quaternary ammonium and phosphonium salts. J. Polym. Sci. A Polym. Chem. 2002, 40, 2384–2393. [Google Scholar] [CrossRef]
- Wang, S.-W.; Liu, W.; Colby, R.H. Counterion dynamics in polyurethane-carboxylate ionomers with ionic liquid counterions. Chem. Mater. 2011, 23, 1862–1873. [Google Scholar] [CrossRef]
- Jangu, C.; Long, T.E. Phosphonium cation-containing polymers: From ionic liquids to polyelectrolytes. Polymer 2014, 55, 3298–3304. [Google Scholar] [CrossRef]
- Xue, Y.; Pan, Y.; Xiao, H.; Zhao, Y. Novel quaternary phosphonium-type cationic polyacrylamide and elucidation of dual-functional antibacterial/antiviral activity. RSC Adv. 2014, 4, 46887–46895. [Google Scholar] [CrossRef]
- Xue, Y.; Xiao, H. Characterization and antipathogenic evaluation of a novel quaternary phosphonium tri-polyacrylamide and elucidation of the inactivation mechanisms. J. Biomed. Mater. Res. A 2015. [Google Scholar] [CrossRef]
- Kougia, E.; Tselepi, M.; Vasilopoulos, G.; Lainioti, G.; Koromilas, N.; Druvari, D.; Bokias, G.; Vantarakis, A.; Kallitsis, J. Evaluation of antimicrobial efficiency of new polymers comprised by covalently attached and/or electrostatically bound bacteriostatic species, based on quaternary ammonium compounds. Molecules 2015, 20, 21313–21327. [Google Scholar] [CrossRef] [PubMed]
- Coelho, J.F.J.; Carvalho, E.Y.; Marques, D.S.; Popov, A.V.; Percec, V.; Gil, M.H. Influence of the isomeric structures of butyl acrylate on its single-electron transfer-degenerative chain transfer living radical polymerization in water catalyzed by Na2S2O4. J. Polym. Sci. A Polym. Chem. 2008, 46, 6542–6551. [Google Scholar] [CrossRef]
- Rocha, N.; Mendonça, P.V.; Mendes, J.P.; Simões, P.N.; Popov, A.V.; Guliashvili, T.; Serra, A.C.; Coelho, J.F.J. Facile synthesis of well-defined telechelic alkyne-terminated polystyrene in polar media using atrp with mixed Fe/Cu transition metal catalyst. Macromol. Chem. Phys. 2013, 214, 76–84. [Google Scholar] [CrossRef]
- Shipp, D.A. Reversible-deactivation radical polymerizations. Polym. Rev. 2011, 51, 99–103. [Google Scholar] [CrossRef]
- Qin, X.; Li, Y.; Zhou, F.; Ren, L.; Zhao, Y.; Yuan, X. Polydimethylsiloxane-polymethacrylate block copolymers tethering quaternary ammonium salt groups for antimicrobial coating. Appl. Surf. Sci. 2015, 328, 183–192. [Google Scholar] [CrossRef]
- Mi, L.; Jiang, S. Integrated antimicrobial and nonfouling zwitterionic polymers. Angew. Chem. Int. Ed. Engl. 2014, 53, 1746–1754. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Mi, L.; Mendiola, J.; Ella-Menye, J.R.; Zhang, L.; Xue, H.; Jiang, S. Reversibly switching the function of a surface between attacking and defending against bacteria. Angew. Chem. Int. Ed. Engl. 2012, 51, 2602–2605. [Google Scholar] [CrossRef] [PubMed]
- Thoma, L.M.; Boles, B.R.; Kuroda, K. Cationic methacrylate polymers as topical antimicrobial agents against Staphylococcus aureus nasal colonization. Biomacromolecules 2014, 15, 2933–2943. [Google Scholar] [CrossRef] [PubMed]
- Diamond, G.; Beckloff, N.; Weinberg, A.; Kisich, K.O. The roles of antimicrobial peptides in innate host defense. Curr. Pharm. Des. 2009, 15, 2377–2392. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef] [PubMed]
- Exley, S.E.; Paslay, L.C.; Sahukhal, G.S.; Abel, B.A.; Brown, T.D.; McCormick, C.L.; Heinhorst, S.; Koul, V.; Choudhary, V.; Elasri, M.O.; et al. Antimicrobial peptide mimicking primary amine and guanidine containing methacrylamide copolymers prepared by raft polymerization. Biomacromolecules 2015, 16, 3845–3852. [Google Scholar] [CrossRef] [PubMed]
- Yount, N.Y.; Yeaman, M.R. Emerging themes and therapeutic prospects for anti-infective peptides. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 337–360. [Google Scholar] [CrossRef] [PubMed]
- Fjell, C.D.; Hiss, J.A.; Hancock, R.E.; Schneider, G. Designing antimicrobial peptides: Form follows function. Nat. Rev. Drug Discov. 2012, 11, 37–51. [Google Scholar] [CrossRef]
- Lienkamp, K.; Madkour, A.E.; Musante, A.; Nelson, C.F.; Nüsslein, K.; Tew, G.N. Antimicrobial polymers prepared by romp with unprecedented selectivity: A molecular construction kit approach. J. Am. Chem. Soc. 2008, 130, 9836–9843. [Google Scholar] [CrossRef] [PubMed]
- Dohm, M.T.; Mowery, B.P.; Czyzewski, A.M.; Stahl, S.S.; Gellman, S.H.; Barron, A.E. Biophysical mimicry of lung surfactant protein b by random nylon-3 copolymers. J. Am. Chem. Soc. 2010, 132, 7957–7967. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, G.J.; Madkour, A.E.; Dabkowski, J.M.; Nelson, C.F.; Nüsslein, K.; Tew, G.N. Synthetic mimic of antimicrobial peptide with nonmembrane-disrupting antibacterial properties. Biomacromolecules 2008, 9, 2980–2983. [Google Scholar] [CrossRef] [PubMed]
- Bernatowicz, M.S.; Wu, Y.; Matsueda, G.R. 1H-pyrazole-1-carboxamidine hydrochloride an attractive reagent for guanylation of amines and its application to peptide synthesis. J. Org. Chem. 1992, 57, 2497–2502. [Google Scholar] [CrossRef]
- Michl, T.D.; Locock, K.E.S.; Stevens, N.E.; Hayball, J.D.; Vasilev, K.; Postma, A.; Qu, Y.; Traven, A.; Haeussler, M.; Meagher, L.; et al. Raft-derived antimicrobial polymethacrylates: Elucidating the impact of end-groups on activity and cytotoxicity. Polym. Chem. 2014, 5, 5813–5822. [Google Scholar] [CrossRef]
- Mowery, B.P.; Lindner, A.H.; Weisblum, B.; Stahl, S.S.; Gellman, S.H. Structure-activity relationships among random nylon-3 copolymers that mimic antibacterial host-defense peptides. J. Am. Chem. Soc. 2009, 131, 9735–9745. [Google Scholar] [CrossRef] [PubMed]
- Locock, K.E.S.; Michl, T.D.; Valentin, J.D.P.; Vasilev, K.; Hayball, J.D.; Qu, Y.; Traven, A.; Griesser, H.J.; Meagher, L.; Haeussler, M. Guanylated polymethacrylates: A class of potent antimicrobial polymers with low hemolytic activity. Biomacromolecules 2013, 14, 4021–4031. [Google Scholar] [CrossRef] [PubMed]
- Gruenheid, S.; Le Moual, H. Resistance to antimicrobial peptides in gram-negative bacteria. FEMS Microbiol. Lett. 2012, 330, 81–89. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Heine, E.; Keusgen, N.; Keul, H.; Möller, M. Synthesis and characterization of amphiphilic monodisperse compounds and poly(ethylene imine)s: Influence of their microstructures on the antimicrobial properties. Biomacromolecules 2012, 13, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Heine, E.T.; Keul, H.; Möller, M. Multifunctional poly(vinyl amine)s bearing azetidinium groups: One pot preparation in water and antimicrobial properties. Macromol. Biosci. 2014, 14, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Mourya, V.K.; Inamdar, N.N. Trimethyl chitosan and its applications in drug delivery. J. Mater. Sci. Mater. Med. 2009, 20, 1057–1079. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A.A.; Tanfani, F. The n-permethylation of chitosan and the preparation of n-trimethyl chitosan iodide. Carbohydr. Polym. 1985, 5, 297–307. [Google Scholar] [CrossRef]
- Dragostin, O.M.; Samal, S.K.; Dash, M.; Lupascu, F.; Pânzariu, A.; Tuchilus, C.; Ghetu, N.; Danciu, M.; Dubruel, P.; Pieptu, D.; et al. New antimicrobial chitosan derivatives for wound dressing applications. Carbohydr. Polym. 2016, 141, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Cheng, H.; Li, J.; Zhang, W.; Shen, Y.; Chen, S.; Ge, Z.; Chen, S. Enhanced water-solubility and antibacterial activity of novel chitosan derivatives modified with quaternary phosphonium salt. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-H.; Liu, W.-S.; Sun, J.-F.; Hou, G.-G.; Chen, Q.; Cong, W.; Zhao, F. Non-toxic o-quaternized chitosan materials with better water solubility and antimicrobial function. Int. J. Biol. Macromol. 2016, 84, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Inta, O.; Yoksan, R.; Limtrakul, J. Hydrophobically modified chitosan: A bio-based material for antimicrobial active film. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 42, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, I.M. Current perspectives of nanoparticles in medical and dental biomaterials. J. Biomed. Res. 2012, 26, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, D.K.; Behari, J.; Sen, P. Application of nanoparticles in waste water treatment. World Appl. Sci. J. 2008, 3, 417–433. [Google Scholar]
- Donaldson, K.; Stone, V.; Tran, C.L.; Kreyling, W.; Borm, P.J.A. Nanotoxicology. Occup. Environ. Med. 2004, 61, 727–728. [Google Scholar] [CrossRef] [PubMed]
- Hussain, F.; Hojjati, M.; Okamoto, M.; Gorga, R.E. Review article: Polymer-matrix nanocomposites, processing, manufacturing, and application: An overview. J. Compos. Mater. 2006, 40, 1511–1575. [Google Scholar] [CrossRef]
- Anh, D.H.; Dumri, K.; Anh, N.T.; Punyodom, W.; Rachtanapun, P. Facile fabrication of polyethylene/silver nanoparticle nanocomposites with silver nanoparticles traps and holds early antibacterial effect. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Madkour, T.M.; Abdelazeem, E.A.; Tayel, A.; Mustafa, G.; Siam, R. In situ polymerization of polyurethane-silver nanocomposite foams with intact thermal stability, improved mechanical performance, and induced antimicrobial properties. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Khare, P.; Sharma, A.; Verma, N. Synthesis of phenolic precursor-based porous carbon beads in situ dispersed with copper-silver bimetal nanoparticles for antibacterial applications. J. Colloid Interface Sci. 2014, 418, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Quiros, J.; Borges, J.P.; Boltes, K.; Rodea-Palomares, I.; Rosal, R. Antimicrobial electrospun silver-, copper- and zinc-doped polyvinylpyrrolidone nanofibers. J. Hazard. Mater. 2015, 299, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Palza, H.; Quijada, R.; Delgado, K. Antimicrobial polymer composites with copper micro- and nanoparticles: Effect of particle size and polymer matrix. J. Bioact. Compat. Polym. 2015, 30, 366–380. [Google Scholar] [CrossRef]
- Rahman, P.M.; Muraleedaran, K.; Mujeeb, V.M.A. Applications of chitosan powder with in situ synthesized nano zno particles as an antimicrobial agent. Int. J. Biol. Macromol. 2015, 77, 266–272. [Google Scholar]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [PubMed]
- Sharma, N.; Jandaik, S.; Kumar, S.; Chitkara, M.; Sandhu, I.S. Synthesis, characterisation and antimicrobial activity of manganese- and iron-doped zinc oxide nanoparticles. J. Exp. Nanosci. 2016, 11, 54–71. [Google Scholar] [CrossRef]
- Yamamoto, O. Influence of particle size on the antibacterial activity of zinc oxide. Int. J. Inorg. Mater. 2001, 3, 643–646. [Google Scholar] [CrossRef]
- Brayner, R.; Ferrari-Iliou, R.; Brivois, N.; Djediat, S.; Benedetti, M.F.; Fiévet, F. Toxicological impact studies based on Escherichia coli bacteria in ultrafine zno nanoparticles colloidal medium. Nano Lett. 2006, 6, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Xu, Y.; Huang, C.-C.; Ma, Y.; Shannon, K.B.; Chen, D.-R.; Huang, Y.-W. Toxicity of nano- and micro-sized zno particles in human lung epithelial cells. J. Nanopart. Res. 2008, 11, 25–39. [Google Scholar] [CrossRef]
- Brooks, B.D.; Brooks, A.E. Therapeutic strategies to combat antibiotic resistance. Adv. Drug Deliv. Rev. 2014, 78, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Ng, V.W.L.; Chan, J.M.W.; Sardon, H.; Ono, R.J.; García, J.M.; Yang, Y.Y.; Hedrick, J.L. Antimicrobial hydrogels: A new weapon in the arsenal against multidrug-resistant infections. Adv. Drug Deliv. Rev. 2014, 78, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.-H.; Bao, Y.; Yang, X.-Z.; Zhu, Y.-H.; Wang, J. Delivery of antibiotics with polymeric particles. Adv. Drug Deliv. Rev. 2014, 78, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Jassim, S.A.; Limoges, R.G. Natural solution to antibiotic resistance: Bacteriophages ‘the living drugs’. World J. Microbiol. Biotechnol. 2014, 30, 2153–2170. [Google Scholar] [CrossRef] [PubMed]
- Landers, T.F.; Cohen, B.; Wittum, T.E.; Larson, E.L. A review of antibiotic use in food animals: Perspective, policy, and potential. Public Health Rep. 2012, 127, 4–22. [Google Scholar] [PubMed]
- Stebbins, N.D.; Ouimet, M.A.; Uhrich, K.E. Antibiotic-containing polymers for localized, sustained drug delivery. Adv. Drug Deliv. Rev. 2014, 78, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Madureira, A.R.; Pereira, A.; Pintado, M. Current state on the development of nanoparticles for use against bacterial gastrointestinal pathogens. Focus on chitosan nanoparticles loaded with phenolic compounds. Carbohydr. Polym. 2015, 130, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Briones, E.; Isabel Colino, C.; Lanao, J.M. Delivery systems to increase the selectivity of antibiotics in phagocytic cells. J. Control. Release 2008, 125, 210–227. [Google Scholar] [CrossRef] [PubMed]
- Kalhapure, R.S.; Suleman, N.; Mocktar, C.; Seedat, N.; Govender, T. Nanoengineered drug delivery systems for enhancing antibiotic therapy. J. Pharm. Sci. 2015, 104, 872–905. [Google Scholar] [CrossRef] [PubMed]
- Goganian, A.M.; Hamishehkar, H.; Arsalani, N.; Khiabani, H.K. Microwave-promoted synthesis of smart superporous hydrogel for the development of gastroretentive drug delivery system. Adv. Powder Technol. 2015, 34. [Google Scholar] [CrossRef]
- Gustafson, C.T.; Boakye-Agyeman, F.; Brinkman, C.L.; Reid, J.M.; Patel, R.; Bajzer, Z.; Dadsetan, M.; Yaszemski, M.J. Controlled delivery of vancomycin via charged hydrogels. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Steffensen, S.L.; Vestergaard, M.H.; Møller, E.H.; Groenning, M.; Alm, M.; Franzyk, H.; Nielsen, H.M. Soft hydrogels interpenetrating silicone—A polymer network for drug-releasing medical devices. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Guadalupe, E.; Ramos, D.; Shelke, N.B.; James, R.; Gibney, C.; Kumbar, S.G. Bioactive polymeric nanofiber matrices for skin regeneration. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Jalvandi, J.; White, M.; Truong, Y.B.; Gao, Y.; Padhye, R.; Kyratzis, I.L. Release and antimicrobial activity of levofloxacin from composite mats of poly(ε-caprolactone) and mesoporous silica nanoparticles fabricated by core-shell electrospinning. J. Mater. Sci. 2015, 50, 7967–7974. [Google Scholar] [CrossRef]
- Zupančič, Š.; Sinha-Ray, S.; Sinha-Ray, S.; Kristl, J.; Yarin, A.L. Long-term sustained ciprofloxacin release from pmma and hydrophilic polymer blended nanofibers. Mol. Pharm. 2016, 13, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Dizman, B.; Elasri, M.O.; Mathias, L.J. Synthesis, characterization, and antibacterial activities of novel methacrylate polymers containing norfloxacin. Biomacromolecules 2005, 6, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Srinivasan, S.; Kelly, A.M.; Chiu, D.Y.; Daugherty, B.K.; Ratner, D.M.; Stayton, P.S.; Convertine, A.J. Raft polymerization of ciprofloxacin prodrug monomers for the controlled intracellular delivery of antibiotics. Polym. Chem. 2016, 7, 826–837. [Google Scholar] [CrossRef]
- Adler-Moore, J.P.; Proffitt, R.T. Novel drug delivery systems for antifungal agents. In Human Fungal Pathogens; Domer, J.E., Kobayashi, G.S., Eds.; Springer: Berlin/Heidleberg, Germany, 2004. [Google Scholar]
- Güngör, S.; Erdal, M.S.; Aksu, B. New formulations strategies in topical antifungal therapy. JCDSA 2013, 3, 56–65. [Google Scholar] [CrossRef]
- Johal, H.S.; Garg, T.; Rath, G.; Goyal, A.K. Advanced topical drug delivery system for the management of vaginal candidiasis. Drug Deliv. 2016, 23, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Mendes, S.; Camacho, F.; Silva, T.; Calado, C.R.C.; Serra, A.C.; Rocha Gonsalves, A.M.D.A.; Roxo-Rosa, M. A nonionic porphyrin as a noninterfering DNA antibacterial agent. Photochem. Photobiol. 2011, 87, 1395–1404. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, Â.; Almeida, A. Photodynamic inactivation of mammalian viruses and bacteriophages. Viruses 2012, 4, 1034–1074. [Google Scholar] [CrossRef] [PubMed]
- Noimark, S.; Allan, E.; Parkin, I.P. Light-activated antimicrobial surfaces with enhanced efficacy induced by a dark-activated mechanism. Chem. Sci. 2014, 5, 2216–2223. [Google Scholar] [CrossRef]
- Winter, S.; Tortik, N.; Kubin, A.; Krammer, B.; Plaetzer, K. Back to the roots: Photodynamic inactivation of bacteria based on water-soluble curcumin bound to polyvinylpyrrolidone as a photosensitizer. Photochem. Photobiol. Sci. 2013, 12, 1795–1802. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yin, B.; Ma, H.; Yuan, H.; Fu, B.; Liu, L. Synthesis of a novel quinoline skeleton introduced cationic polyfluorene derivative for multimodal antimicrobial application. ACS Appl. Mater. Interfaces 2015, 7, 25390–25395. [Google Scholar] [CrossRef] [PubMed]

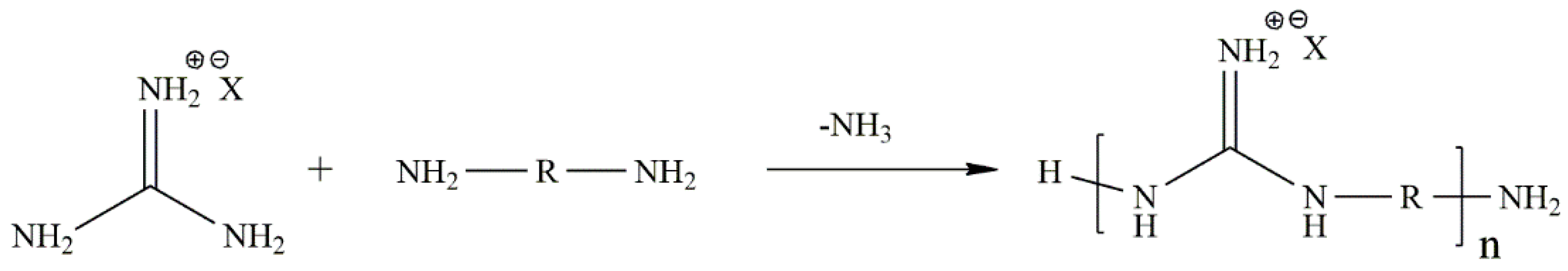
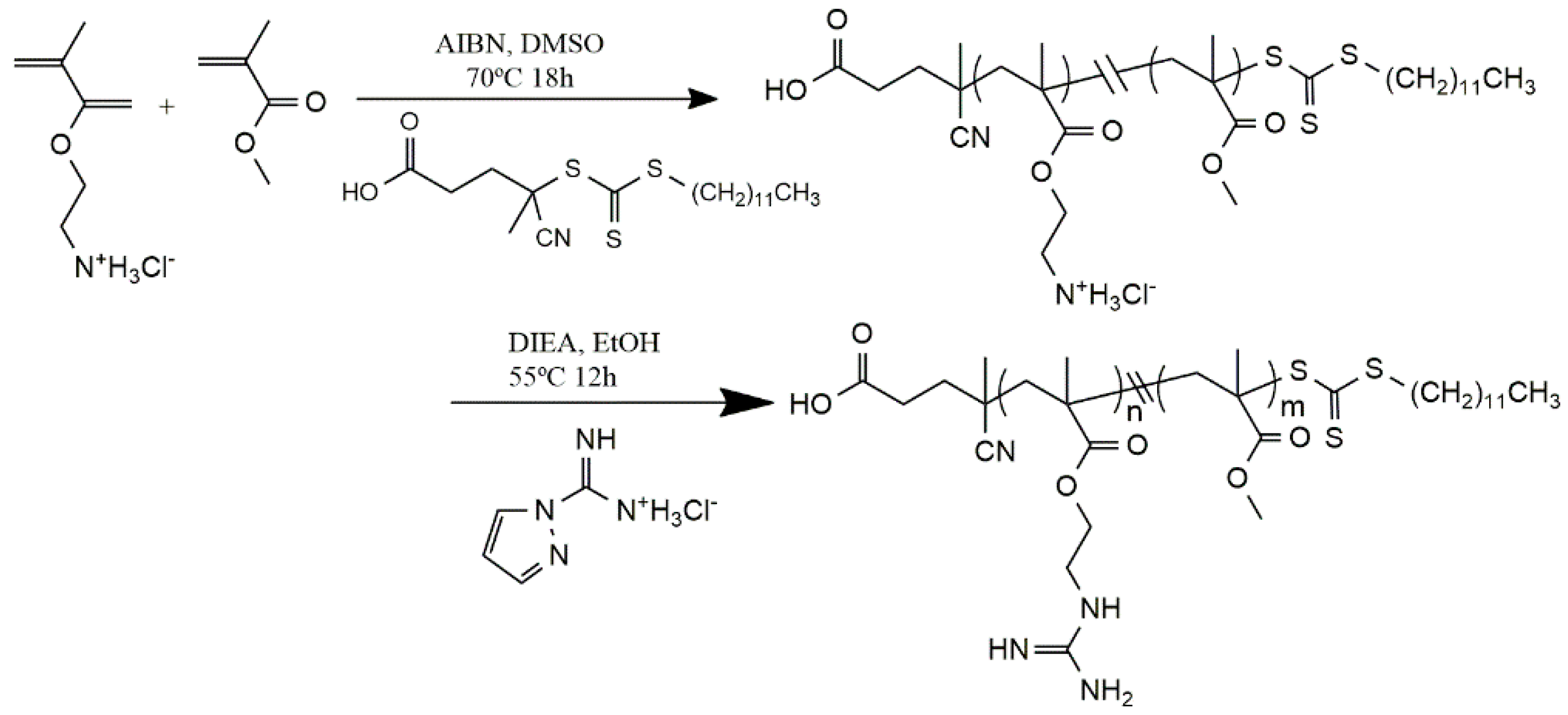
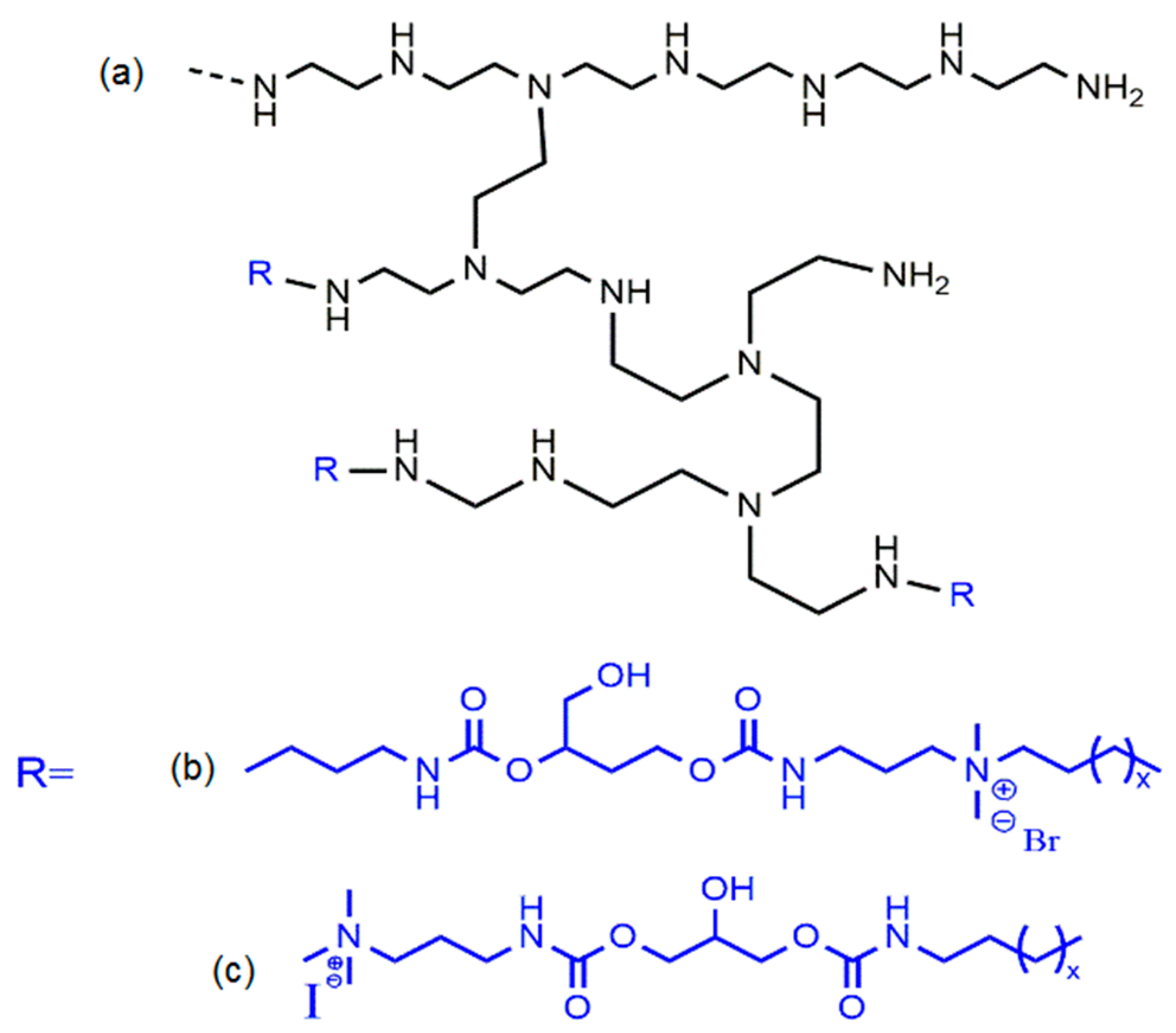
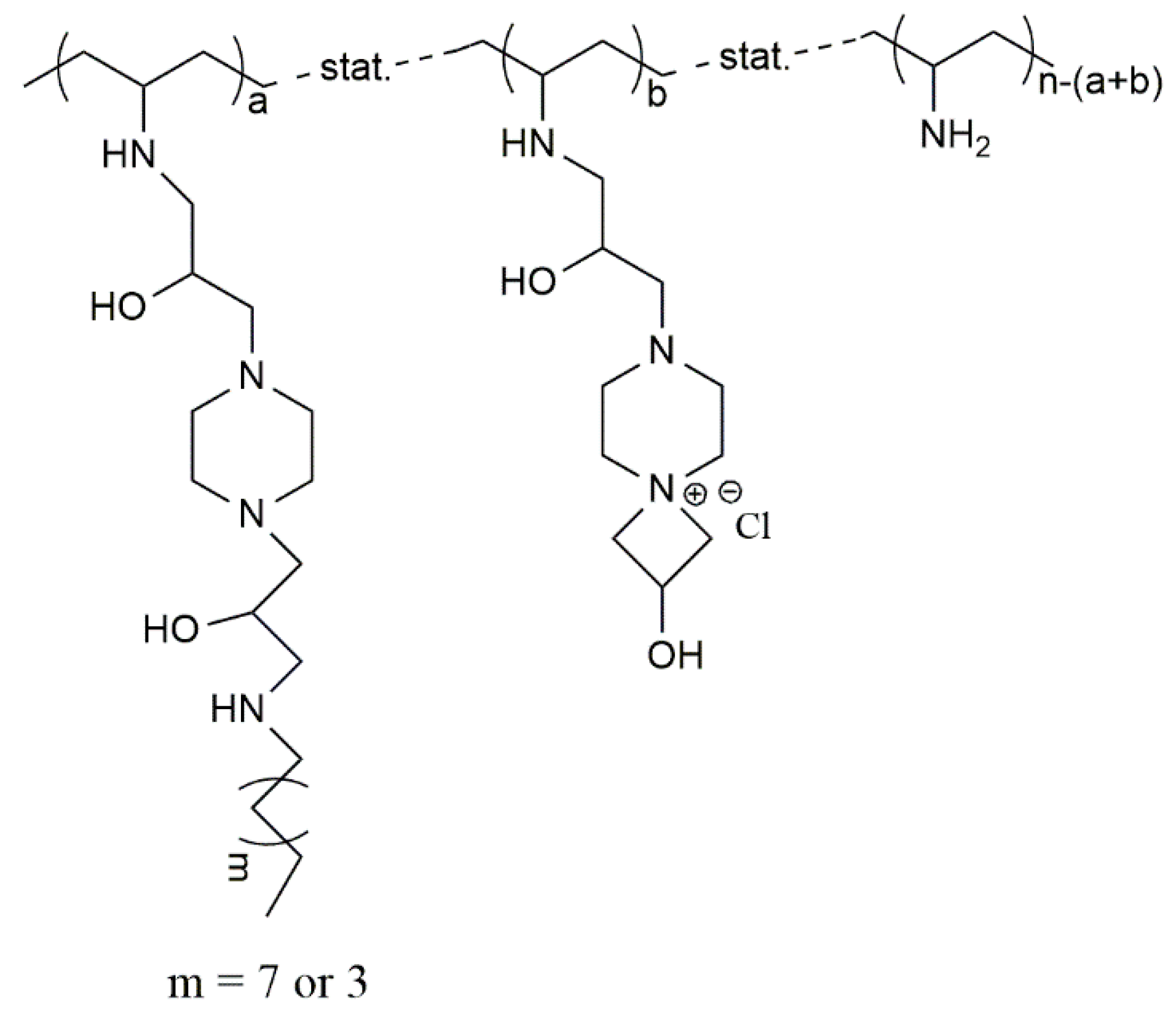
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, M.R.E.; Fonseca, A.C.; Mendonça, P.V.; Branco, R.; Serra, A.C.; Morais, P.V.; Coelho, J.F.J. Recent Developments in Antimicrobial Polymers: A Review. Materials 2016, 9, 599. https://doi.org/10.3390/ma9070599
Santos MRE, Fonseca AC, Mendonça PV, Branco R, Serra AC, Morais PV, Coelho JFJ. Recent Developments in Antimicrobial Polymers: A Review. Materials. 2016; 9(7):599. https://doi.org/10.3390/ma9070599
Chicago/Turabian StyleSantos, Madson R. E., Ana C. Fonseca, Patrícia V. Mendonça, Rita Branco, Arménio C. Serra, Paula V. Morais, and Jorge F. J. Coelho. 2016. "Recent Developments in Antimicrobial Polymers: A Review" Materials 9, no. 7: 599. https://doi.org/10.3390/ma9070599
APA StyleSantos, M. R. E., Fonseca, A. C., Mendonça, P. V., Branco, R., Serra, A. C., Morais, P. V., & Coelho, J. F. J. (2016). Recent Developments in Antimicrobial Polymers: A Review. Materials, 9(7), 599. https://doi.org/10.3390/ma9070599