Spectroscopic Study of Plasma Polymerized a-C:H Films Deposited by a Dielectric Barrier Discharge
Abstract
:1. Introduction
2. Experimental
2.1. Fourier Transform Infrared Reflection Absorption Spectroscopy (FT-IRRAS)
2.2. Raman Spectroscopy
2.3. Spectroscopic Ellipsometry (SE)
3. Results and Discussion
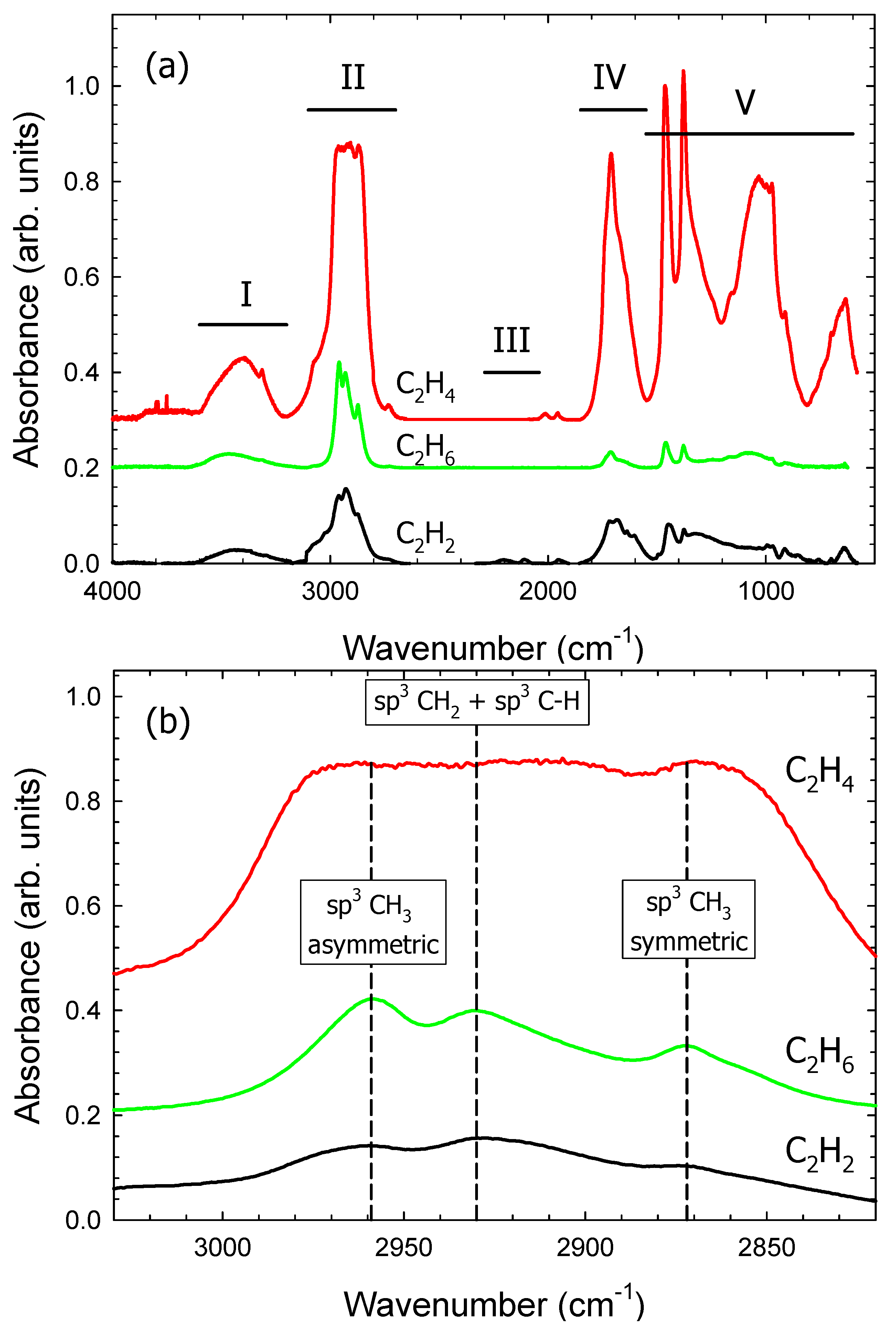
3.1. Fourier Transform Infrared Reflection Absorption Spectroscopy (FT-IRRAS)
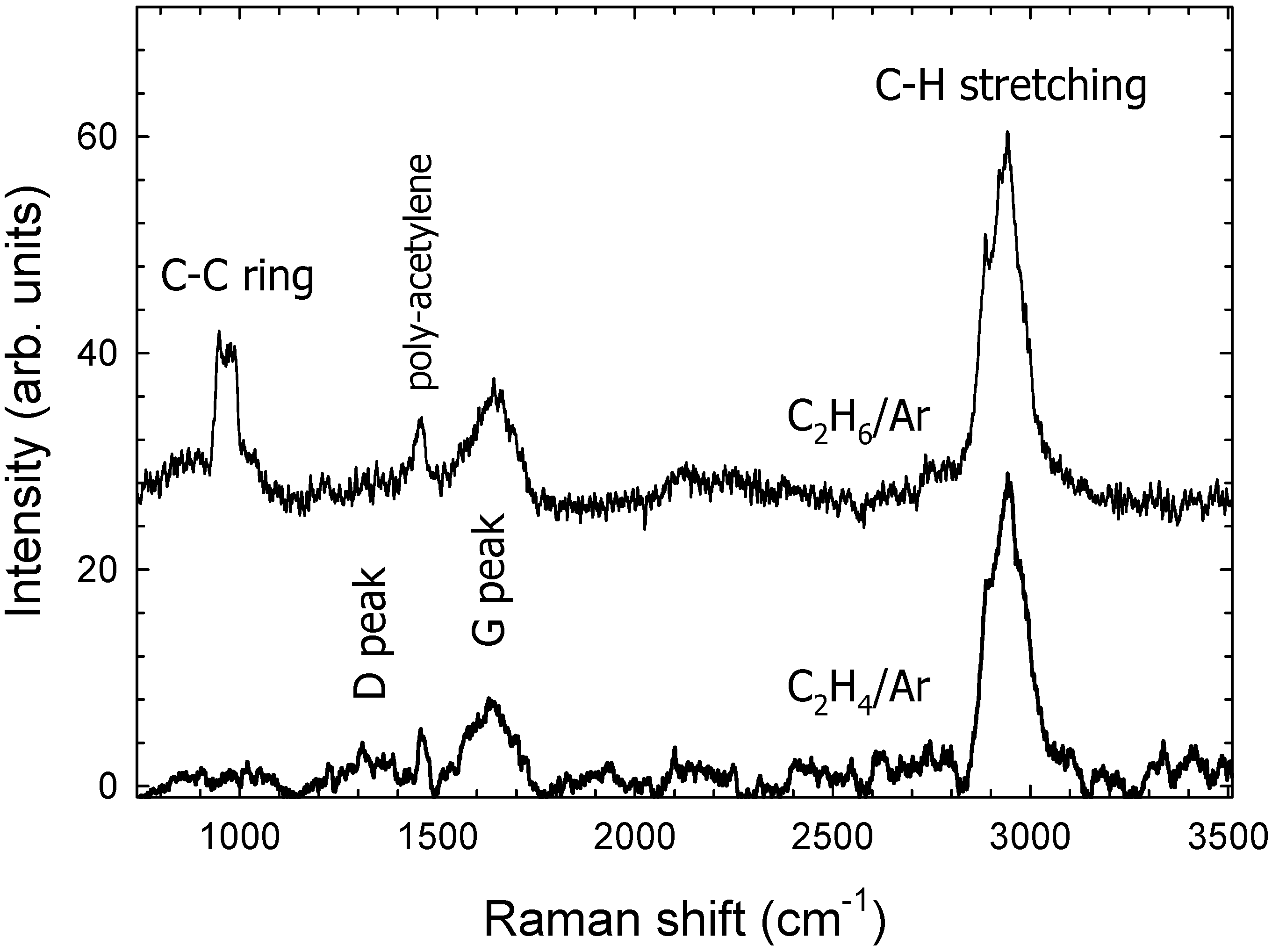
3.2. Raman Spectroscopy
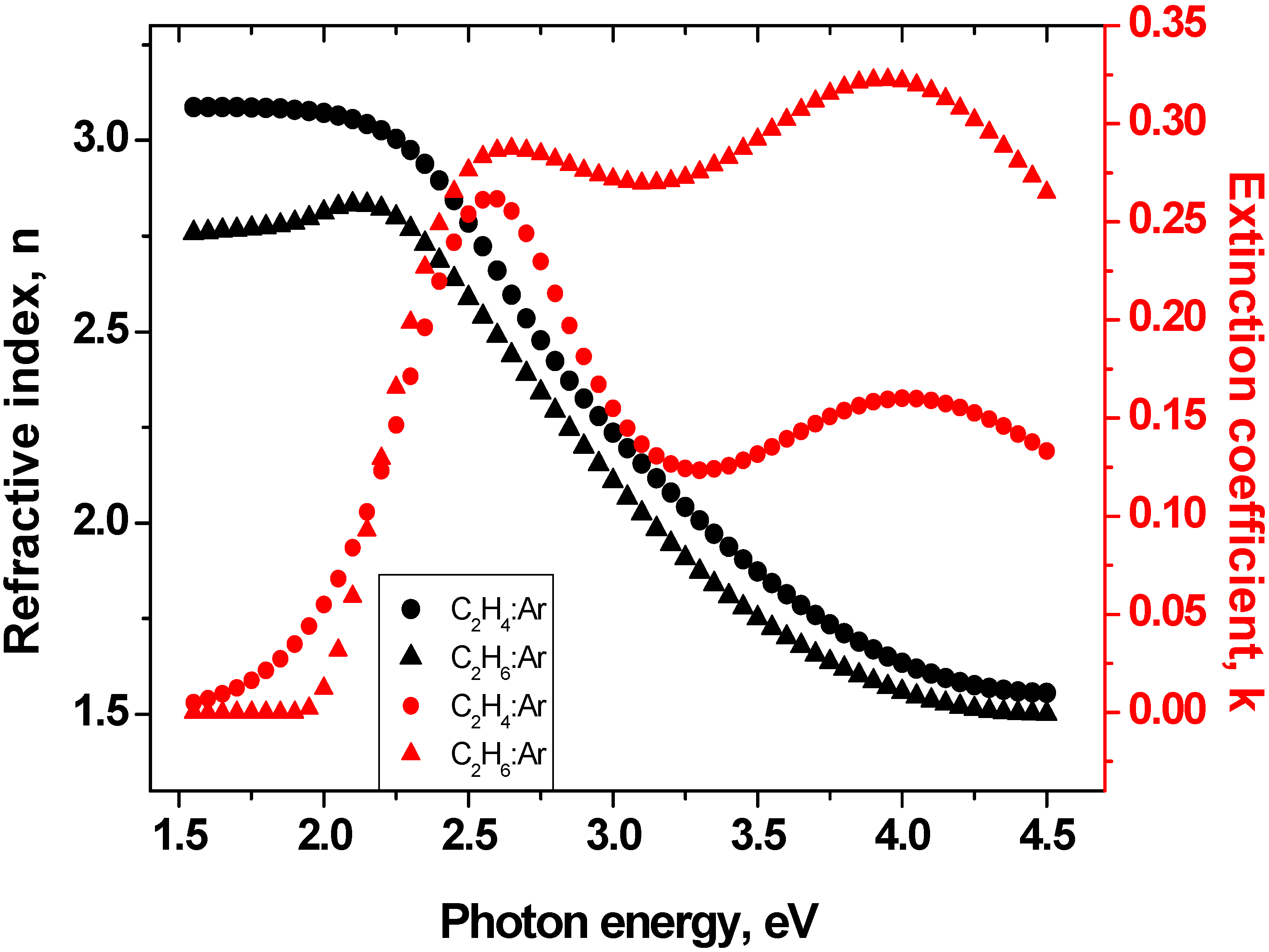
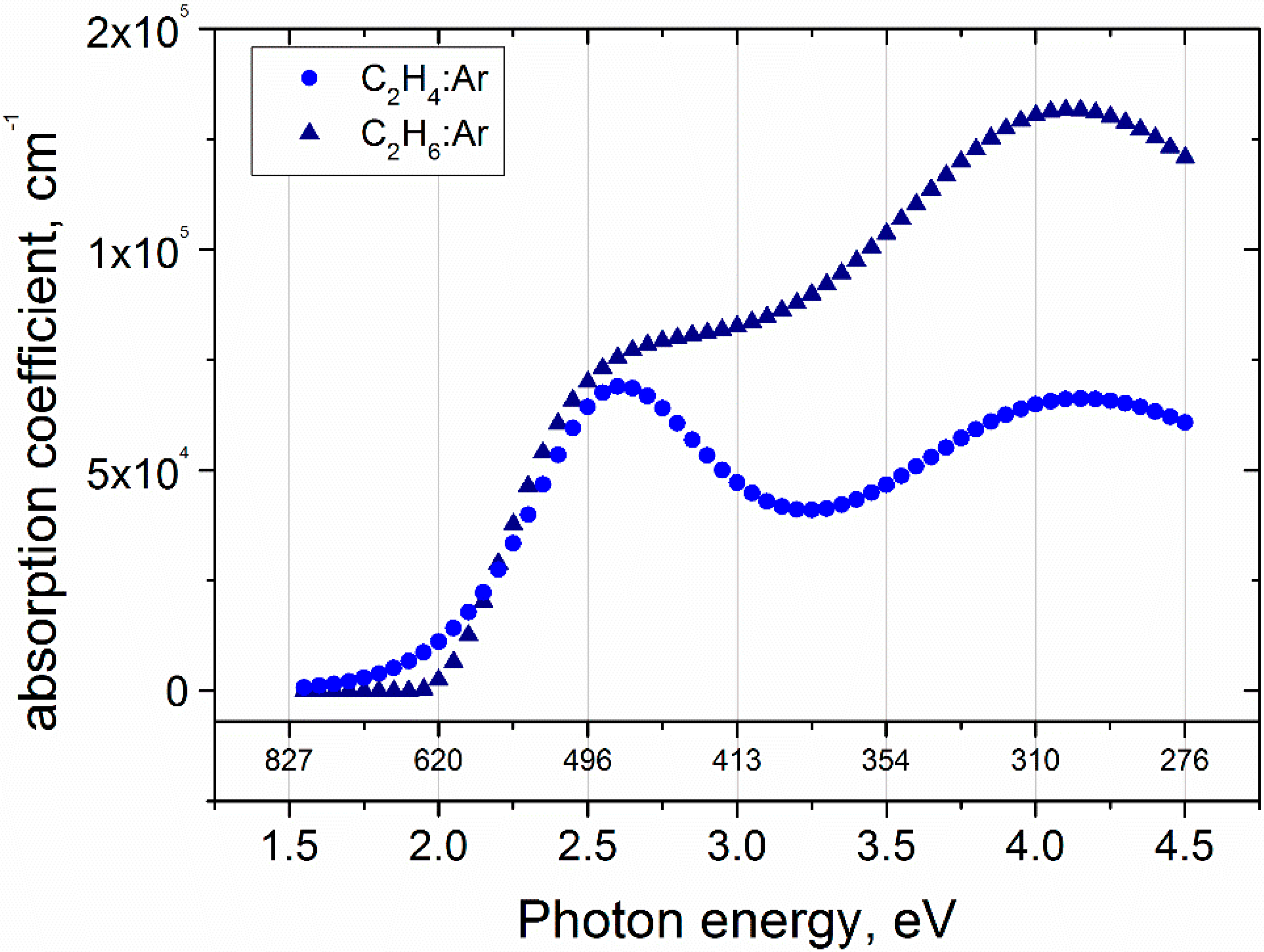
3.3. Ellipsometry
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, A.Y.; Cohen, M.L. Prediction of New Low Compressibility Solids. Science 1989, 245, 841. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.Y.; Cohen, M.L. Structural properties and electronic structure of low-compressibility materials: β-Si3N4 and hypothetical β-C3N4. Phys. Rev. B 1990, 41, 10727. [Google Scholar] [CrossRef]
- Rodil, S.E.; Ferrari, A.C.; Robertson, J.; Muhl, S. Infrared spectra of carbon nitride films. Thin Solid Films 2002, 420, 122. [Google Scholar] [CrossRef]
- Thejaswini, H.C.; Hoskinson, A.R.; Agasanapura, B.; Grunde, M.; Hopwood, J. Deposition and characterization of diamond-like carbon films by microwave resonator microplasma at one atmosphere. Diam. Rel. Mater. 2014, 48, 24. [Google Scholar] [CrossRef]
- Pothiraja, R.; Bibinov, N.; Awakowicz, P. Plasma-Chemical Kinetics of Film Deposition in Argon-Methane and Argon-Acetylene Mixtures under Atmospheric Pressure Conditions. In Chemical Kinetics; Patel, V., Ed.; InTech: Rijeka, Croatia, 2012; Chapter 11; Available online: http://www.intechopen.com/books/chemical-kinetics/plasma-chemical-kinetics-of-film-deposition-in-argonmethane-and-argon-acetylene-mixtures-under-atmo (accessed on 21 April 2016).
- Casiraghi, C.; Piazza, F.; Ferrari, A.C.; Grambole, D.; Robertson, J. Bonding in hydrogenated diamond-like carbon by Raman spectroscopy. Diam. Rel. Mater. 2005, 14, 1098. [Google Scholar] [CrossRef]
- Cui, F.Z.; Qing, X.L.; Zhao, J. Biomedical investigations on CNx coating. Surf. Coat. Technol. 2005, 200, 1009. [Google Scholar] [CrossRef]
- Kato, K.; Koide, H.; Umehara, N. Micro-wear properties of carbon nitride coatings. Wear 2000, 238, 40. [Google Scholar] [CrossRef]
- Godet, C.; Adamopoulos, G.; Kumar, S.; Katsuno, T. Optical and electronic properties of plasma-deposited hydrogenated amorphous carbon nitride and carbon oxide films. Thin Solid Films 2005, 482, 24. [Google Scholar] [CrossRef]
- Godet, C.; Kleider, J.P.; Gudovskikh, A.S. Scaling analysis of field-enhanced bandtail hopping transport in amorphous carbon nitride. Phys. Status Solidi B 2007, 244, 2081. [Google Scholar] [CrossRef]
- Kumar, A.; Srivastava, A.; Galaev, I.Y.; Mattiasson, B. Smart polymers: Physical forms and bioengineering applications. Prog. Polym. Sci. 2007, 32, 1205–1237. [Google Scholar] [CrossRef]
- Pucci, A.; Ruggeri, G.; Bronco, S.; Signori, F.; Donati, F.; Bernabo, M.; Ciardelli, F. Colour responsive smart polymers and biopolymers films through nanodispersion of organic chromophores and metal particles. Prog. Org. Coat. 2011, 72, 21–25. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, H.S.; Augustine, S.; Kang, J.K. Nanopores in carbon nitride nanotubes: Reversible hydrogen storage sites. Appl. Phys. Lett. 2006, 89, 253119. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Z.; Li, H. Crystalline carbon nitride films formation by chemical vapor deposition. Appl. Phys. Lett. 1996, 68, 634. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.; Liu, Y.; Chen, T.P.; Sritharan, T.; Xu, C. Magnetron sputtered nc-Al/α-Al2O3 nanocomposite thin films for nonvolatile memory application. J. Nanosci. Nanotech. 2009, 9, 4116. [Google Scholar] [CrossRef]
- Wu, D.; Fu, D.J.; Guo, H.; Zhang, Z.; Meng, X.; Fan, X. Structure and characteristics of C3N4 thin films prepared by rf plasma-enhanced chemical vapor deposition. Phys. Rev. B 1997, 56, 4949. [Google Scholar]
- Merel, P.; Chaker, M.; Tabbal, M.; Moisan, M. The influence of atomic nitrogen flux on the composition of carbon nitride thin films. Appl. Phys. Lett. 1997, 71, 3814. [Google Scholar] [CrossRef]
- Reitz, U.; Salge, J.G.H.; Schwarz, R. Pulsed barrier discharges for thin film production at atmospheric pressure. Surf. Coat. Technol. 1993, 59, 144. [Google Scholar] [CrossRef]
- Goossens, O.; Dekempeneer, E.; Vangeneugden, D.; van de Leest, R.; Leys, C. Application of atmospheric pressure dielectric barrier discharges in deposition, cleaning and activation. Surf. Coat. Technol. 2001, 142–144, 474. [Google Scholar] [CrossRef]
- Massines, F.; Sarra-Bournet, C.; Fanelli, F.; Naude, N.; Gherardi, N. Atmospheric Pressure Low Temperature Direct Plasma Technology: Status and Challenges for Thin Film Deposition. Plasma Process. Polym. 2012, 9, 1041. [Google Scholar] [CrossRef]
- Kogelschatz, U. Dielectric-Barrier Discharges: Their History, Discharge Physics, and Industrial Applications. Plasma Chem. Plasma Process. 2003, 23, 1. [Google Scholar] [CrossRef]
- Thejaswini, H.C.; Peglow, S.; Martens, U.; Sushkov, V.; Hippler, R. Comparative Plasma Chemical Reaction Studies of CH4/Ar and C2Hm/Ar (m = 2, 4, 6) Gas Mixtures in a Dielectric Barrier Discharge. Contrib. Plasma Phys. 2014, 54, 683–696. [Google Scholar] [CrossRef]
- Thejaswini, H.C.; Majumdar, A.; Tun, T.M.; Hippler, R. Plasma chemical reactions in C2H2/N2, C2H4/N2, and C2H6/N2 gas mixtures of a laboratory dielectric barrier discharge. Adv. Space Res. 2011, 48, 857. [Google Scholar] [CrossRef]
- Majumdar, A.; Behnke, J.F.; Hippler, R.; Matyash, K.; Schneider, R. Chemical Reaction Studies in CH4/Ar and CH4/N2 Gas Mixtures of a Dielectric Barrier Discharge. J. Phys. Chem. A 2005, 109, 9371. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, A.; Hippler, R. Development of dielectric barrier discharge plasma processing apparatus for mass spectrometry and thin film deposition. Rev. Sci. Instr. 2007, 78, 075103. [Google Scholar] [CrossRef] [PubMed]
- Martens, U.; Thejaswini, H.C.; Majumdar, A.; Hippler, R. Deposition of Amorphous Hydrogenated Carbon Nitride Films with a Dielectric Barrier Discharge. Plasma Proc. Polym. 2012, 9, 647. [Google Scholar] [CrossRef]
- Shirakura, A.; Nakaya, M.; Koga, Y.; Kodama, H.; Hasebe, T.; Suzuki, T. Diamond-like carbon films for PET bottles and medical applications. Thin Solid Films 2006, 494, 84–91. [Google Scholar] [CrossRef]
- Girard, H.A.; Arnault, J.C.; Perruchas, S.; Saada, S.; Gacoin, T.; Boilot, J.P.; Bergonozo, P. Hydrogenation of nanodiamonds using MPCVD: A new route toward organic functionalization. Diam. Rel. Mater. 2010, 19, 1117–1123. [Google Scholar] [CrossRef]
- Robertson, J. Diamond-like amorphous carbon. Mater. Sci. Eng. R 2002, 37, 129. [Google Scholar] [CrossRef]
- Lazar, G.; Zellama, K.; Vascan, I.; Stamate, M.; Lazar, I.; Rusu, I. Infrared absorption properties of amorphous carbon films. J. Optoelectron. Adv. Mater. 2005, 7, 647. [Google Scholar]
- Stan, G.E.; Marcov, D.A.; Popa, A.C.; Husanu, M.A. Polymer-like and diamond-like carbon coatings prepared by RF-PECVD for biomedical applications. Dig. J. Nanaomater. Biostruct. 2010, 5, 705. [Google Scholar]
- Bian, X.; Chen, Q.; Zhang, Y.; Sang, L.; Tang, W. Deposition of nano-diamond-like carbon films by an atmospheric pressure plasma gun and diagnostic by optical emission spectrum on the process. Surf. Coat. Tech. 2008, 202, 5383. [Google Scholar] [CrossRef]
- Li, H.; Xu, T.; Wang, C.; Chen, J.; Zhou, H.; Liu, H. Annealing effect on the structure, mechanical and tribological properties of hydrogenated diamond-like carbon films. Thin Solid Films 2006, 515, 2153. [Google Scholar] [CrossRef]
- Bugaev, S.P.; Korotaev, A.D.; Oskomov, K.V.; Sochugov, N.S. a-C:H films deposited in the plasma of barrier and surface discharges at atmospheric pressure. Surf. Coat. Tech. 1997, 96, 123. [Google Scholar] [CrossRef]
- Thejaswini, H.C.; Bogdanowicz, R.; Schaefer, J.; Danilov, V.; Meichsner, J.; Hippler, R. Deposition and characterization of organic polymer thin films using a dielectric barrier discharge with different C2Hm/N2 (m = 2, 4, 6) gas mixtures. Eur. Phys. J. D 2015, 69, 142. [Google Scholar]
- Hobert, H.; Dunken, H.H.; Meinschien, J.; Stafast, H. Infrared and Raman spectroscopic investigation of thin films of AlN and SiC on Si substrates. Vib. Spectrosc. 1999, 19, 205. [Google Scholar] [CrossRef]
- Musa, G.; Vladoiu, R.; Ciupina, V.; Janik, J. Raman spectra of carbon thin films. J. Optoelectron. Adv. Mater. 2006, 8, 621. [Google Scholar]
- Marcinauskas, L.; Grigonis, A.; Valincius, V.; Valatkevicius, P. Surface and Structural Analysis of Carbon Coatings Produced by Plasma Jet CVD. Mater. Sci. 2007, 13, 269. [Google Scholar]
- Oliveira, É.C.; Cruz, S.A.; Aguiar, P.H.L. Effect of PECVD deposition parameters on the DLC/PLC composition of a-C:H thin films. J. Braz. Chem. Soc. 2012, 23, 1657. [Google Scholar] [CrossRef]
- Gioti, M.; Papadimitriou, D.; Logothetidis, S. Optical properties and new vibrational modes in carbon films. Diam. Rel. Mater. 2000, 9, 741. [Google Scholar] [CrossRef]
- Logothetidis, S.; Gioti, M.; Patsalas, P.; Charitidis, C. Insights on the deposition mechanism of sputtered amorphous carbon films. Carbon 1999, 37, 765. [Google Scholar] [CrossRef]
- Gioti, M.; Logothetidis, S. Dielectric function, electronic properties and optical constants of amorphous carbon and carbon nitride films. Diam. Rel. Mater. 2003, 12, 957. [Google Scholar] [CrossRef]
- Jellison, G.E.; Modine, F.A. Parameterization of the optical functions of amorphous materials in the interband region. Appl. Phys. Lett. 1996, 69, 371. [Google Scholar] [CrossRef]
- Palik, E.D. Handbook of Optical Constants of Solids II; Academic Press: San Diego, CA, USA, 1991. [Google Scholar]
- Tompkins, H.G.; Irene, E.A. Handbook of Ellipsometry; William Andrew: Norwich, UK, 2005. [Google Scholar]
- Benmassaoud, A.A.; Paynter, R.W. Influence of the power and pressure on the growth rate and refractive index of a-C:H thin films deposited by r.f. plasma-enhanced chemical vapour deposition. Thin Solid Films 1996, 287, 125–129. [Google Scholar] [CrossRef]
- Hong, J.; Goullet, A.; Turban, G. Ellipsometry and Raman study on hydrogenated amorphous carbon (a-C:H) films deposited in a dual ECR-r.f. plasma. Thin Solid Films 1999, 352, 41–48. [Google Scholar] [CrossRef]
- Tolmachiev, V.A.; Konshina, E.A. Ellipsometric study of a-C:H films. Diam. Relat. Mater. 1996, 5, 1397–1401. [Google Scholar] [CrossRef]
- Zaharia, T.; Sullivan, J.L.; Saied, S.O.; Bosch, R.C.M.; Bijker, M.D. Fast deposition of diamond-like hydrogenated carbon films. Diam. Relat. Mater. 2007, 16, 623–629. [Google Scholar] [CrossRef]
- Xu, S.; Tay, B.K.; Tan, H.S.; Zhong, L.; Tu, Y.Q.; Silva, S.R.P.; Milne, W.I. Properties of carbon ion deposited tetrahedral amorphous carbon films as a function of ion energy. J. Appl. Phys. 1996, 79, 7234–7240. [Google Scholar] [CrossRef]
- Tauc, J.; Abelès, F. The Optical Properties of Solids; Academic Press: New York, NY, USA, 1972. [Google Scholar]
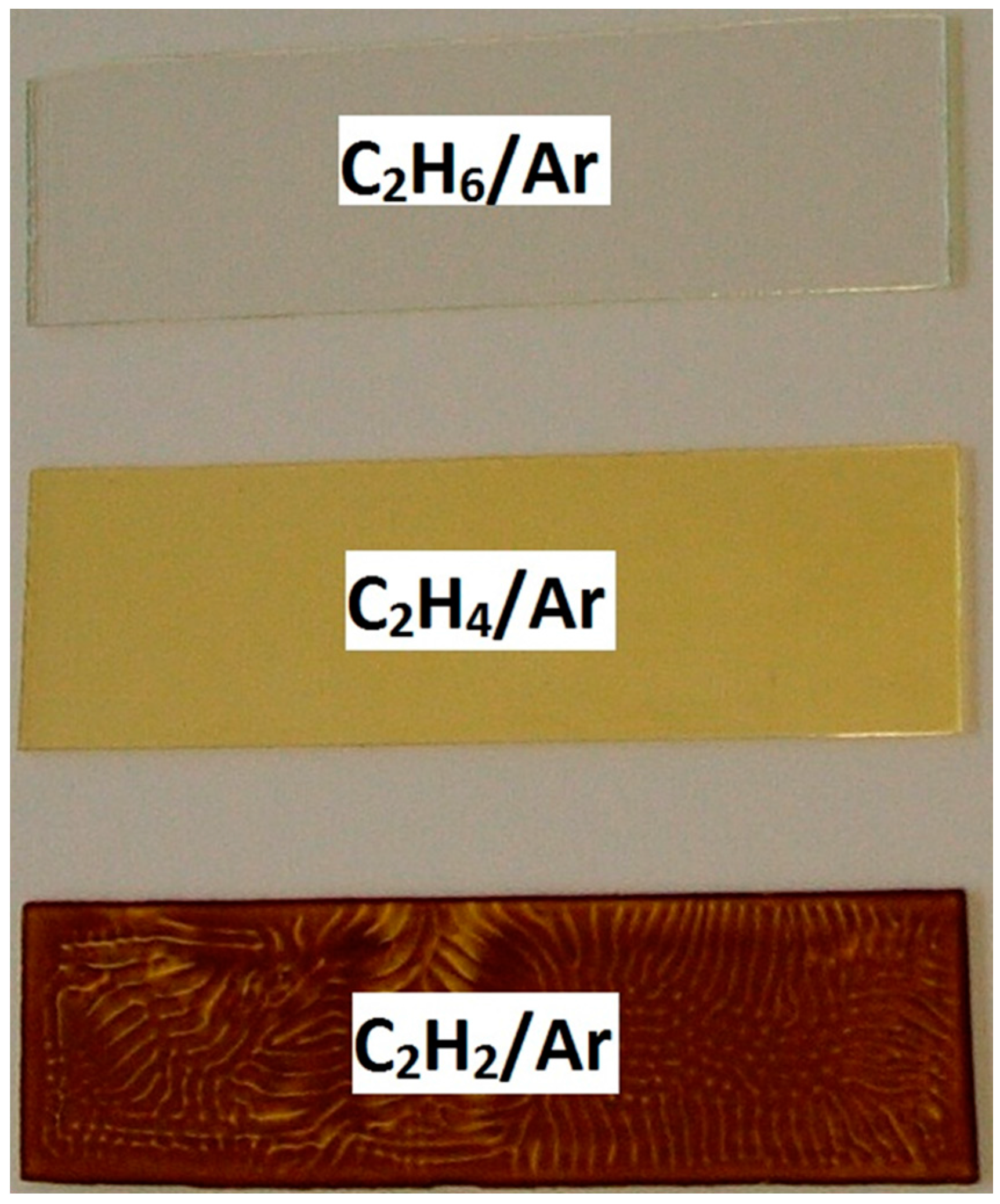
| Gas Mixture | Deposition Rate in 10−5 kg/(s·m2) |
|---|---|
| C2H6/Ar | 0.08 |
| C2H4/Ar | 0.18 |
| C2H2/Ar | 0.28 |
| Region | Wavenumber (cm−1) | Relative Absorbance | ||
|---|---|---|---|---|
| C2H2 | C2H4 | C2H6 | ||
| I | 3200–3600 | 0.11 | 0.09 | 0.19 |
| II | 2700–3100 | 0.40 | 0.35 | 0.58 |
| III | 2040–2290 | 0.01 | 0.00 | 0.00 |
| IV | 1550–1850 | 0.22 | 0.20 | 0.07 |
| V | 1200–1550 | 0.26 | 0.36 | 0.15 |
| Sample | n (@ 2 eV) | Eog (eV) | ID/IG Ratio |
|---|---|---|---|
| C2H4/Ar | 3.07 | 1.75 | 0.45 |
| C2H6/Ar | 2.81 | 1.91 | 0.3 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandrashekaraiah, T.H.; Bogdanowicz, R.; Rühl, E.; Danilov, V.; Meichsner, J.; Thierbach, S.; Hippler, R. Spectroscopic Study of Plasma Polymerized a-C:H Films Deposited by a Dielectric Barrier Discharge. Materials 2016, 9, 594. https://doi.org/10.3390/ma9070594
Chandrashekaraiah TH, Bogdanowicz R, Rühl E, Danilov V, Meichsner J, Thierbach S, Hippler R. Spectroscopic Study of Plasma Polymerized a-C:H Films Deposited by a Dielectric Barrier Discharge. Materials. 2016; 9(7):594. https://doi.org/10.3390/ma9070594
Chicago/Turabian StyleChandrashekaraiah, Thejaswini Halethimmanahally, Robert Bogdanowicz, Eckart Rühl, Vladimir Danilov, Jürgen Meichsner, Steffen Thierbach, and Rainer Hippler. 2016. "Spectroscopic Study of Plasma Polymerized a-C:H Films Deposited by a Dielectric Barrier Discharge" Materials 9, no. 7: 594. https://doi.org/10.3390/ma9070594
APA StyleChandrashekaraiah, T. H., Bogdanowicz, R., Rühl, E., Danilov, V., Meichsner, J., Thierbach, S., & Hippler, R. (2016). Spectroscopic Study of Plasma Polymerized a-C:H Films Deposited by a Dielectric Barrier Discharge. Materials, 9(7), 594. https://doi.org/10.3390/ma9070594






