Effect of Transition Metal Substitution on the Structure and Properties of a Clathrate-Like Compound Eu7Cu44As23
Abstract
:1. Introduction
2. Results
2.1. Synthesis and Homogeneity Ranges
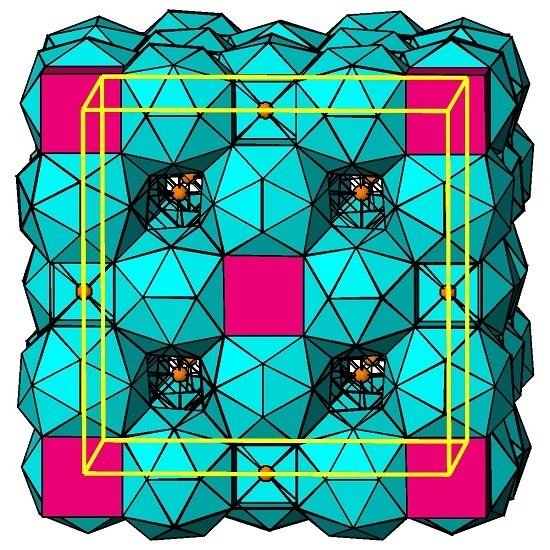
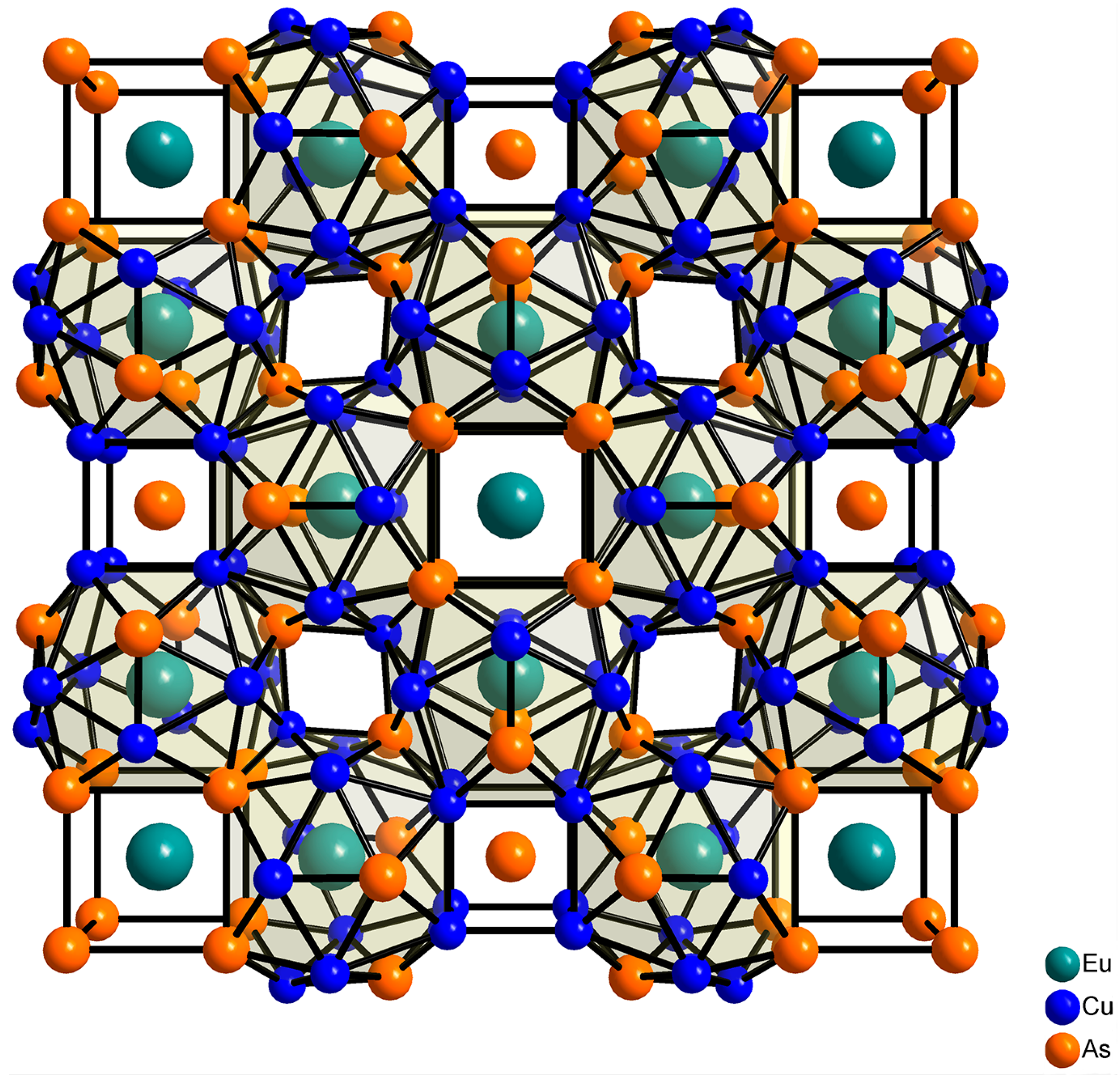
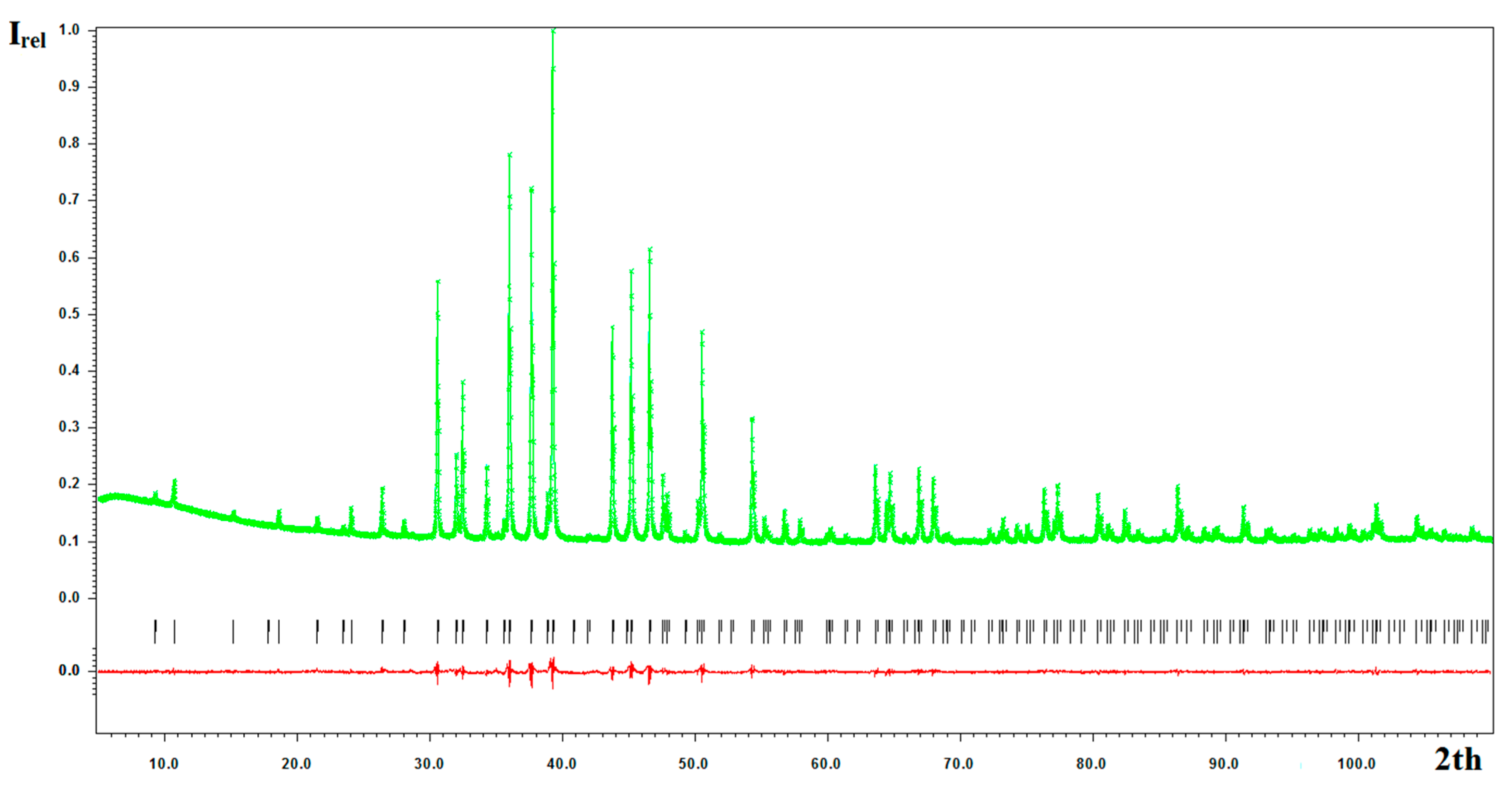
2.2. Crystal Structure Refinement and Description
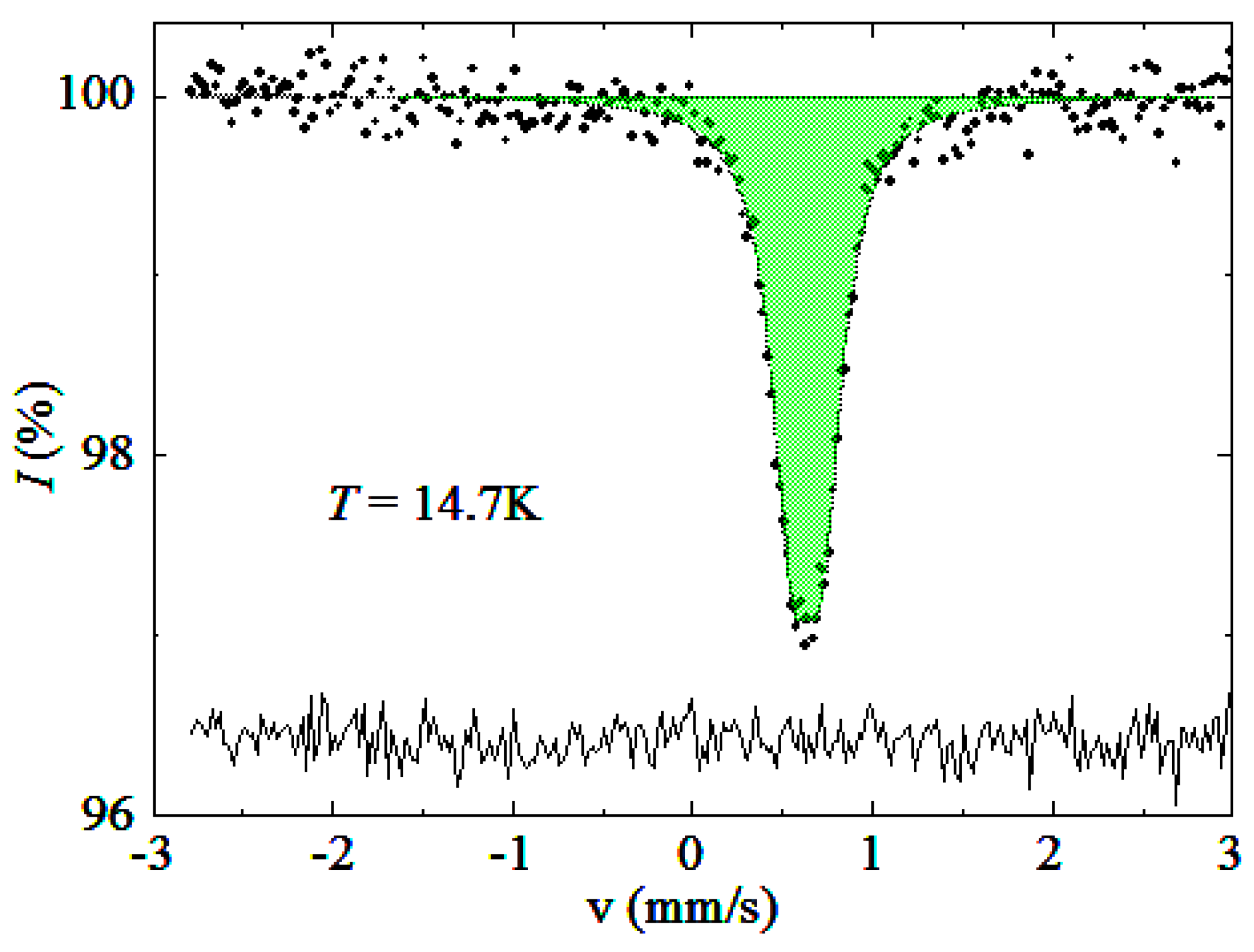
2.3. Mössbauer Spectra
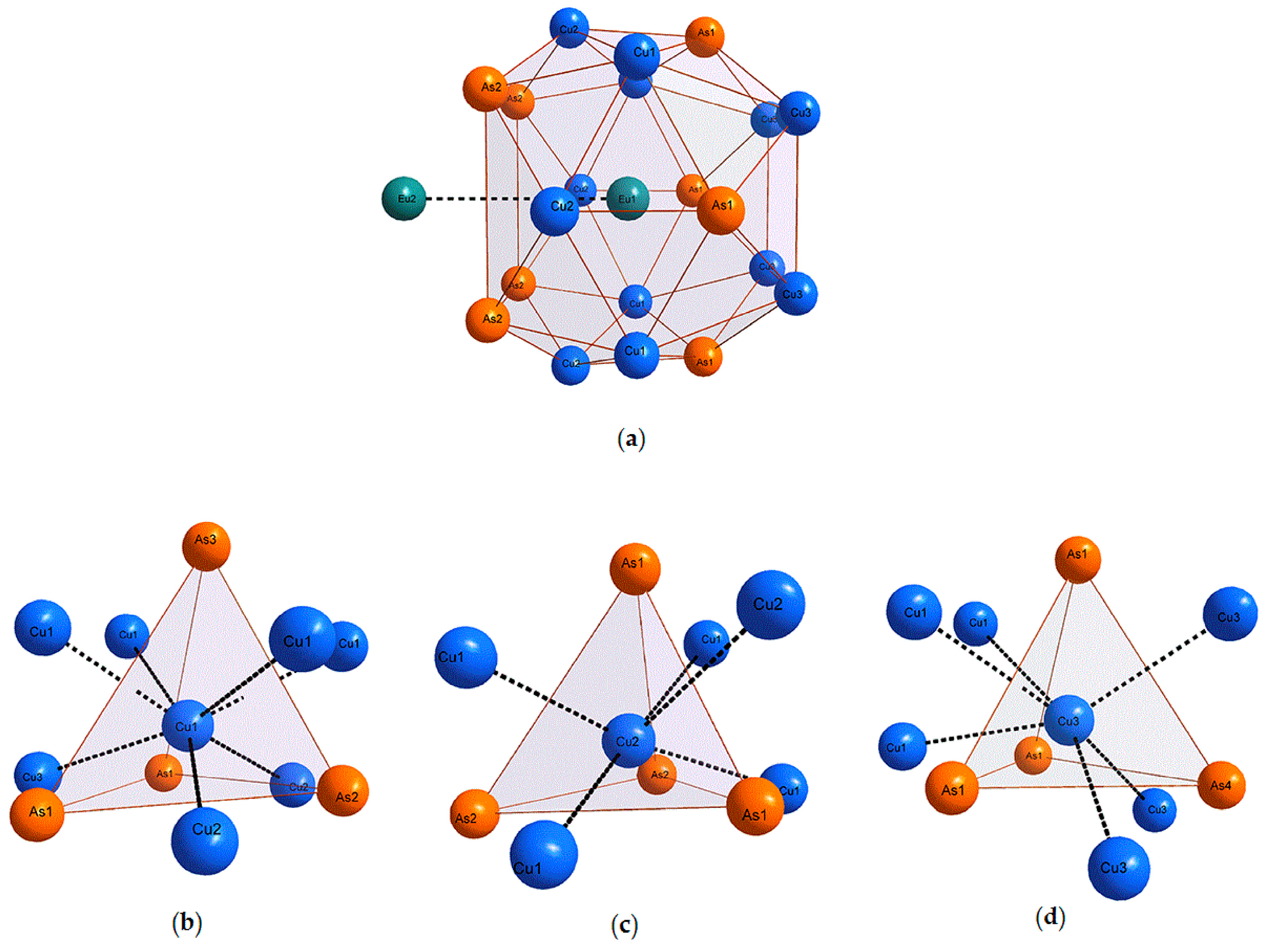
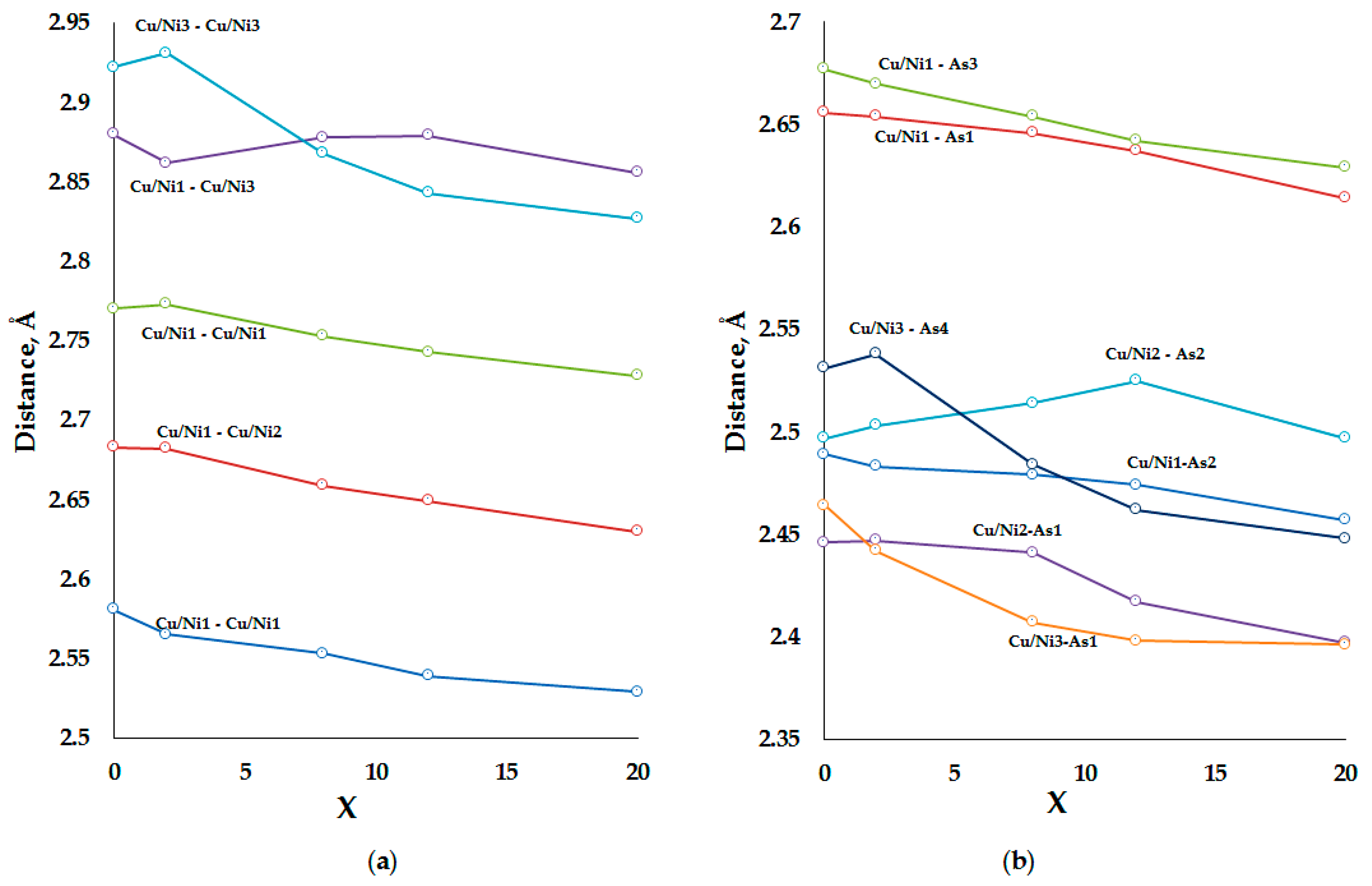
2.4. Interatomic Distances
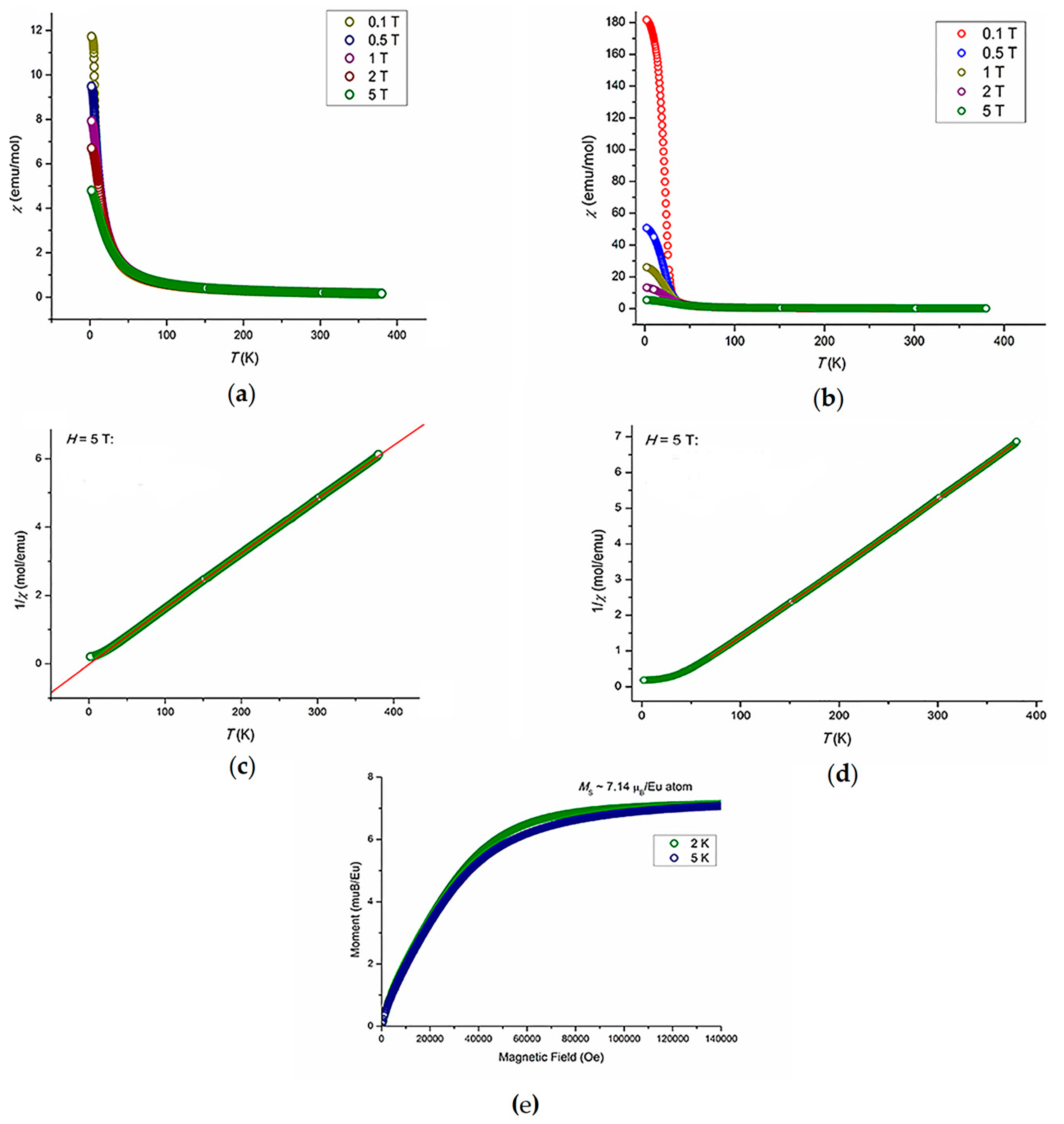
2.5. Magnetic Properties
3. Discussion
4. Materials and Methods
4.1. Synthesis and Primary Characterization
4.2. Crystal Structure Determination
4.3. Magnetic Properties
4.4. Mössbauer Study
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| T | Fe, Co, Ni |
| FM | Ferromagnetic |
| AFM | Antiferromagnetic |
References
- Shevelkov, A.V.; Kovnir, K. Zintl clathrates. Struct. Bond. 2011, 139, 97–144. [Google Scholar] [CrossRef]
- Prokofiev, A.; Sidorenko, A.; Hradil, K.; Ikeda, M.; Svagera, R.; Waas, M.; Winkler, H.; Neumaier, K.; Paschen, S. Thermopower enhancement by encapsulating cerium in clathrate cages. Nat. Mater. 2013, 12, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, V.; Bentien, A.; Carrillo-Cabrera, W.; Paschen, S.; Steglich, F.; Grin, Y. Relationship between composition and charge carrier concentration in Eu8Ga16−xGe30+xclathrates. Phys. Rev. B 2005, 71, 165205. [Google Scholar] [CrossRef]
- Bentien, A.; Pacheco, V.; Paschen, S.; Grin, Y.; Steglich, F. Transport properties of composition tuned α- and β-Eu8Ga16−xGe30+x. Phys. Rev. B 2005, 71, 165206. [Google Scholar] [CrossRef]
- Srinath, S.; Gass, J.; Rebar, D.J.; Woods, G.T.; Srikanth, H. Giant magnetocaloric effect in clathrates. J. Appl. Phys. 2006, 99, 08K902. [Google Scholar] [CrossRef]
- Phan, M.H.; Woods, G.T.; Chaturvedi, A.; Stefanoski, S.; Nolas, G.S.; Srikanth, H. Long-range ferromagnetism and giant magnetocaloric effect in type VIII Eu8Ga16Ge30clathrates. Appl. Phys. Lett. 2008, 93, 252505. [Google Scholar] [CrossRef]
- Munevar, J.; Micklitz, H.; Alzamora, M.; Argüello, C.; Goko, T.; Ning, F.L. Magnetism in superconducting EuFe2As1.4P0.6 single crystals studied by local probes. Solid State Commun. 2014, 187, 18–22. [Google Scholar] [CrossRef]
- Motomitsu, E.; Yanagi, H.; Kamiya, T.; Hirano, M.; Hosono, H. Synthesis, structure and physical properties of layered semiconductors MCuFCh (M = Sr, Eu; Ch = S, Se). J. Solid State Chem. 2006, 179, 1668–1673. [Google Scholar] [CrossRef]
- Charkin, D.O.; Urmanov, A.V.; Kazakov, S.M.; Batuk, D.; Abakumov, A.M.; Knöner, S.; Gati, E.; Wolf, B.; Lang, M.; Shevelkov, A.V.; et al. Synthesis, crystal structure, transport, and magnetic properties of novel ternary copper phosphides, A2Cu6P5 (A = Sr, Eu) and EuCu4P3. Inorg. Chem. 2012, 51, 8948–8955. [Google Scholar] [CrossRef] [PubMed]
- Kovnir, K.; Köhler, U.; Budnyk, S.; Prots, Y.; Baitinger, M.; Paschen, S.; Shevelkov, A.V.; Grin, Y. Introducing a magnetic guest to a tetrel-free clathrate: Synthesis, structure, and properties of EuxBa8–xCu16P30 (0 ≤ x ≤ 1.5). Inorg. Chem. 2011, 50, 10387–10396. [Google Scholar] [CrossRef] [PubMed]
- Charkin, D.O.; Demchyna, R.; Prots, Y.; Borrmann, H.; Burkhardt, U.; Schwarz, U.; Schnelle, W.; Plokhikh, I.V.; Kazakov, S.M.; Abakumov, A.M.; et al. Two new arsenides, Eu7Cu44As23 and Sr7Cu44As23, with a new filled variety of the BaHg11 structure. Inorg. Chem. 2014, 53, 11173–11184. [Google Scholar] [CrossRef] [PubMed]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic Computing System JANA2006: General features. Z. Kristallogr. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Peyronel, G. Struttura della fase BaHg11. Gazz. Chim. Ital. 1952, 82, 679. [Google Scholar]
- Verchenko, V.Y.; Tsirlin, A.A.; Sobolev, A.V.; Presniakov, I.A.; Shevelkov, A.V. Ferromagnetic order, strong magnetocrystalline anisotropy and magnetocaloric effect in the layered telluride Fe3−δGeTe2. Inorg. Chem. 2015, 54, 8598–8607. [Google Scholar] [CrossRef] [PubMed]
- Takagi, S.; Yasuoka, H.; Ogawa, S.; Wernick, J.H. 29Si NMR studies of an “unusual” paramagnet FeSi–Anderson localized state model. J. Phys. Soc. Jpn. 1981, 50, 2539–2546. [Google Scholar] [CrossRef]
- Litvinenko, O.N.; Kuznetsov, A.N.; Olenev, A.V.; Popovkin, B.A. New mixed tellurides of nickel and Group 13–14 metals Ni3−δMTe2 (M = Sn, In, Ga). Russ. Chem. Bull. 2007, 56, 1945–1947. [Google Scholar] [CrossRef]
- Baranov, A.I.; Kloo, L.; Olenev, A.V.; Popovkin, B.A.; Romanenko, A.I.; Shevelkov, A.V. Unique metallic wires in a novel quasi-1D compound: Synthesis, crystal and electronic structure, and properties of Ni8Bi8SI. J. Am. Chem. Soc. 2001, 123, 12375–12379. [Google Scholar] [CrossRef] [PubMed]
- Matsnev, M.E.; Rusakov, V.S. SpectrRelax: An application for Mössbauer spectra modeling and fitting. AIP Conf. Proc. 2012, 1489, 178. [Google Scholar] [CrossRef]
| Composition (T, x) | Ni, 2 | Ni, 8 | Ni, 12 | Ni, 20 | Co, 8 | Fe, 8 |
|---|---|---|---|---|---|---|
| Z Cell parameters | 4 | |||||
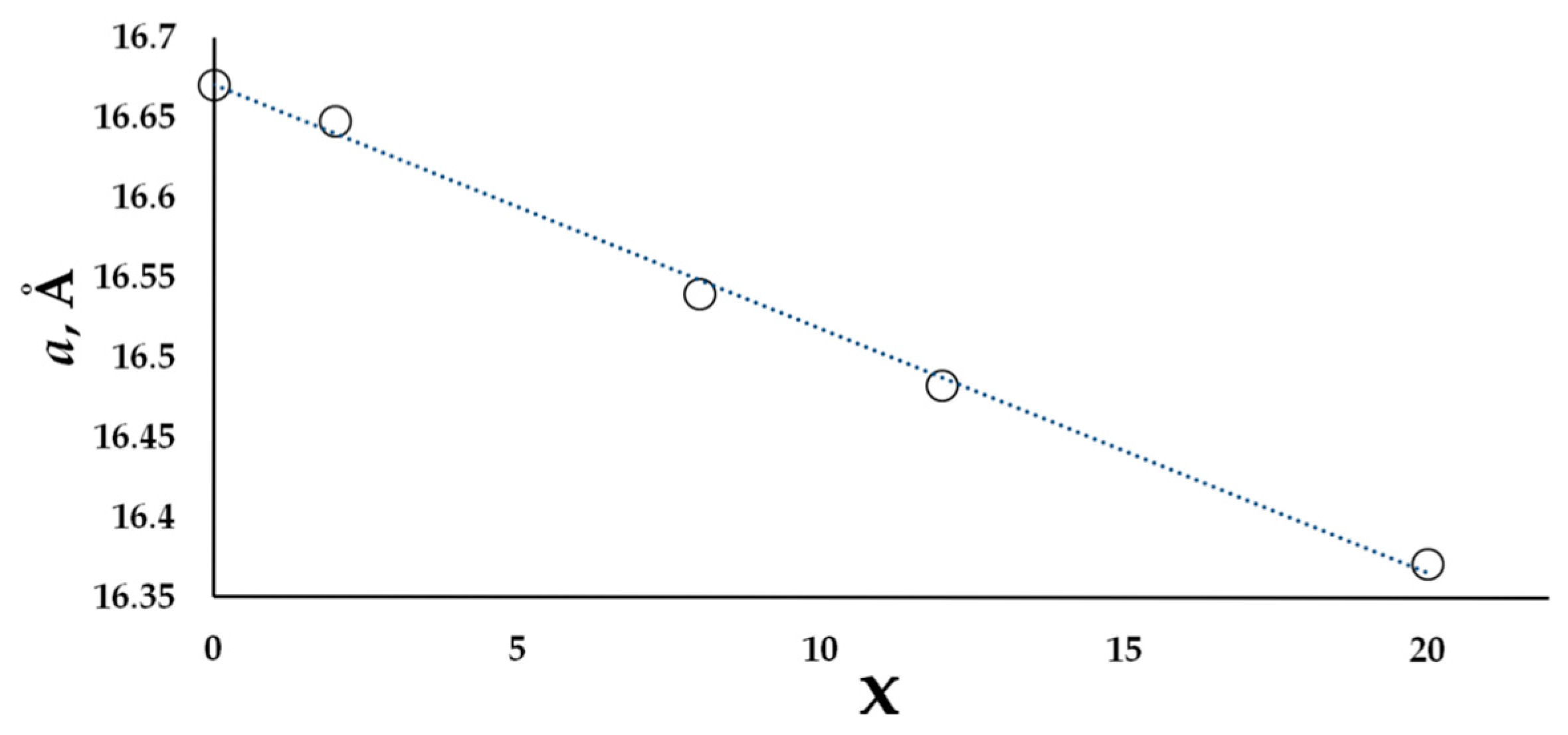
| a, Å | 16.6487(2) | 16.5407(1) | 16.4830(1) | 16.3719(1) | 16.5421(1) | 16.6251(1) |
| V, Å3 | 4614.65(8) | 4525.42(3) | 4478.25(3) | 4388.34(6) | 4526.57(3) | 4595.11(1) |
| Calculated density, g/cm3 | 8.0359 | 8.1253 | 8.1451 | 8.2506 | 8.1599 | 7.9947 |
| Radiation | CuKα1,2 | |||||
| 2θ range | 5–140 | 5–110 | 5–110 | 5–120 | 5–110 | 5–110 |
| Data points/reflections | 10,282/271 | 7996/186 | 7996/184 | 8758/205 | 7996/186 | 1997/187 |
| Overall/structural parameters | 44/17 | 43/17 | 52/17 | 62/17 | 56/17 | 46/20 |
| Analyzing package R values 1 (%): | Jana 2006 [12] | |||||
| RB | 3.60 | 1.92 | 2.03 | 1.62 | 1.94 | 1.76 |
| Rp | 1.32 | 1.13 | 1.51 | 1.15 | 0.81 | 1.35 |
| Rexp | 0.81 | 0.74 | 1.44 | 1.13 | 0.55 | 1.23 |
| GOF | 2.39 | 2.26 | 1.38 | 1.30 | 2.47 | 1.47 |
| T = Ni, x = 2 | |||||
| Atom | Position | x/a | y/b | z/c | Uiso |
| Eu1 | 24e(x, 0, 0) | 0.2447(1) | - | - | 0.0101(5) |
| Eu2 | 4a(0, 0, 0) | - | - | - | 0.005(1) |
| Cu/Ni1 | 96k(x, x, z) | 0.13666(4) | - | 0.2544(1) | 0.0168(6) |
| Cu/Ni2 | 48h(0, y, y) | - | 0.1825(1) | - | 0.0161(9) |
| Cu/Ni3 | 32f(x, x, x) | 0.41199(9) | - | - | 0.021(1) |
| As1 | 48i(½, y, y) | - | 0.17097(8) | - | 0.0080(6) |
| As2 | 32f(x, x, x) | 0.11008(7) | - | - | 0.0072(7) |
| As3 | 8c(¼, ¼, ¼) | - | - | - | 0.0034(9) |
| As4 | 4b(½, ½, ½) | - | - | - | 0.042(3) |
| T = Ni, x = 8 | |||||
| Atom | Position | x/a | y/b | z/c | Uiso |
| Eu1 | 24e(x, 0, 0) | 0.24356(9) | - | - | 0.0066(3) |
| Eu2 | 4a(0, 0, 0) | - | - | - | 0.0024(9) |
| Cu/Ni1 | 96k(x, x, z) | 0.13658(4) | - | 0.2543(1) | 0.0105(3) |
| Cu/Ni2 | 48h(0, y, y) | - | 0.18399(7) | - | 0.0124(6) |
| Cu/Ni3 | 32f(x, x, x) | 0.41328(8) | - | - | 0.0121(8) |
| As1 | 48i(½, y, y) | - | 0.16919(6) | - | 0.0072(4) |
| As2 | 32f(x, x, x) | 0.10942(5) | - | - | 0.0085(5) |
| As3 | 8c(¼, ¼, ¼) | - | - | - | 0.0087(7) |
| As4 | 4b(½, ½, ½) | - | - | - | 0.048(2) |
| T = Ni, x = 12 | |||||
| Atom | Position | x/a | y/b | z/c | Uiso |
| Eu1 | 24e(x, 0, 0) | 0.2434(1) | - | - | 0.0054(4) |
| Eu2 | 4a(0, 0, 0) | - | - | - | 0.002(1) |
| Cu/Ni1 | 96k(x, x, z) | 0.13670(5) | - | 0.2544(1) | 0.0083(4) |
| Cu/Ni2 | 48h(0, y, y) | - | 0.18523(9) | - | 0.0104(8) |
| Cu/Ni3 | 32f(x, x, x) | 0.4138(1) | - | - | 0.0095(10) |
| As1 | 48i(½, y, y) | - | 0.16905(7) | - | 0.0039(5) |
| As2 | 32f(x, x, x) | 0.10934(7) | - | - | 0.0066(7) |
| As3 | 8c(¼, ¼, ¼) | - | - | - | 0.0023(9) |
| As4 | 4b(½, ½, ½) | - | - | - | 0.020(2) |
| T = Ni, x = 20 | |||||
| Atom | Position | x/a | y/b | z/c | Uiso |
| Eu1 | 24e(x, 0, 0) | 0.2425(1) | - | - | 0.0032(3) |
| Eu2 | 4a(0, 0, 0) | - | - | - | 0.009(1) |
| Cu/Ni1 | 96k(x, x, z) | 0.13647(3) | - | 0.2543(1) | 0.0047(3) |
| Cu/Ni2 | 48h(0, y, y) | - | 0.18447(8) | - | 0.0016(7) |
| Cu/Ni3 | 32f(x, x, x) | 0.41368(8) | - | - | 0.0073(9) |
| As1 | 48i(½, y, y) | - | 0.16986(6) | - | 0.0035(4) |
| As2 | 32f(x, x, x) | 0.10923(5) | - | - | 0.0042(5) |
| As3 | 8c(¼, ¼, ¼) | - | - | - | 0.0016(7) |
| As4 | 4b(½, ½, ½) | - | - | - | 0.004(2) |
| T = Co, x = 8 | |||||
| Atom | Position | x/a | y/b | z/c | Uiso |
| Eu1 | 24e(x, 0, 0) | 0.24150(9) | - | - | 0.0094(3) |
| Eu2 | 4a(0, 0, 0) | - | - | - | 0.010(1) |
| Cu/Co1 | 96k(x, x, z) | 0.13653(4) | - | 0.25467(1) | 0.0161(8) |
| Cu/Co2 | 48h(0, y, y) | - | 0.18542(8) | - | 0.018(7) |
| Cu/Co3 | 32f(x, x, x) | 0.41383(8) | - | - | 0.014(5) |
| As1 | 48i(½, y, y) | - | 0.16785(6) | - | 0.0110(5) |
| As2 | 32f(x, x, x) | 0.10955(6) | - | - | 0.0122(6) |
| As3 | 8c(¼, ¼, ¼) | - | - | - | 0.0092(8) |
| As4 | 4b(½, ½, ½) | - | - | - | 0.023(2) |
| T = Fe, x = 8 | |||||
| Atom | Position | x/a | y/b | z/c | Uiso |
| Eu1 | 24e(x, 0, 0) | 0.23912(9) | - | - | 0.0088(3) |
| Eu2 | 4a(0, 0, 0) | - | - | - | 0.010(1) |
| Cu/Fe1 | 96k(x, x, z) | 0.13663(4) | - | 0.2547(1) | 0.0140(4) |
| Cu/Fe2 | 48h(0, y, y) | - | 0.18436(8) | - | 0.0118(7) |
| Cu/Fe3 | 32f(x, x, x) | 0.41385(8) | - | - | 0.0115(8) |
| As1 | 48i(½, y, y) | - | 0.16843(6) | - | 0.0074(5) |
| As2 | 32f(x, x, x) | 0.11001(6) | - | - | 0.0048(5) |
| As3 | 8c(¼, ¼, ¼) | - | - | - | 0.0033(8) |
| As4 | 4b(½, ½, ½) | - | - | - | 0.020(2) |
| T, K (±1 K) | δ (mm/s) | ∆ (mm/s) | W (mm/s) |
|---|---|---|---|
| 15 | 0.636(3) | 0.169(7) | 0.28(2) |
| 27 | 0.638(3) | 0.176(6) | 0.27(2) |
| 41 | 0.633(2) | 0.166(7) | 0.29(2) |
| Composition (T, x) | Undoped | Ni, 2 | Ni, 8 | Ni, 12 | Ni, 20 | Co, 8 | Fe, 8 | |
|---|---|---|---|---|---|---|---|---|
| Eu1 | 1 × Eu2 | 4.0863(2) | 4.074(2) | 4.029(2) | 4.012(2) | 3.970(2) | 3.993(2) | 3.975(2) |
| 4 × Cu/T1 | 3.2217(3) | 3.2217(7) | 3.1999(6) | 3.1917(8) | 3.1657(6) | 3.2010(8) | 3.2227(7) | |
| 4 × Cu/T2 | 3.2092(2) | 3.210(2) | 3.199(1) | 3.200(2) | 3.166(1) | 3.203(2) | 3.197(1) | |
| 4 × Cu/T3 | 3.4702(2) | 3.471(2) | 3.464(2) | 3.453(2) | 3.442(2) | 3.493(2) | 3.541(2) | |
| 4 × As1 | 3.1814(1) | 3.174(2) | 3.149(1) | 3.138(1) | 3.129(1) | 3.157(1) | 3.194(1) | |
| 4 × As2 | 3.4326(2) | 3.427(2) | 3.387(1) | 3.373(2) | 3.340(1) | 3.365(2) | 3.361(1) | |
| Eu2 | 8 × As2 | 3.1659(3) | 3.174(1) | 3.1346(9) | 3.122(1) | 3.0974(9) | 3.139(1) | 3.1677(9) |
| 6 × Eu1 | 4.0863(2) | 4.074(2) | 4.029(2) | 4.012(2) | 3.970(2) | 3.992(2) | 3.975(2) | |
| Cu/T1 | 2 × Cu/T1 | 2.5811(5) | 2.565(2) | 2.553(2) | 2.539(2) | 2.529(2) | 2.544(2) | 2.556(2) |
| 2 × Cu/T1 | 2.7703(5) | 2.773(2) | 2.752(2) | 2.743(2) | 2.728(2) | 2.766(2) | 2.775(2) | |
| 2 × Cu/T2 | 2.6830(2) | 2.682(1) | 2.659(1) | 2.649(1) | 2.630(1) | 2.659(1) | 2.675(1) | |
| 1 × Cu/T3 | 2.8802(5) | 2.862(2) | 2.878(2) | 2.856(2) | 2.856(2) | 2.881(3) | 2.900(2) | |
| 2 × As1 | 2.6560(2) | 2.654(1) | 2.646(1) | 2.637(1) | 2.614(1) | 2.647(1) | 2.660(1) | |
| 1 × As2 | 2.4887(4) | 2.483(2) | 2.479(2) | 2.474(2) | 2.457(2) | 2.483(2) | 2.485(2) | |
| 1 × As3 | 2.6765(3) | 2.6696(7) | 2.6540(6) | 2.6420(7) | 2.6295(6) | 2.6563(7) | 2.6667(7) | |
| 1 × Eu1 | 3.2217(3) | 3.2217(7) | 3.1999(6) | 3.1917(8) | 3.1657(6) | 3.2010(8) | 3.2227(7) | |
| Cu/T2 | 2 × As1 | 2.4462(3) | 2.447(2) | 2.441(2) | 2.417(2) | 2.397(2) | 2.445(2) | 2.462(2) |
| 2 × As2 | 2.4968(3) | 2.503(2) | 2.514(1) | 2.525(2) | 2.497(1) | 2.536(2) | 2.530(1) | |
| 4 × Cu/T1 | 2.6830(2) | 2.682(1) | 2.659(1) | 2.649(1) | 2.630(1) | 2.659(1) | 2.674(1) | |
| 1 × Eu1 | 3.2092(2) | 3.210(2) | 3.199(1) | 3.200(2) | 3.166(1) | 3.203(2) | 3.197(1) | |
| Cu/T3 | 3 × As1 | 2.4643(3) | 2.442(2) | 2.404(2) | 2.398(2) | 2.396(2) | 2.383(2) | 2.407(2) |
| 1 × As4 | 2.5305(5) | 2.538(1) | 2.484(1) | 2.462(2) | 2.448(1) | 2.472(2) | 2.481(1) | |
| 3 × Cu/T1 | 2.8802(5) | 2.862(2) | 2.878(2) | 2.856(2) | 2.856(2) | 2.881(3) | 2.900(2) | |
| 3 × Cu/T3 | 2.9220(6) | 2.931(2) | 2.868(2) | 2.843(2) | 2.827(2) | 2.854(2) | 2.864(2) | |
| 3 × Eu1 | 3.4702(2) | 3.471(2) | 3.464(2) | 3.453(2) | 3.442(2) | 3.493(2) | 3.541(2) | |
| As1 | 4 × Cu/T1 | 2.6560(2) | 2.654(1) | 2.646(1) | 2.637(1) | 2.614(1) | 2.647(1) | 2.660(1) |
| 2 × Cu/T2 | 2.4462(3) | 2.447(2) | 2.441(2) | 2.417(2) | 2.397(2) | 2.445(2) | 2.462(2) | |
| 2 × Cu/T3 | 2.4643(3) | 2.442(2) | 2.404(2) | 2.398(2) | 2.396(2) | 2.383(2) | 2.407(2) | |
| 2 × Eu1 | 3.1814(1) | 3.174(2) | 3.149(1) | 3.138(1) | 3.129(1) | 3.157(1) | 3.194(1) | |
| As2 | 3 × Cu/T1 | 2.4887(4) | 2.483(2) | 2.479(2) | 2.474(2) | 2.457(2) | 2.483(2) | 2.485(2) |
| 3 × Cu/T2 | 2.4968(3) | 2.503(2) | 2.514(1) | 2.525(2) | 2.497(1) | 2.536(2) | 2.530(1) | |
| 3 × Eu1 | 3.4326(2) | 3.427(2) | 3.387(1) | 3.373(2) | 3.340(1) | 3.365(2) | 3.361(1) | |
| 1 × Eu2 | 3.1659(3) | 3.174(1) | 3.1346(9) | 3.122(1) | 3.0974(9) | 3.139(1) | 3.1677(9) | |
| As3 | 12 × Cu/T1 | 2.6765(3) | 2.6696(7) | 2.6540(6) | 2.6420(7) | 2.6295(6) | 2.6563(7) | 2.6667(7) |
| As4 | 8 × Cu/T3 | 2.5305(5) | 2.538(1) | 2.484(1) | 2.462(2) | 2.448(1) | 2.472(2) | 2.481(1) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plokhikh, I.V.; Charkin, D.O.; Verchenko, V.Y.; Ignatyev, I.A.; Kazakov, S.M.; Sobolev, A.V.; Presniakov, I.A.; Tsirlin, A.A.; Shevelkov, A.V. Effect of Transition Metal Substitution on the Structure and Properties of a Clathrate-Like Compound Eu7Cu44As23. Materials 2016, 9, 587. https://doi.org/10.3390/ma9070587
Plokhikh IV, Charkin DO, Verchenko VY, Ignatyev IA, Kazakov SM, Sobolev AV, Presniakov IA, Tsirlin AA, Shevelkov AV. Effect of Transition Metal Substitution on the Structure and Properties of a Clathrate-Like Compound Eu7Cu44As23. Materials. 2016; 9(7):587. https://doi.org/10.3390/ma9070587
Chicago/Turabian StylePlokhikh, Igor V., Dmitri O. Charkin, Valeriy Yu. Verchenko, Ivan A. Ignatyev, Sergey M. Kazakov, Alexey V. Sobolev, Igor A. Presniakov, Alexander A. Tsirlin, and Andrei V. Shevelkov. 2016. "Effect of Transition Metal Substitution on the Structure and Properties of a Clathrate-Like Compound Eu7Cu44As23" Materials 9, no. 7: 587. https://doi.org/10.3390/ma9070587
APA StylePlokhikh, I. V., Charkin, D. O., Verchenko, V. Y., Ignatyev, I. A., Kazakov, S. M., Sobolev, A. V., Presniakov, I. A., Tsirlin, A. A., & Shevelkov, A. V. (2016). Effect of Transition Metal Substitution on the Structure and Properties of a Clathrate-Like Compound Eu7Cu44As23. Materials, 9(7), 587. https://doi.org/10.3390/ma9070587