Repair Bond Strength of Aged Resin Composite after Different Surface and Bonding Treatments
Abstract
:1. Introduction
2. Results
3. Discussion
4. Experimental Section
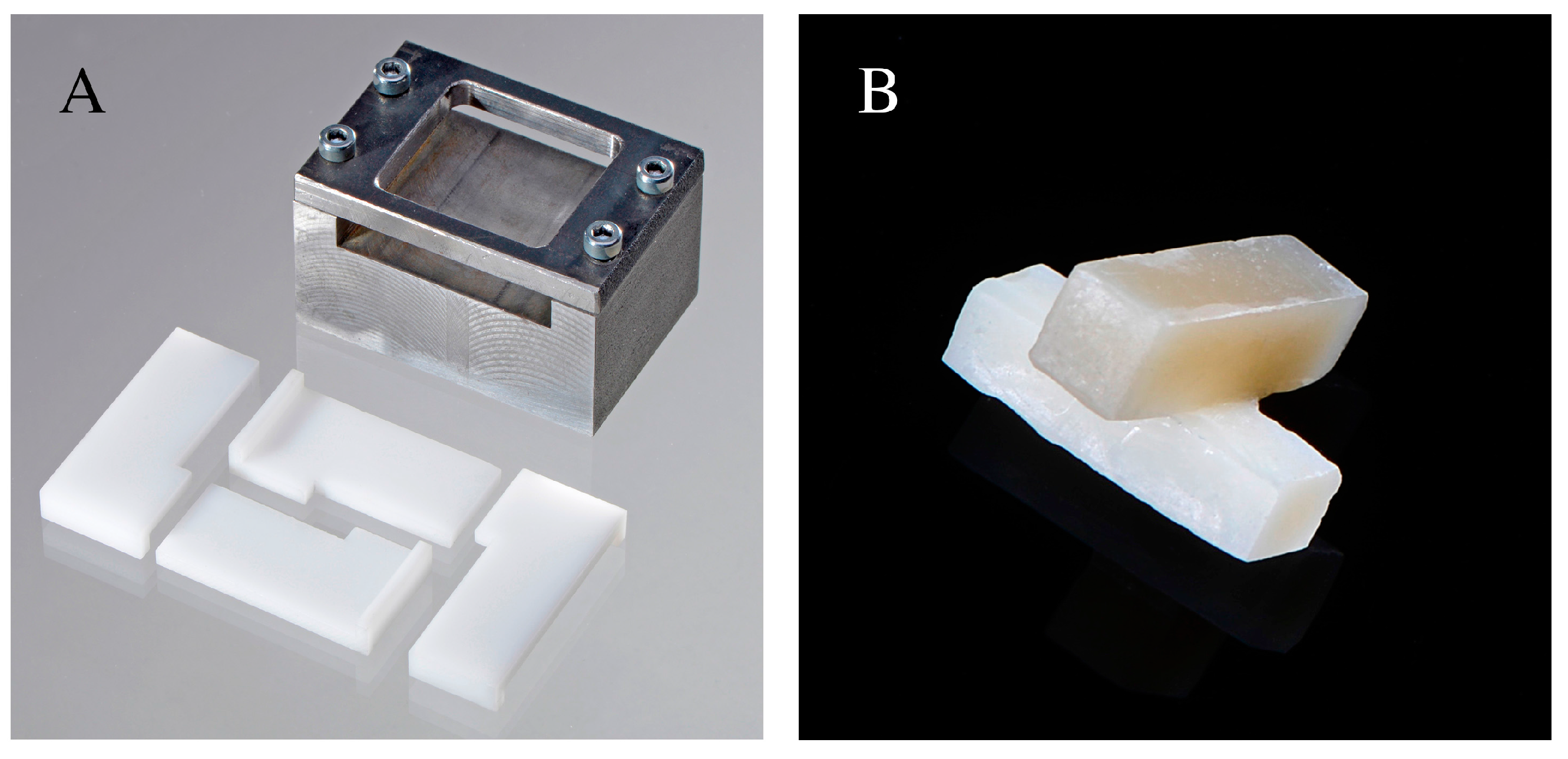
4.1. Specimen Fabrication and Aging Procedure
4.2. Surface Treatment and Resin Composite Bonding
4.3. Tensile Bond Strength (TBS)
4.4. Surface Characterization: SEM and Profilometric Evaluation
4.5. Degree of Conversion: FTIR-ATR Spectroscopy
5. Conclusions
- -
- A considerable decrease in the availability of unreacted carbon double bonds was observed after aging.
- -
- The increase in TBS values was not directly correlated to the surface roughness profiles measured.
- -
- Cleaning with phosphoric acid significantly improved TBS.
- -
- The use of a bonding system resulted critical to achieve reliable bond strengths.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gordan, V.V.; Riley, J.L.; Geraldeli, S.; Rindal, D.B.; Qvist, V.; Fellows, J.L.; Kellum, H.P.; Gilbert, G.H. Repair or replacement of defective restorations by dentists in the dental practice-based research network. J. Am. Dent. Assoc. 2012, 143, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, E.; Martin, J.; Vildosola, P.; Oliveira, O.B.J.; Gordan, V.; Mjor, I.; Bersezio, C.; Estay, J.; de Andrade, M.F.; Moncada, G. Can repair increase the longevity of composite resins? Results of a 10-year clinical trial. J. Dent. 2015, 43, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.; Fernandez, E.; Estay, J.; Gordan, V.V.; Mjor, I.A.; Moncada, G. Minimal invasive treatment for defective restorations: Five-year results using sealants. Oper. Dent. 2013, 38, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Gordan, V.V.; Shen, C.; Riley, J.L.; Mjor, I.A. Two-year clinical evaluation of repair versus replacement of composite restorations. J. Esthet. Restor. Dent. 2006, 18, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Moncada, G.; Martin, J.; Fernandez, E.; Hempel, M.C.; Mjor, I.A.; Gordan, V.V. Sealing, refurbishment and repair of class i and class ii defective restorations: A three-year clinical trial. J. Am. Dent. Assoc. 2009, 140, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Popoff, D.A.; de Magalhaes, C.S.; de Freitas Oliveira, W.; Soares, L.A.; de Almeida Santa Rosa, T.T.; Ferreira, R.C.; Moreira, A.N.; Mjor, I.A. Two-year clinical performance of dimethacrylatebased composite restorations repaired with a silorane-based composite. J. Adhes. Dent. 2014, 16, 575–583. [Google Scholar] [PubMed]
- Opdam, N.J.; Bronkhorst, E.M.; Loomans, B.A.; Huysmans, M.C. Longevity of repaired restorations: A practice based study. J. Dent. 2012, 40, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Imbery, T.A.; Gray, T.; DeLatour, F.; Boxx, C.; Best, A.M.; Moon, P.C. Evaluation of flexural, diametral tensile, and shear bond strength of composite repairs. Oper. Dent. 2014, 39, E250–E260. [Google Scholar] [CrossRef] [PubMed]
- Brosh, T.; Pilo, R.; Bichacho, N.; Blutstein, R. Effect of combinations of surface treatments and bonding agents on the bond strength of repaired composites. J. Prosthet. Dent. 1997, 77, 122–126. [Google Scholar] [CrossRef]
- Bonstein, T.; Garlapo, D.; Donarummo, J., Jr.; Bush, P.J. Evaluation of varied repair protocols applied to aged composite resin. J. Adhes. Dent. 2005, 7, 41–49. [Google Scholar] [PubMed]
- Shahdad, S.A.; Kennedy, J.G. Bond strength of repaired anterior composite resins: An in vitro study. J. Dent. 1998, 26, 685–694. [Google Scholar] [CrossRef]
- Costa, T.R.; Ferreira, S.Q.; Klein-Junior, C.A.; Loguercio, A.D.; Reis, A. Durability of surface treatments and intermediate agents used for repair of a polished composite. Oper. Dent. 2010, 35, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Hannig, C.; Laubach, S.; Hahn, P.; Attin, T. Shear bond strength of repaired adhesive filling materials using different repair procedures. J. Adhes. Dent. 2006, 8, 35–40. [Google Scholar] [PubMed]
- Rodrigues, S.A., Jr.; Ferracane, J.L.; Della Bona, A. Influence of surface treatments on the bond strength of repaired resin composite restorative materials. Dent. Mater. 2009, 25, 442–451. [Google Scholar] [PubMed]
- Ozcan, M.; Barbosa, S.H.; Melo, R.M.; Galhano, G.A.; Bottino, M.A. Effect of surface conditioning methods on the microtensile bond strength of resin composite to composite after aging conditions. Dent. Mater. 2007, 23, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Lung, C.Y.; Matinlinna, J.P. Aspects of silane coupling agents and surface conditioning in dentistry: An overview. Dent. Mater. 2012, 28, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Rinastiti, M.; Ozcan, M.; Siswomihardjo, W.; Busscher, H.J. Immediate repair bond strengths of microhybrid, nanohybrid and nanofilled composites after different surface treatments. J. Dent. 2010, 38, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Rathke, A.; Tymina, Y.; Haller, B. Effect of different surface treatments on the composite-composite repair bond strength. Clin. Oral Investig. 2009, 13, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.F.; Ferreira, S.F.; Catelan, A.; Palialol, A.R.; Goncalves, L.S.; Aguiar, F.H.; Marchi, G.M. The effect of surface treatment and bonding procedures on the bond strength of silorane composite repairs. Acta Odontol. Scand. 2014, 72, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Papacchini, F.; Dall’Oca, S.; Chieffi, N.; Goracci, C.; Sadek, F.T.; Suh, B.I.; Tay, F.R.; Ferrari, M. Composite-to-composite microtensile bond strength in the repair of a microfilled hybrid resin: Effect of surface treatment and oxygen inhibition. J. Adhes. Dent. 2007, 9, 25–31. [Google Scholar] [PubMed]
- Tezvergil, A.; Lassila, L.V.; Vallittu, P.K. Composite-composite repair bond strength: Effect of different adhesion primers. J. Dent. 2003, 31, 521–525. [Google Scholar] [CrossRef]
- Sau, C.W.; Oh, G.S.; Koh, H.; Chee, C.S.; Lim, C.C. Shear bond strength of repaired composite resins using a hybrid composite resin. Oper. Dent. 1999, 24, 156–161. [Google Scholar] [PubMed]
- Loomans, B.A.; Cardoso, M.V.; Roeters, F.J.; Opdam, N.J.; De Munck, J.; Huysmans, M.C.; Van Meerbeek, B. Is there one optimal repair technique for all composites? Dent. Mater. 2011, 27, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Li, J. Effects of surface properties on bond strength between layers of newly cured dental composites. J. Oral Rehabil. 1997, 24, 358–360. [Google Scholar] [CrossRef] [PubMed]
- Truffier-Boutry, D.; Place, E.; Devaux, J.; Leloup, G. Interfacial layer characterization in dental composite. J. Oral Rehabil. 2003, 30, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Dall’Oca, S.; Papacchini, F.; Goracci, C.; Cury, A.H.; Suh, B.I.; Tay, F.R.; Polimeni, A.; Ferrari, M. Effect of oxygen inhibition on composite repair strength over time. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 81, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Dall’oca, S.; Papacchini, F.; Radovic, I.; Polimeni, A.; Ferrari, M. Repair potential of a laboratory-processed nano-hybrid resin composite. J. Oral Sci. 2008, 50, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, V.E.; Pfeifer, C.S.; Froes-Salgado, N.R.; Boaro, L.C.; Braga, R.R. Monomers used in resin composites: Degree of conversion, mechanical properties and water sorption/solubility. Braz. Dent. J. 2012, 23, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Cornelio, R.B.; Wikant, A.; Mjosund, H.; Kopperud, H.M.; Haasum, J.; Gedde, U.W.; Ortengren, U.T. The influence of bis-ema vs bis gma on the degree of conversion and water susceptibility of experimental composite materials. Acta Odontol. Scand. 2014, 72, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Alshali, R.Z.; Silikas, N.; Satterthwaite, J.D. Degree of conversion of bulk-fill compared to conventional resin-composites at two time intervals. Dent. Mater. 2013, 29, e213–e217. [Google Scholar] [CrossRef] [PubMed]
- Par, M.; Gamulin, O.; Marovic, D.; Klaric, E.; Tarle, Z. Raman spectroscopic assessment of degree of conversion of bulk-fill resin composites—Changes at 24 hours post cure. Oper. Dent. 2015, 40, E92–E101. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.F.; Cavalcante, L.M.; Silikas, N.; Watts, D.C. Degradation resistance of silorane, experimental ormocer and dimethacrylate resin-based dental composites. J. Oral Sci. 2011, 53, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Ortengren, U.; Wellendorf, H.; Karlsson, S.; Ruyter, I.E. Water sorption and solubility of dental composites and identification of monomers released in an aqueous environment. J. Oral Rehabil. 2001, 28, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Brendeke, J.; Ozcan, M. Effect of physicochemical aging conditions on the composite-composite repair bond strength. J. Adhes. Dent. 2007, 9, 399–406. [Google Scholar] [PubMed]
- Ferracane, J.L.; Berge, H.X.; Condon, J.R. In vitro aging of dental composites in water—Effect of degree of conversion, filler volume, and filler/matrix coupling. J. Biomed. Mater. Res. 1998, 42, 465–472. [Google Scholar] [CrossRef]
- Yesilyurt, C.; Kusgoz, A.; Bayram, M.; Ulker, M. Initial repair bond strength of a nano-filled hybrid resin: Effect of surface treatments and bonding agents. J. Esthet. Restor. Dent. 2009, 21, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Kupiec, K.A.; Barkmeier, W.W. Laboratory evaluation of surface treatments for composite repair. Oper. Dent. 1996, 21, 59–62. [Google Scholar] [PubMed]
- Da Costa, T.R.; Serrano, A.M.; Atman, A.P.; Loguercio, A.D.; Reis, A. Durability of composite repair using different surface treatments. J. Dent. 2012, 40, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, A.N.; De Lima, A.F.; Peris, A.R.; Mitsui, F.H.; Marchi, G.M. Effect of surface treatments and bonding agents on the bond strength of repaired composites. J. Esthet. Restor. Dent. 2007, 19, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, S.T.; Tibballs, J.; Dahl, J.E. Effect of different surface treatments and adhesives on repair bond strength of resin composites after one and 12 months of storage using an improved microtensile test method. Oper. Dent. 2014, 39, E206–E216. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, J.M.; Dickens, S.H.; Fowler, B.O.; Xu, H.H.K.; McDonough, W.G. Chemistry of silanes: Interfaces in dental polymers and composites. J. Res. Natl. Inst. Stand. Technol. 2005, 110, 541–558. [Google Scholar] [CrossRef] [PubMed]
- Hisamatsu, N.; Atsuta, M.; Matsumura, H. Effect of silane primers and unfilled resin bonding agents on repair bond strength of a prosthodontic microfilled composite. J. Oral Rehabil. 2002, 29, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Maneenut, C.; Sakoolnamarka, R.; Tyas, M.J. The repair potential of resin composite materials. Dent. Mater. 2011, 27, e20–e27. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, A.S.; El-Askary, F.S.; Amer, M.A. Effect of surface treatments on the tensile bond strength of repaired water-aged anterior restorative micro-fine hybrid resin composite. J. Dent. 2008, 36, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Magni, E.; Ferrari, M.; Papacchini, F.; Hickel, R.; Ilie, N. Influence of ozone on the composite-to-composite bond. Clin. Oral Investig. 2011, 15, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Lucena-Martin, C.; Gonzalez-Lopez, S.; Navajas-Rodriguez de Mondelo, J.M. The effect of various surface treatments and bonding agents on the repaired strength of heat-treated composites. J. Prosthet. Dent. 2001, 86, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Papacchini, F.; Magni, E.; Radovic, I.; Mazzitelli, C.; Monticellia, F.; Goracci, C.; Polimeni, A.; Ferrari, M. Effect of intermediate agents and pre-heating of repairing resin on composite-repair bonds. Oper. Dent. 2007, 32, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Papacchini, F.; Radovic, I.; Magni, E.; Goracci, C.; Monticelli, F.; Chieffi, N.; Polimeni, A.; Ferrari, M. Flowable composites as intermediate agents without adhesive application in resin composite repair. Am. J. Dent. 2008, 21, 53–58. [Google Scholar] [PubMed]
- Hannig, C.; Hahn, P.; Thiele, P.P.; Attin, T. Influence of different repair procedures on bond strength of adhesive filling materials to etched enamel in vitro. Oper. Dent. 2003, 28, 800–807. [Google Scholar] [PubMed]
- Lohbauer, U.; Zipperle, M.; Rischka, K.; Petschelt, A.; Muller, F.A. Hydroxylation of dental zirconia surfaces: Characterization and bonding potential. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 87, 461–467. [Google Scholar] [CrossRef] [PubMed]

| Group | Surface Treatment | TBS (SD) |
|---|---|---|
| Group 1 Negative Reference | Red code diamond bur (grain size 27–76 µm) | 4.86 a (±1.06) |
| Group 2 | Etching with 35% phosphoric acid (Scotchbond Etchant, 3M ESPE, St. Paul, MN, USA) for 15 s | 6.75 b (±1.40) |
| Group 3 | Blue code diamond bur (grain size 64–126 µm) | 7.15 b (±1.85) |
| Group 4 | Sandblasting (CoJet-System, 3M ESPE, Seefeld, Germany) with 35 µm Al2O3 particles (2.8 bar for 4 s at 20 mm distance) | 7.9 b (±1.64) |
| Group 5 | Silane application for 60 s | 6.62 b (±1.59) |
| Group 6 | Sandblasting with 30 µm CoJet Sand (2.8 bar for 4 s at 20 mm distance) and subsequent silane application for 60 s | 7.75 b (±1.87) |
| Group 7 | Application of Syntac Primer for 15 s and careful drying with compressed air | 9.82 c,d (±1.76) |
| Group 8 | Application of Syntac Adhesive for 10 s and careful drying with compressed air | 10.03 c,d (±1.51) |
| Group 9 | Application of Heliobond for 60 s, careful drying with compressed air and light polymerization for 40 s | 9.35 c (±2.05) |
| Group 10 | Application of Syntac Primer + Adhesive and subsequent application of Heliobond | 9.67 c,d (±1.88) |
| Group 11 | Etching with 35% phosphoric acid followed by Syntac Primer + Adhesive and Heliobond | 11.33 d (±2.03) |
| Positive reference | No surface treatment, no aging after repair | 10.07 c,d (±1.54) |
| Positive reference | No surface treatment. After repair, aged in distilled water for 30 days | 10.54 c,d (±2.04) |
| Group | Ra (µm) | Rz (µm) |
|---|---|---|
| Group 1—Negative reference Red code bur | 1.07 ± 0.05 | 3.69 ± 0.25 |
| Group 2 Phosphoric acid | 1.15 ± 0.21 | 3.92 ± 0.77 |
| Group 3 Blue code bur | 3.36 ± 0.51 | 10.36 ± 1.55 |
| Group 4 Al2O3 sandblasting | 0.73 ± 0.05 | 2.5 ± 0.18 |
| Group 6 CoJet Sand | 0.81 ± 0.07 | 2.81 ± 0.21 |
| Material | Composition |
|---|---|
| Grandio SO * Voco Cuxhaven, Germany | Filler (89 wt %): 0.5–3 µm glass ceramic particles 0–40 nm SiO2 nanoparticles Matrix: Bis-GMA, Bis-EMA, TEGDMA |
| CoJet Sand 3M ESPE Seefeld, Germany | 30 µm Al2O3 silicatized particles |
| Monobond Plus Ivoclar Vivadent Schaan, Lichtenstein | 3-trimethoxysilylpropyl methacrylate (<2.5%) Methacrylated phosphoric acid ester (<2.5%) Ethanol (50%–100%) |
| Syntac Ivoclar Vivadent Schaan, Lichtenstein | Primer: TEGDMA, PEGDMA (25%) Maleic acid (2.5%–10%) Acetone (25%–50%) Adhesive: PEGDMA (25%–50%) Glutaraldehyde (2.5%–10%) Water (60%) |
| Heliobond Ivoclar Vivadent Schaan, Lichtenstein | Bis-GMA (50%–60%) TEGDMA (25%–50%) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wendler, M.; Belli, R.; Panzer, R.; Skibbe, D.; Petschelt, A.; Lohbauer, U. Repair Bond Strength of Aged Resin Composite after Different Surface and Bonding Treatments. Materials 2016, 9, 547. https://doi.org/10.3390/ma9070547
Wendler M, Belli R, Panzer R, Skibbe D, Petschelt A, Lohbauer U. Repair Bond Strength of Aged Resin Composite after Different Surface and Bonding Treatments. Materials. 2016; 9(7):547. https://doi.org/10.3390/ma9070547
Chicago/Turabian StyleWendler, Michael, Renan Belli, Reinhard Panzer, Daniel Skibbe, Anselm Petschelt, and Ulrich Lohbauer. 2016. "Repair Bond Strength of Aged Resin Composite after Different Surface and Bonding Treatments" Materials 9, no. 7: 547. https://doi.org/10.3390/ma9070547
APA StyleWendler, M., Belli, R., Panzer, R., Skibbe, D., Petschelt, A., & Lohbauer, U. (2016). Repair Bond Strength of Aged Resin Composite after Different Surface and Bonding Treatments. Materials, 9(7), 547. https://doi.org/10.3390/ma9070547





