1. Introduction
Sewage sludge is the waste material that is produced during the treatment of industrial or municipal wastewater. As the amount of sludge increased with the implementation of environmental programs in many countries, handling of sewage sludge began to attract considerable attention. In agriculture, treated sewage sludge is used as biosolids to spread over land for non-food agriculture or food agriculture. However, this practice raises many disputes due to health and environmental concerns [
1].
Containing SiO
2 and Al
2O
3 and characterized as light porous particles with large water absorbing ability, sewage sludge has potentials as high recycling value for construction industry. Researchers have investigated the use of sewage sludge ash (SSA) for various construction applications, such as components of bricks and tiles, raw material for cement production, aggregates of concrete and mortar, a synthesized component of lightweight materials, and substitutes for sand and/or cement in road pavement [
2].
Among these applications, applying SSA to produce bricks and tiles has been a major area with promising potentials and has attracted much attention in academia. Tay [
3] was one of the first pioneers to mix dry sewage sludge with clay to fabricate blocks, finding 40% dry sludge replacement a feasible solution. Tay and Goh [
4] later found some blemishes appeared in the tiles with a high sludge replacement ratio. In order to better understand the properties of incinerated sewage sludge ash (ISSA) for possible construction use, Lin [
5] studied the mineralogy and microstructures of SSA and found that the bulk density of the sintered monoliths reduced as the sintering temperature increased from 1050 to 1100 °C. Lin and Weng [
6] added various percentages of SSA, ranging from 0% to 50% to clay in producing brick samples. After a series of tests, the results showed that an appropriate quantity of sewage sludge ash replacement was below 20%. Weng and Lin [
7] later investigated SSA tiles further and discovered some major shortcomings of SSA tiles, including excessive water absorption, deficient abrasion, and extensive porosity. Many attempts have been made to resolve such shortcomings. Lin et al. [
8] applied glaze with different colorants to ISSA biscuit tile specimens and found that glaze improved the bending strength, abrasion resistance, and acid-alkali resistance of tiles. Wiebusch and Seyfried [
9], mixing sewage sludge with clay to manufacture bricks and tiles, noticed that different SSAs have different influences such as the ceramic qualities of bricks or tiles. Monteiro et al. [
10] used SSA contents of 0, 3, 5, and 10 wt % to manufacture tiles at different kiln temperatures and found that the addition of 10 wt % SSA to the tiles improved their water absorption behavior but slightly reduced their bending strength. Zhou et al. [
11] performed a series of formulation experiments and characterized the physical and chemical properties of tiles and found that the maximum quantity of sewage sludge used in the spilt tiles is 60%. Lin et al. [
12] applied nano-SiO
2 as an additive to tiles containing SSA. They proved that tile bending strength improved with increased nano-SiO
2 amount. Moreover, Lin et al. [
13] studied the influences of kiln temperature and the amount of nanomaterial additive on the properties of SSA tiles and concluded that tile bending strength increased with increasing temperature when kiln temperatures were lower than 1100 °C, while both bending strength and firing shrinkage decreased as temperature went beyond 1150 °C.
Glass has also become a useful additive for improving SSA tiles. Lin and Cheng [
14] mixed clay and different amount of solar panel waste glass to manufacture eco-tiles, and used XRD (X-ray diffraction), FTIR (Fourier transform infrared spectroscopy), and SEM (scanning electron microscopy) to investigate the characteristics of the microstructures of the specimens. Kim et al. [
15] used LCD (liquid crystal display) waste glass as a flux material to replace the traditional feldspar in the manufacture of the ceramic tile specimens. They found that the calcined tile body containing LCD waste glass had a dense microstructure and had positive influences on tile specimens such as water absorption and the thermal expansion coefficient. Rozenstrauha et al. [
16] spotted dense glass-ceramics composite on SSA/glass ceramics at the temperature between 1120 and 1140 °C. The crystalline phases of quartz (SiO
2), anorthite (CaAl
2Si
2O
8), and hematite (Fe
2O
3) were observed by XRD analysis, and these mineralogical compositions were proved to improve compressive strength of the glass-ceramics specimens. Fan and Li demonstrated that the LCD waste glass as the fluxing agent and MgO as the modifying agent can be used as a raw material for producing insulating glass ceramics [
17].
To further examine the properties of reclaimed tile with SSA and waste glass, this study probed the persistence of the tile in extreme environments. Industrialization has led to acid rain and other harmful pollutants in major metropolitan areas, and the effects of acid and pollutants on reclaimed tiles have not been carefully studied. The main purpose of this study was thus to perform acid-alkali resistance tests on reclaimed tiles to inspect the variations in tile properties caused by the amount of SSA/glass replacement. The goal was to obtain a better SSA/glass replacement ratio benefiting the manufacture of the reclaimed tile to meet more demanding requirements. Before the acid-alkali resistance tests, a series of tests to identify basic material attributes including thermal characteristics, physical and mechanical properties, and mineralogical features was performed. Discussion and explanations are made herein to link and justify acid-alkali resistance test results and the basic material attributes.
2. Materials and Methods
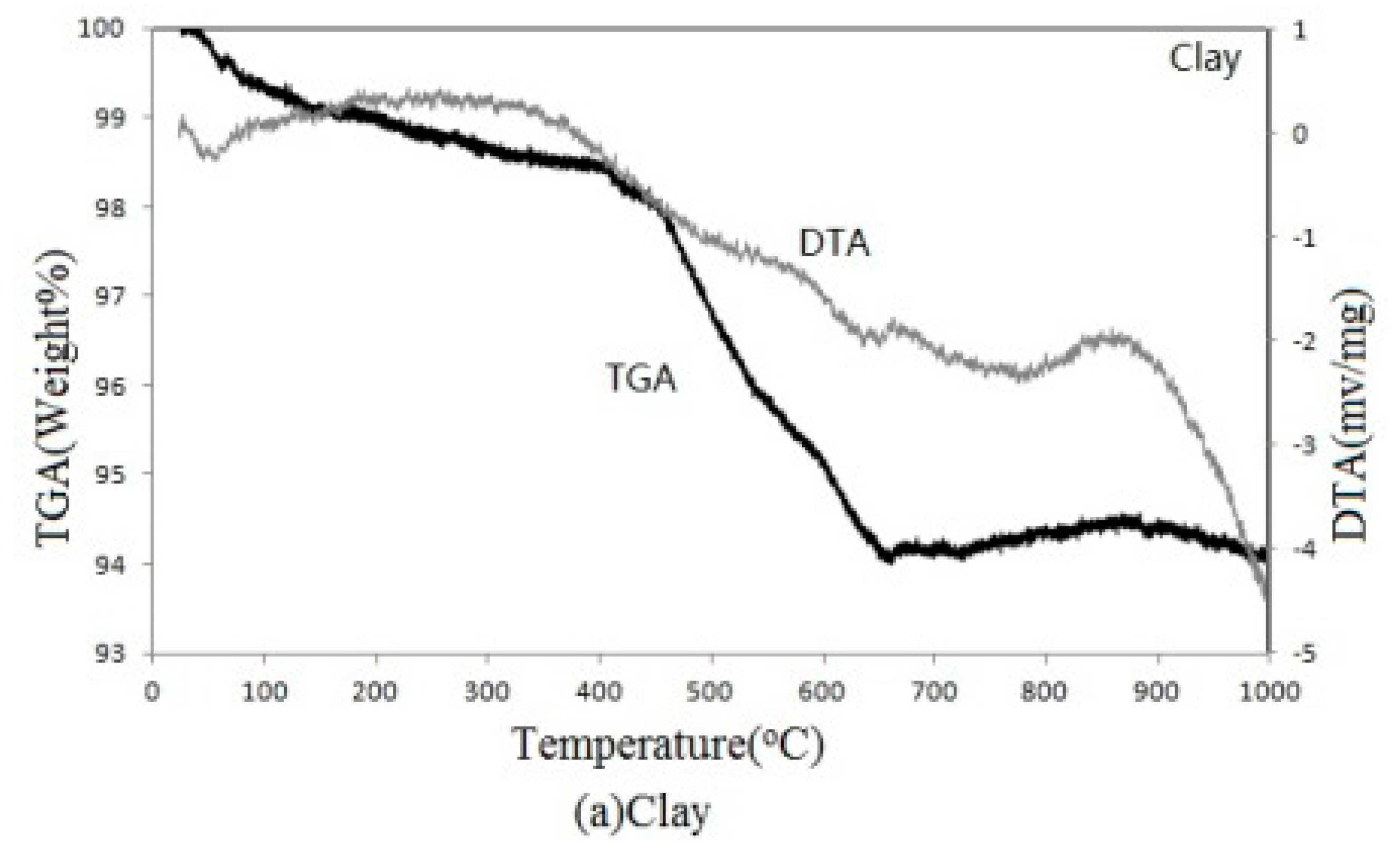
The clay used in this study was a gray-white powder with a specific gravity of 2.33. Its chemical compositions were O (45.02%), Si (23.52%), Al (14.11%), K (7.52%), and Ca (4.77%). The compositions were determined by energy dispersive X-ray analysis (EDXA) (Oxford’s Instrument, Abingdon, UK), which detects unique sets of peaks with different atomic structures on a sample’s X-ray emission spectrum.
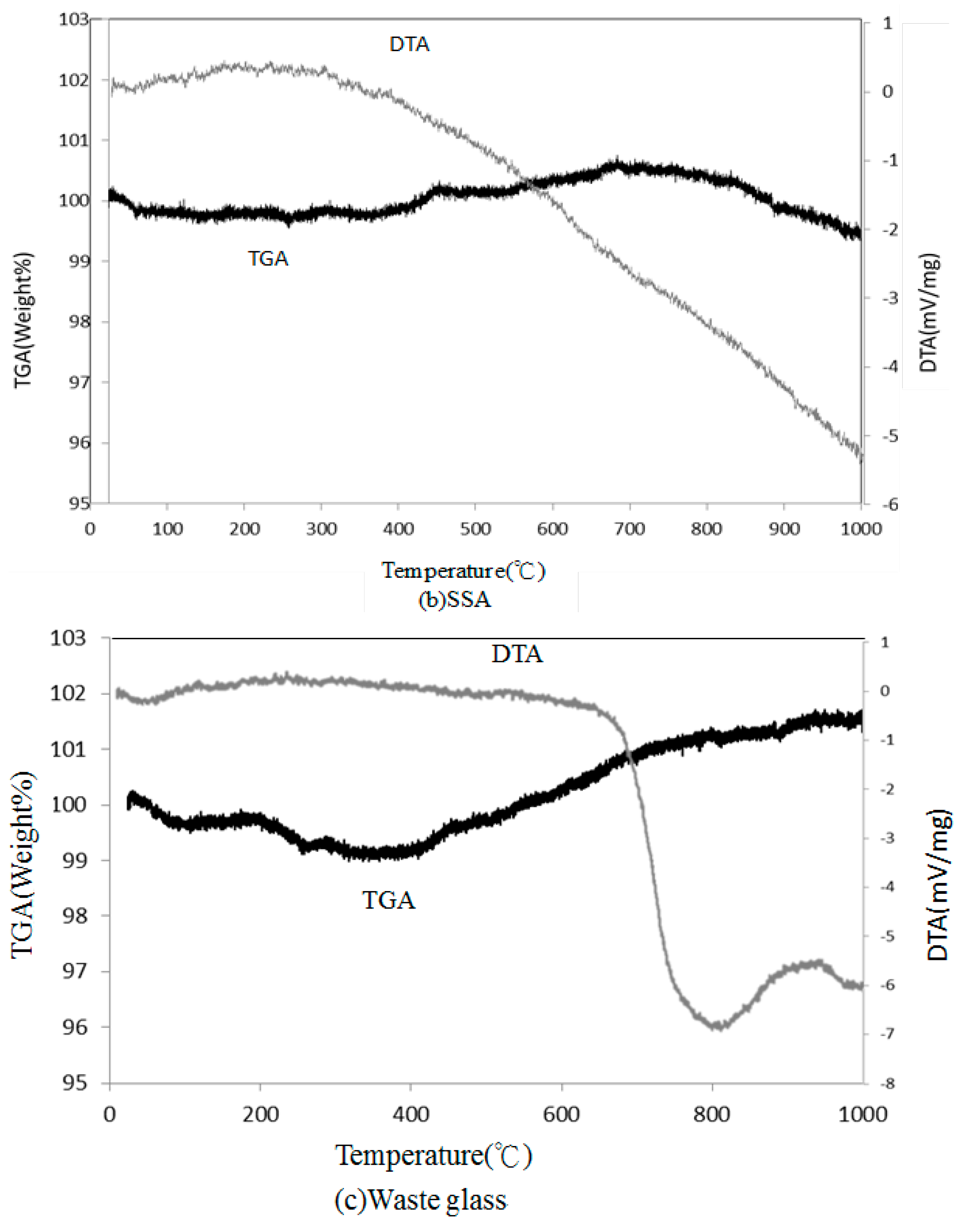
The sewage sludge cakes were acquired from the Kaohsiung City wastewater treatment plant with a specific gravity of 2.33 and moisture content of 75%. These cakes were naturally dried for 1 to 2 days before initially incinerated in a kiln at 800 °C for 20 h. After kiln incineration, the cakes were ground into a powder to pass through #200 sieves by a multi-spinning powder-grinding machine. The chemical compositions of sewage sludge ash (SSA) were then analyzed with EDXA; the SSA was found to include O (50.34%), Si (37.86%), Ca (2.66%), Fe (2.95%), and Al (2.38%), as well as slight amounts of Mg, K, Cr, and Zn. Waste glass sample used in this study was acquired from a recycling company near Kaohsiung City and had a specific gravity of 2.39. The chemical compositions of the waste glass sample were found to be O (46.87%), Si (28.44%), Na (7.85%), and Ca (7.12%), and trace amounts of Mg, Al, and C were detected.
Before being sent for making specimens, the SSA and waste glass samples underwent a toxicity characteristic leaching procedure (TCLP), which is to ensure that the raw materials are free of toxic hazards. TCLP simulates the conditions that may be present in a landfill, where water may pass through the land-filled waste and travel into the groundwater carrying the soluble materials with it. The test was performed according to NIEA R201.14C [
18] (National Institute of Environmental Analysis, Taipei, Taiwan), which is equivalent to EPA Test Method 1311 [
19] (Environmental Protection Agency, Washington, DC, USA). TCLP test results showed that the leached metal concentrations of these materials complied with the requirements set by the Environmental Protection Agency of Taiwan, as shown in
Table 1.
In this study, the amounts of clay replaced with waste glass were 0%, 10%, 20%, 40%, and 60% for the fabrication of reclaimed tile specimens with 10% SSA replacement. In the process of fabrication, clay, SSA, and different amounts of waste glass were uniformly mixed in a shaft clay mixer first, and the mixtures were kneaded with a de-airing vacuum pug mill to reduce extra interior pores. Afterwards, the well-kneaded mixtures were placed in a mold with the size of 12 × 6 × 1 cm
3 and compressed by a pressing machine with a normal pressure of 34.32 ± 0.5 MPa to produce the specimens. These molded specimens were next placed in a wooden plate and dried down in room temperature. After drying, the specimens were left in a kiln at a temperature of 1000 °C. Kiln temperature was controlled at a rate of 2 °C/min increase. Then, the specimens were cooled to room temperature in the kiln to complete the fabrication. The tests conducted during the course of this study included tests of abrasion resistance, weight loss on ignition, shrinkage, and bending strength to determine the relevant physical and chemical properties of the reclaimed tile specimens based on the CNS (Chinese National Standard) 3299 standards [
20], which are equivalent to ISO (the International Organization for Standardization) 10,545 standards [
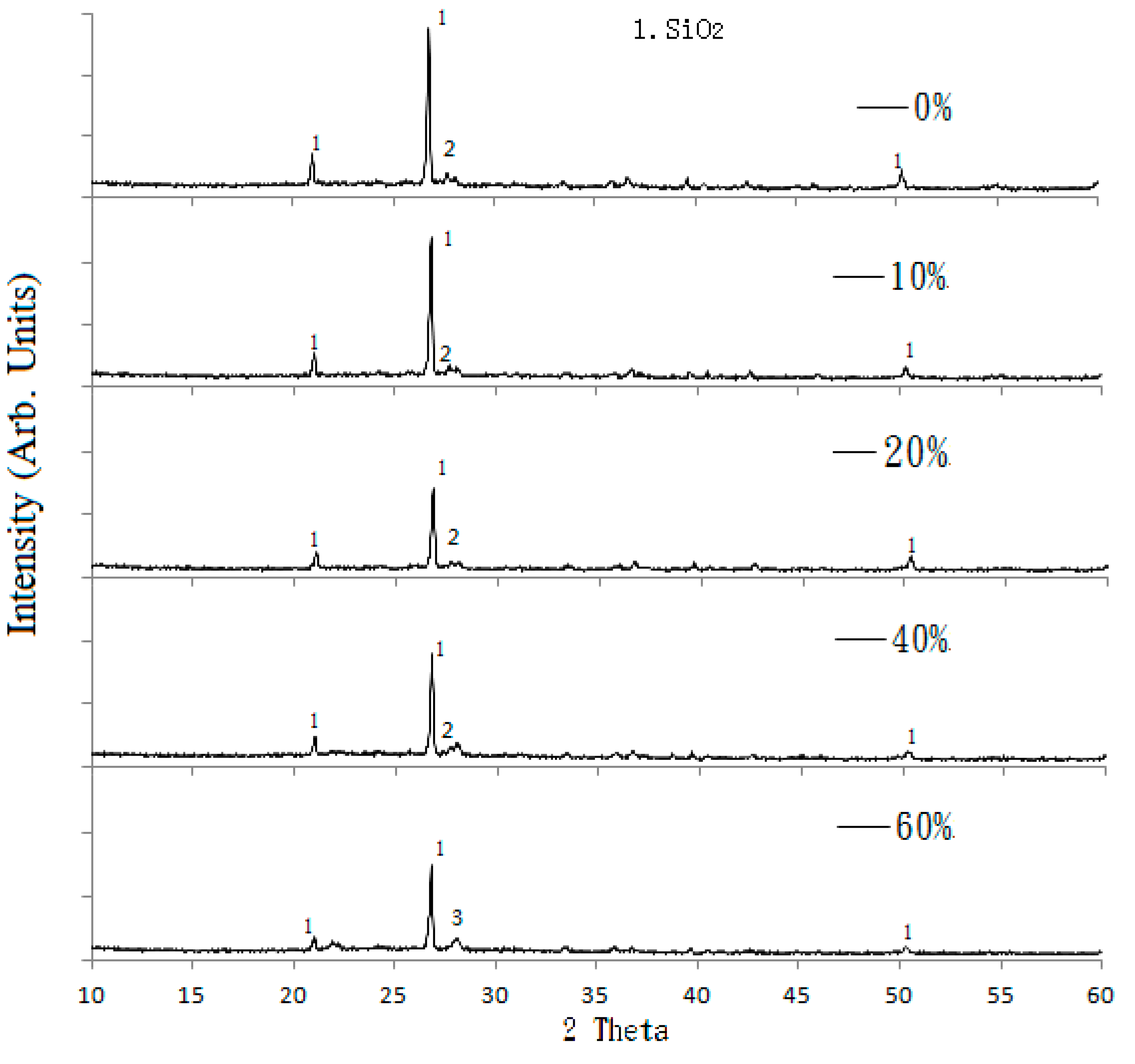
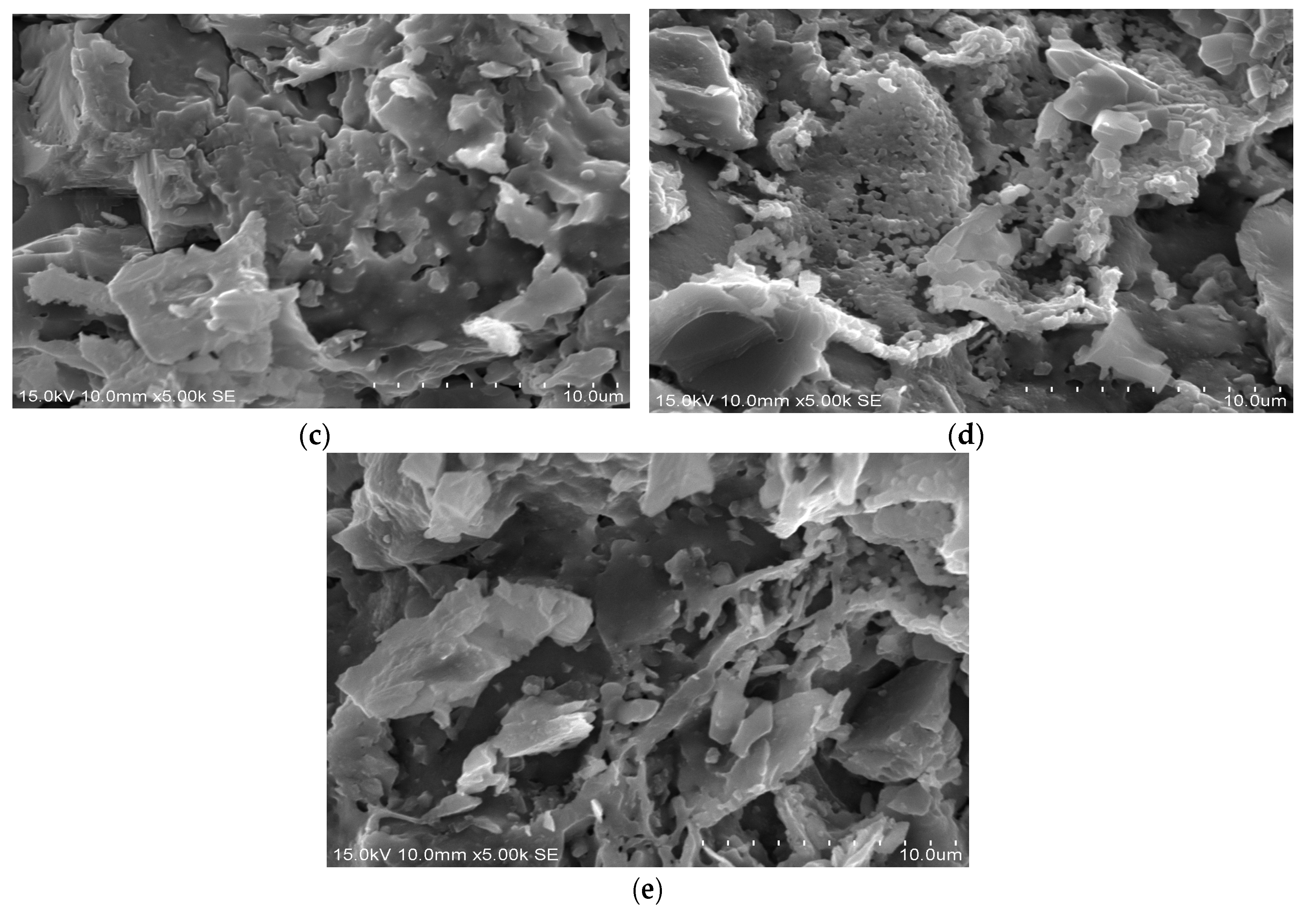
21]. Furthermore, a Shimadzu X-ray diffractometer (model XRD-6000) (Tokyo, Japan) was used for X-ray diffraction (XRD) analysis at a scan rate of 2°/min, a diffraction angle (2θ) of 10 to 60 degrees, an electric current of 30 mA, and an operating voltage of 40 kV. Scanning electron microscopy (SEM) equipment (model S-4700, Hitachi, Tokyo, Japan) was used to study the microstructure of the reclaimed tile specimens.
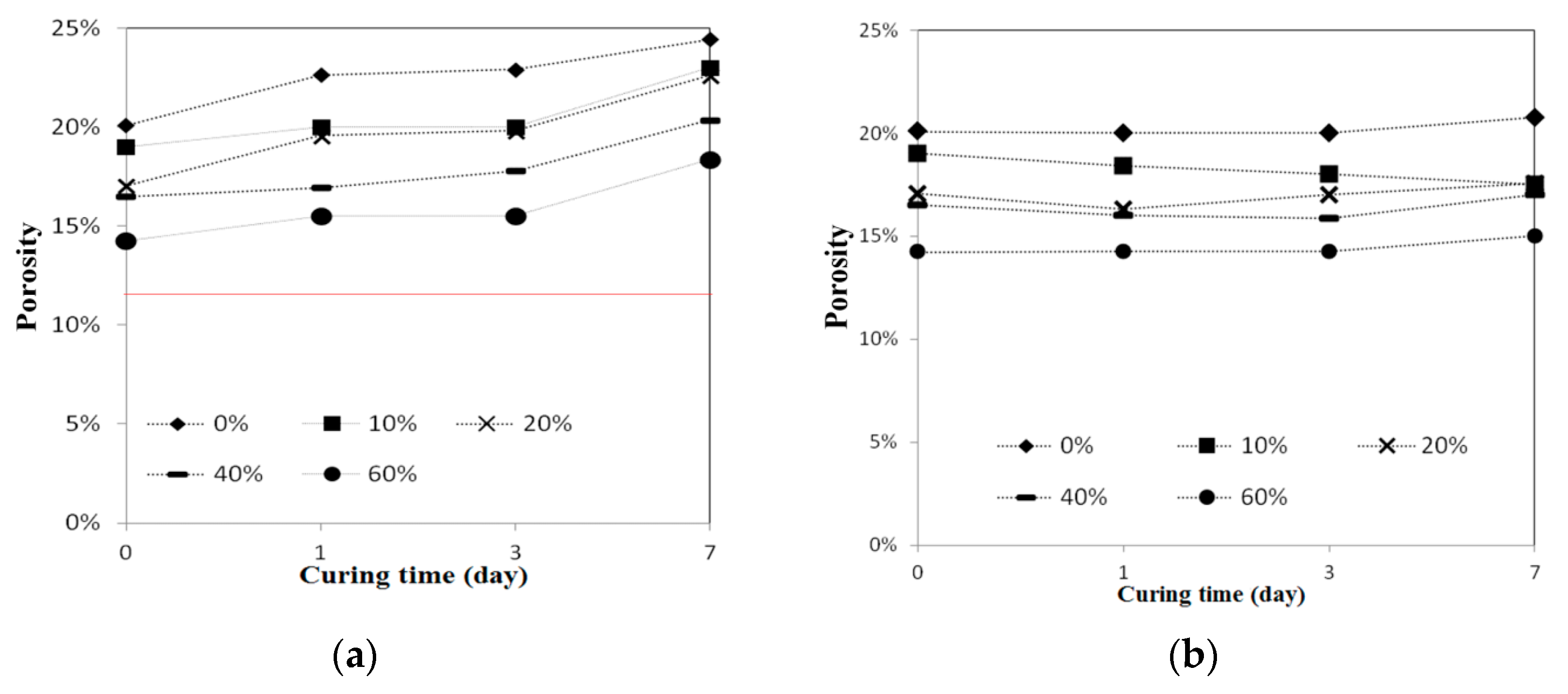
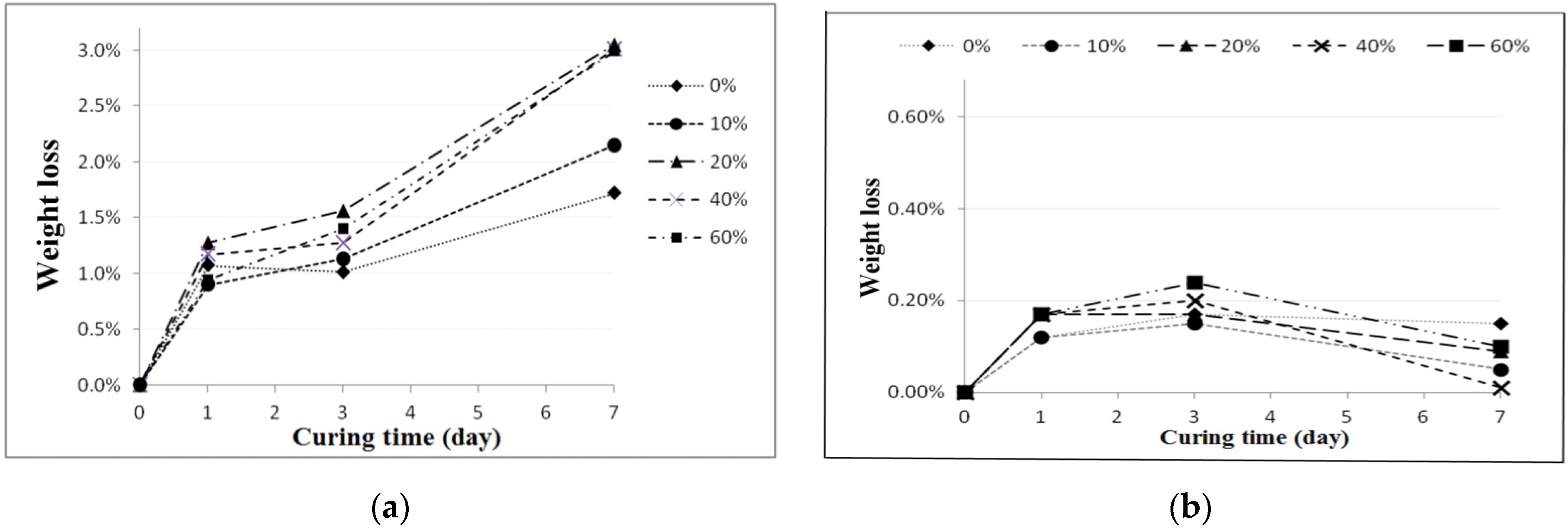
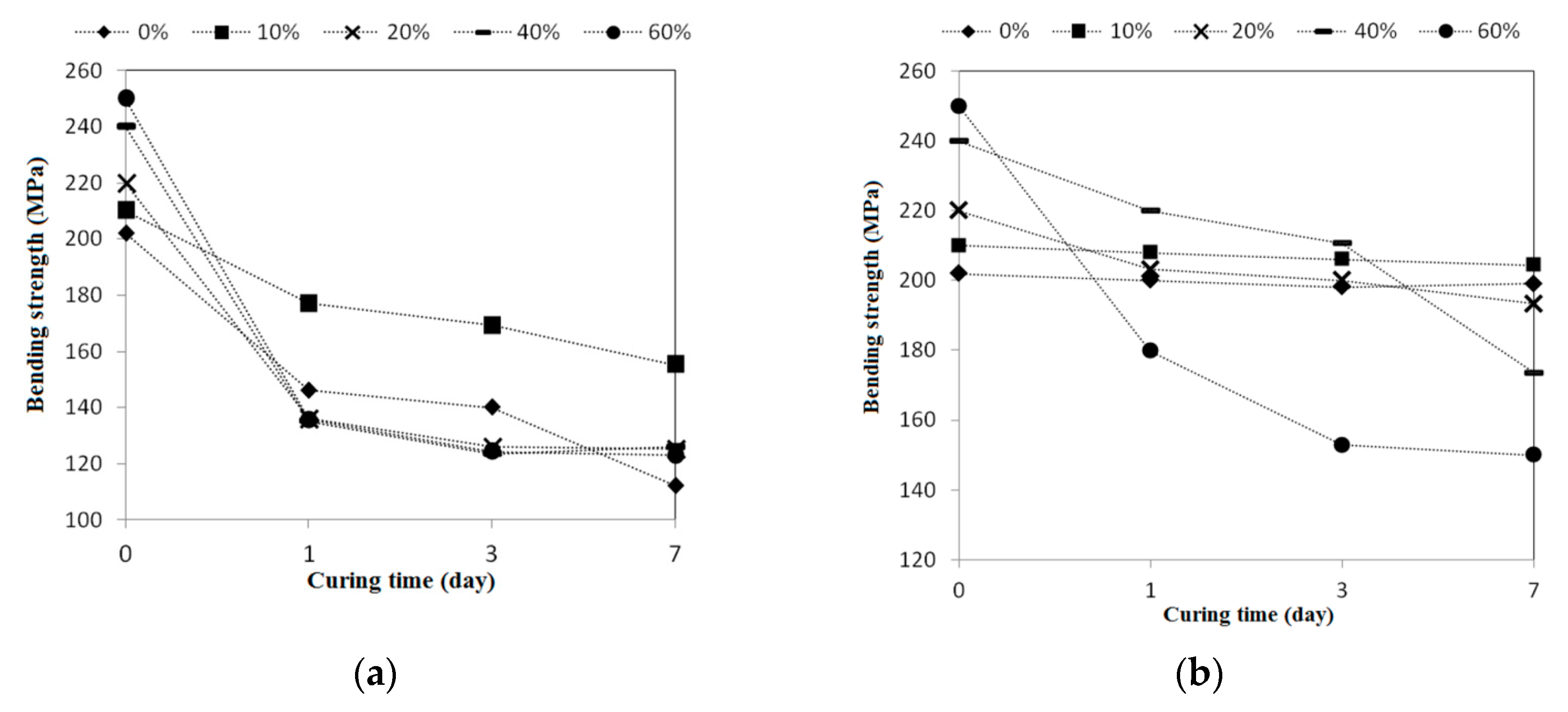
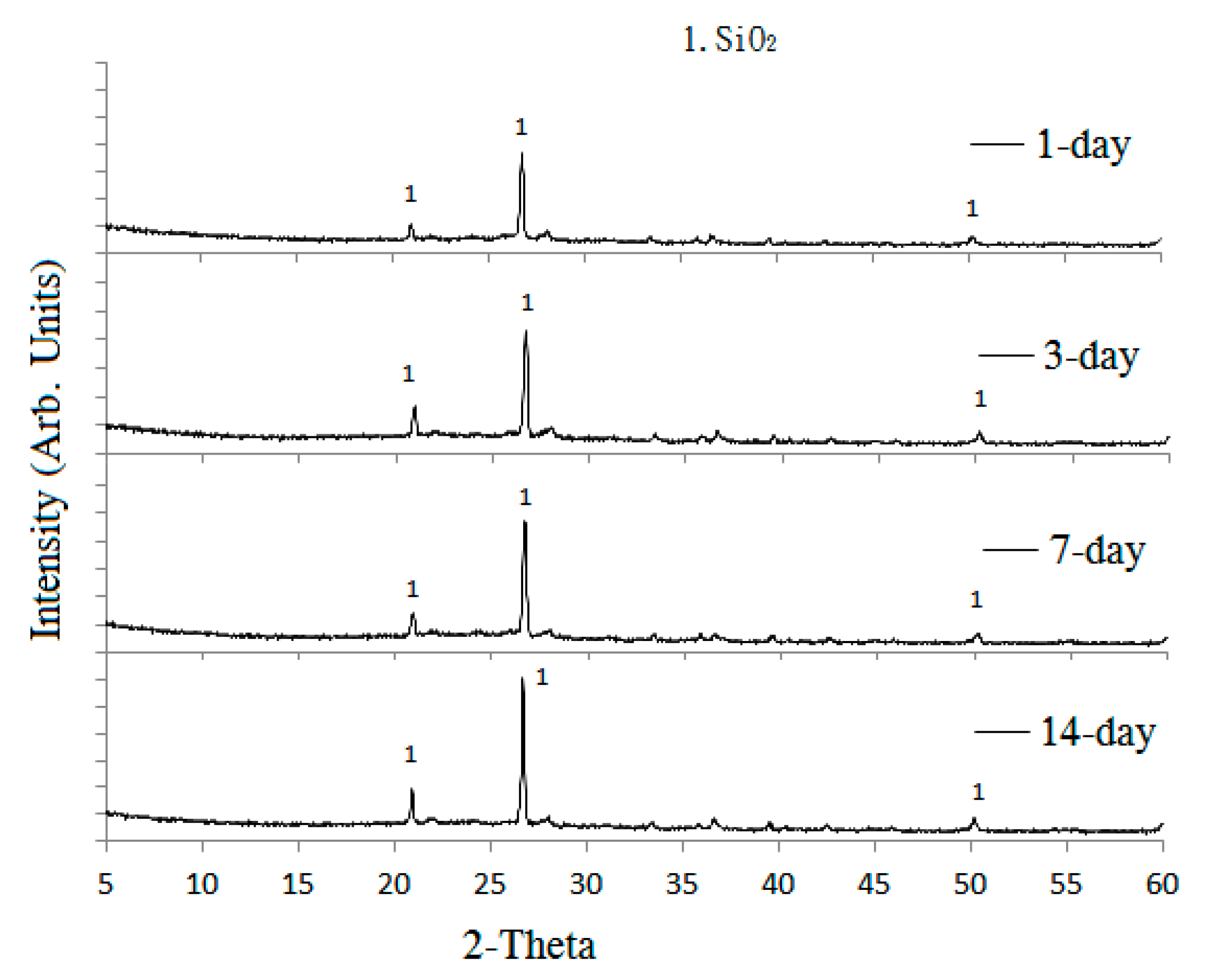
To perform the acid-alkali resistance tests, the reclaimed tiles specimens containing different amounts of waste glass were submerged in HCl and KOH solutions. The pH values of acidic and alkaline solutions were maintained at 4 and 10, respectively. The resistance tests were performed at room temperature, and the specimens were retained in the acidic and alkaline solutions for 1-, 3-, and 7-day periods. At the end of each experimental period, tests including porosity, weight measurement, bending strength, and X-ray diffraction (XRD) tests were carried out for the particular specimens.
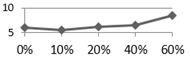
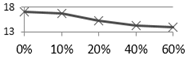
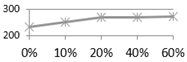
4. Conclusions
In this study, the possible application of using waste glass and sewage sludge ash (SSA) to replace part of the clay in the manufacture of reclaimed tiles was further investigated. Test results performed in the normal environment show that the main chemical components of the waste glass and SSA were silicon dioxide and alumina, respectively. The addition of waste glass to the reclaimed tile specimens decreased the porosity and water absorption of the tiles and enhanced their bending strength. Moreover, after the completion of the calcination of the reclaimed tile specimens containing waste glass and SSA, the weight loss of the tile bodies was apparently reduced. When 10% SSA replacement was applied in the reclaimed tile specimens, increasing the waste glass content resulted in higher shrinkage and reduced weight loss on ignition in the bodies of the reclaimed tiles. Furthermore, the XRD spectra show that the SiO2 peak intensity decreased with an increasing level of waste glass replacement. The Al2O3 content played an important role in crystallization in the bodies of the reclaimed tiles. These findings suggest that the production of crystalline structures in the tile bodies might be affected by the waste glass content. The results of this study indicate that, for reclaimed tile specimens with 10% SSA replacement, the optimal level of waste glass replacement is between 10% and 20%. Acid-alkali resistance tests were performed on the reclaimed tiles. It was found that the reclaimed tiles performed well in the acid-alkali resistance tests, especially in the alkali resistance test. Hence, it is suggested that the reclaimed tiles can be applied to the alkaline environment. Furthermore, extra weight loss of the reclaimed tiles was noticed in the acidic environment. The weight loss increased with the increased amount of the waste glass replacement. The reduction in bending strength of the reclaimed tiles was larger in the acidic environment than that in the alkaline environment. The amount of the waste glass replacement had an impact on the bending strength of the reclaimed tiles. Based on the results of this study, it is not recommended that reclaimed tiles be placed in acidic environments over a long period of time.